Introduction
Unexplained head and neck masses are common and all require prompt evaluation to exclude malignancy. Tissue samples are required for diagnosis in all such cases and this is usually obtained by fine needle aspiration cytology (FNAC). However, if FNAC is inconclusive, an open surgical biopsy is required.
Surgical excision has well described disadvantages, including the requirement for in-patient admission, scar formation, the risk of neurovascular injury and the dangers associated with general anaesthesia.Reference Kleid and Millar1 Core-needle biopsy has emerged as a viable alternative; it can safely provide high quality tissue samples within an out-patient setting using local anaesthetic.
Core-needle biopsy has been used by interventional radiologists for over 25 years and has now become a well described and accepted technique in the head and neck clinic.Reference Bearcroft, Berman and Grant2–Reference Pfeiffer, Kayser, Technau-Ihling, Boedeker and Ridder6 Evidence for the diagnostic accuracy of core-needle biopsy in the head and neck region has previously relied on image guidance, primarily using ultrasonography. However, this facility is not readily accessible in many otolaryngology clinics, and referral to local radiological services can result in unwanted diagnostic delay. We describe the diagnostic utility of freehand core-needle biopsy in select patients to assess unexplained head and neck masses.
Materials and methods
A retrospective chart review was performed on all patients who underwent core-needle biopsy for the assessment of an unknown cervicofacial mass in the Department of Otolaryngology, Edinburgh between August 2006 and June 2008. Clinical information was obtained from the case record files as part of a service evaluation.
Patient characteristics
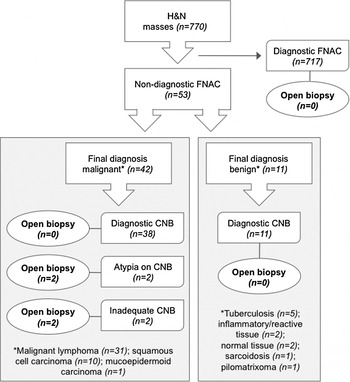
In total, 770 patients with neck masses were evaluated; 53 of these patients (7 per cent) had an unexplained mass and had undergone core-needle biopsy and formed the study cohort (Figure 1). The study comprised 29 males and 24 females (a ratio of 1.2 to 1) with an age range of 21–86 years and a mean age of 58 years. The study cohort included 13 patients (25 per cent) with a clinical history of lymphoma and 3 patients (6 per cent) with a history of carcinoma.

Fig. 1 Flowchart showing patient management using core-needle biopsy. H&N = head and neck; FNAC = fine needle aspiration cytology; CNB = core-needle biopsy
Inclusion criteria
Patients were considered suitable for core-needle biopsy if the assessment by FNAC was non-diagnostic. Forty-four of the patients (83 per cent) had undergone FNAC prior to core-needle biopsy. Fine needle aspiration cytology was not attempted in two patients with inaccessible tongue base masses, in six patients with recurrent cervicofacial masses and a past history of lymphoma, and in one patient in whom tuberculosis (TB) was clinically suspected. If the FNAC results were negative, but there was a continued suspicion of squamous cell carcinoma (SCC) from an unknown primary, FNAC was performed a second time; if still inconclusive, core-needle biopsy was used rather than excision biopsy. Core-needle biopsy was also used in patients with residual neck masses following chemoradiation for SCC if FNAC was inconclusive in excluding recurrent or residual disease.
Core-needle biopsy was deemed suitable and safe by the investigating physician (IG) if the mass was over 1 cm in size, easily accessible and not in close proximity to the carotid sheath. Thirty-six patients (68 per cent) had cross-sectional imaging prior to core-needle biopsy (30 underwent computed tomography (CT), 4 magnetic resonance imaging (MRI), 1 positron emission tomography (PET)-CT (combined approach), and 1 patient underwent both CT and MRI). The remaining 17 patients had their core-needle biopsy performed without radiological studies.
Head and neck mass characteristics
Head and neck masses ranged in size, with a maximal diameter of 1–15 cm (mean 4.3 cm, median 3 cm). The anatomical location of the masses in the 53 patients biopsied is shown in Table I.
Table I Anatomical location of biopsy sites

Biopsy technique
Informed verbal consent was obtained from each patient prior to biopsy. As this was a novel procedure within the department, the initial four cases were performed, without complication, in the operating theatre under general anaesthesia. The technique was subsequently performed in the out-patient setting under local anaesthesia. However, in some cases the patients were undergoing additional assessment that required general anaesthesia (e.g. rigid upper aerodigestive tract endoscopy), and the biopsy was carried out during this procedure.
The core-needle biopsy was performed in the out-patient department in 42 patients (79 per cent). No patients preferentially requested the procedure to be undertaken under general anaesthesia. A coagulation profile was not routinely obtained before the procedure.
All procedures were performed freehand, without image guidance, by the same senior clinician (IG). A 14-gauge Tru-Cut biopsy needle (Cardinal Health, McGaw Park, Illinois, USA) was employed using a standardised percutaneous technique.Reference Bearcroft, Berman and Grant2, Reference Bain, Bearcroft, Berman and Grant3 The masses were sampled transcutaneously, with the exception of the four tongue bass masses which were biopsied transorally. A mean average of 2.2 cores were taken per patient (range 1–5). These were fixed in 10 per cent buffered formalin before being submitted to routine histological examination. Patients were monitored in the department for 30 minutes prior to discharge.
Open surgical biopsy was performed only if the diagnosis from the core-needle biopsy and other available information was in doubt. All core biopsies were reviewed by a pathologist with a special interest in head and neck SCC, with the exception of the core biopsies suspicious for lymphoma which were reviewed by one of two haematopathologists.
Statistical analysis
Sensitivity, specificity and accuracy were calculated from data for each patient, rather than for each individual core taken. Accuracy reflected the sampling error rate in correctly sampled lesions for which sufficient material was obtained for diagnostic purposes. The final diagnosis was based on histological examination of subsequent excised specimens, additional laboratory studies (e.g. serology or microbiological analysis) or from the patient's clinical course (using information obtained from the case records after a minimum one-year follow up). The diagnostic difference of core-needle biopsy in the assessment of benign versus malignant masses was examined using the chi-square test. A difference was considered significant if the p value was less than 0.05.
Ethical considerations
This study was a retrospective chart review. Following a review of the study protocol by the scientific officer from the South East Scotland Research Ethics Service, the authors were advised that the project was a service evaluation and therefore did not require National Health Service ethical review under the terms of the Governance Arrangements for Research Ethics Committees in the UK.
Results
The core biopsy technique was well tolerated and there were no reported complications within the study population. The final histological diagnoses of the patients are shown in Tables II and III. Specimens obtained by core-needle biopsy in the study population were of high quality. All 53 patients (100 per cent) had the target tissue correctly sampled using a freehand technique. Samples in 51 patients (96 per cent) yielded sufficient tissue volume for diagnostic purposes. In total, 4 patients (7 per cent) required open surgical excision prior to commencing treatment (Figure 1).
Table II Benign diagnoses and core-needle biopsy results

*Based on histology.
Table III Malignant diagnoses and core-needle biopsy results

*Based on histology.
In 11 patients, the final histology was benign and for this group the diagnostic accuracy of core-needle biopsy was 100 per cent (Table II). In three patients, the cytopathologist recommended open biopsy on the basis of FNAC, but this was avoided following pathological clarification with core-needle biopsy. Five cases of TB were identified from core biopsy in conjunction with other laboratory investigations that are routinely performed to diagnose this condition (e.g. 24-hour urine collection, induced sputum culture and chest radiograph). No TB patients required open biopsy, thereby avoiding the risk of developing a chronic discharging fistula.
Of the 42 cases in which the final histology was malignant, there were 31 cases of lymphoma and 10 cases of SCC (Table III). The cores taken from this group were diagnostically accurate in 38 patients (90 per cent). In two patients, core-needle biopsy provided insufficient tissue to reach a final diagnosis. In one of these patients, core-needle biopsy yielded insufficient material from an enlarged cervical node; excision biopsy revealed chronic lymphocytic leukaemia. In the second case, core-needle biopsy from a cervical node was suggestive of a malignant B-cell lymphoma but could not be confirmed as the tissue was highly necrotic; surgical excision corroborated this diagnosis. Two further patients required open biopsy before a final histological diagnosis could be made. One of these patients, with a parotid mass and non-diagnostic FNAC, underwent core-needle biopsy that showed chronic inflammation, sclerosis and atypia suggestive of a neoplasm. Subsequent excision provided a final histological diagnosis of low grade mucoepidermoid tumour. The other patient, who presented with a cystic cervical mass in the level II region, had an FNAC that was suggestive of a branchial cyst. Core-needle biopsy demonstrated severe dysplasia and probable SCC; invasive squamous carcinoma was confirmed using surgical excision with frozen section followed by neck dissection.
Ten patients had a final histopathologic diagnosis of SCC. Of these, three had tongue base masses, including one with a history of previously treated tongue base SCC. There was no associated cervical lymphadenopathy. Due to the site of the primary mass, FNAC was only attempted in one of the three patients and this was non-diagnostic. The remaining seven patients all presented with head and neck masses (six cervical, one parotid) with an unknown primary site. There was no previous history of malignancy, and FNAC had proved non-diagnostic on more than one attempt. Core-needle biopsy confirmed SCC, thus avoiding an open biopsy in these patients.
Lymphoma was the final histological diagnosis in 31 patients, 4 of whom had Hodgkin lymphoma (2 primary, 2 recurrent) and 27 had non-Hodgkin lymphoma (17 primary, 10 recurrent). Two of the three patients with inaccurate core-needle biopsy fell within the lymphoma cohort. Of the adequate samples, full sub-classification according to the World Health Organization systemReference Harris, Jaffe, Diebold, Flandrin, Muller-Hermelink and Vardiman7 was achieved in 29 patients from the core biopsy alone, none of whom required open biopsy prior to the initiation of treatment.
If inadequate or equivocal samples are to be regarded as false negatives, the analysis of all 53 patients as a single group provides an accuracy of 96 per cent, and a sensitivity of 92 per cent for the freehand core-needle biopsy technique. There was no statistical difference in diagnostic accuracy between patients with benign or malignant histological diagnoses or, within the malignant group, between those with primary or recurrent tumour.
Discussion
The accurate diagnosis of suspicious head and neck masses requires high quality pathological samples. This should ideally be done using a technique that is technically easy, readily available, inexpensive and acceptable to the patient. Our observational study has shown core-needle biopsy to be effective in diagnosing a variety of benign and malignant aetiologies. The time targets of modern cancer pathways have placed considerable pressure on diagnostic oncology specialties; core-needle biopsy is capable of increasing the speed with which patients are either reassured or their treatment initiated.
In our cohort of 53 patients, only 4 (7 per cent) required open surgical excision before a definitive management plan could be instituted. Avoidance of an open biopsy procedure with resulting scar is preferable to patients. Open biopsy has risks associated with general anaesthesia and neurovascular injury. Other disadvantages include the inherent financial cost and use of in-patient resources.
Core-needle biopsy was first described as a sampling technique in the diagnosis of head and neck masses by Bearcroft et al. in 1995.Reference Bearcroft, Berman and Grant2 Since then, a number of authors have published case series of their experience with the technique. Most of these have involved image-guided targeting, primarily using ultrasonography.Reference Bain, Bearcroft, Berman and Grant3–Reference Pfeiffer, Kayser, Technau-Ihling, Boedeker and Ridder6 Radiological visualisation during the procedure allows precise targeting of the lesion whilst avoiding important surrounding structures. However, in clinics without immediate on-site radiological support, this technique inevitably leads to a logistical diagnostic delay, and increases manpower and financial costs. A recent review of the diagnostic accuracy of ultrasound-guided core-needle biopsy in salivary gland lesions highlighted the increased sensitivity of this technique compared with FNAC.Reference Schmidt, Hall and Layfield8 Its use was reported to result in a lower false negative rate: from 21 per cent for FNAC only, to 1 per cent when FNAC was combined with core-needle biopsy. It also increased the adequacy of repeat biopsies in patients with a previous non-diagnostic FNAC, although it was unclear whether the small incremental difference was cost-effective.
The current series, with a diagnostic accuracy of 96 per cent, compares favourably with those studies using ultrasound guidance. Whilst imaging is undoubtedly the gold standard in sampling small lesions or those adjacent to critical structures, this study demonstrates that high quality pathological samples can be obtained in select patients without image guidance. However, the selection of patients does require an experienced clinician with good anatomical knowledge of important neurovascular structures in the head and neck region in order to avoid any complications.
In patients with SCC, the use of open surgical biopsy for neck masses is largely condemned due to the risk of tumour dissemination within the soft tissues of the neck. Every attempt should be made to obtain the diagnosis by FNAC. If the first FNAC is inconclusive then ultrasound guidance may help to identify areas with solid tissue, thus avoiding a false negative from the aspiration of areas of necrosis. However, there will be a few select patients in whom open biopsy will have to be carried out if FNAC remains inconclusive. This includes patients with a neck mass suspicious for SCC from an unknown primary and patients with residual neck masses treated with chemoradiation for SCC. In this select group of patients, core-needle biopsy can be carried out successfully without resorting to open surgical biopsy. Of the 10 patients with SCC in our study, 9 were definitively diagnosed using core-needle biopsy (8 primary and 1 with recurrent disease), and the other was diagnosed with severe dysplasia and probable invasive disease. Core-needle biopsy is therefore a useful adjunctive assessment tool in patients suspected of having SCC where FNAC has been non-diagnostic or equivocal. We have also found core-needle biopsy to be of benefit when assessing patients who have undergone previous radiotherapy and/or chemotherapy in whom scar tissue can be difficult to separate from recurrent tumour on clinical or radiological grounds alone.
The robust diagnosis of lymphoma in the head and neck relies on the provision of specimens that preserve nodal architecture and that are of sufficient volume for full sub-classification. Modern lymphoma examination employs a number of histological, immunohistochemical and cytogenetic techniques, wherein emphasis is placed on both the quantity and quality of diagnostic tissue. As a consequence, complete nodal excision remains the gold standard for the assessment of probable lymphoma in USA guidelines.Reference Hoppe, Advani, Bierman, Bloomfield, Buadi and Djulgegovic9 This is of particular significance in a tumour where tissue architecture remains diagnostically important, such as low grade non-Hodgkin lymphoma or nodular lymphocyte-predominant Hodgkin lymphoma. The core-needle biopsy sampling in this study provided adequate tissue volume for all necessary histopathological methods to be performed. An advantage of this biopsy technique is that a number of cores can be obtained from a nodal mass, providing a representative cross section of nodal architecture and tissue volume for genetic studies to be performed.
Core-needle biopsy is well established in the diagnosis of inaccessible nodal lymphomas such as those in the chest and retroperitoneum for which the risks of open surgical biopsy are unacceptably high. There has been considerable interest in employing this technique in the assessment of peripheral lymphoma masses. Indeed, a number of studies have shown core-needle biopsy to be effective in the identification and classification of such masses.Reference Ben-Yehuda, Polliack, Okon, Sherman, Fields and Lebenshart10–Reference Kim, Kim, Kim, Yang, Park and Park14 The results of the current study further support this view and suggest that core-needle biopsy has a role in the management of patients with suspected head and neck lymphoma. Whilst excision biopsy remains preferable, core biopsy can be used for the exclusion of other causes of lymphadenopathy, and the rapid diagnosis and sub-classification of lymphoma in patients where open biopsy is undesirable due to co-morbidity. Patients at high risk from general anaesthesia include those with advanced disease or bulky cervical lymphadenopathy, a proportion of whom will have occult mediastinal disease.
The definitive diagnosis of TB requires the identification of either acid-fast bacilli on microscopy or positive culture. Studies investigating the success of core biopsies in diagnosing TB in other anatomical sites have shown variable success.Reference Fukuda, Ibukuro, Tsukiyama and Ishii15 Of the five patients with TB in our series, three had acid-fast bacilli as revealed by core-needle biopsy, and anti-tuberculous treatment was commenced based on this finding alone. The two remaining patients had caseating granulomas according to core-needle biopsy, but the final diagnosis was made after microbiological review of other specimens (induced sputum and urine collection). In these patients the core-needle biopsy findings excluded malignant disease and focused further investigations appropriately without the need for open surgical biopsy.
• Inconclusive fine needle aspiration cytology (FNAC) for head and neck masses traditionally results in open surgical biopsy
• Image-guided core-needle biopsy is an alternative to surgical biopsy in select patients
• This paper describes the diagnostic accuracy of freehand core-needle biopsy
• Nodal tissue was obtained in all 53 patients using this technique
• The routine use of core-needle biopsy (after FNAC) may avoid the need for open surgical biopsy
Sarcoidosis was correctly identified using core-needle biopsy in one patient in our study. Although core biopsies have been utilised for the diagnosis of sarcoidosis outside of the head and neck,Reference Morita, Numata, Tanaka, Mitsui, Matsumoto and Kitamura16 this condition is an uncommon cause of cervical lymphadenopathy and rarely described in head and neck case series. In a retrospective analysis of 181 cervicofacial core-needle biopsies in 88 patients, Pfeiffer et al. successfully diagnosed a single patient with sarcoidosis using this technique.Reference Pfeiffer, Kayser, Technau-Ihling, Boedeker and Ridder6 Our experience suggests that core biopsy is effective in differentiating between various granulomatous disorders and is successful in identifying rarer causes of head and neck masses.
The seeding of tumour cells along the needle track is a potential risk of any percutaneous needle sampling technique. This complication was not identified in any of the patients within this study at follow up (which was a minimum of nine months). The exact risk of this happening when sampling superficial craniofacial masses has not been established, but previous published case series have yet to identify a single case of this occurring.Reference Bearcroft, Berman and Grant2–Reference Pfeiffer, Kayser, Technau-Ihling, Boedeker and Ridder6, Reference Fukuda, Ibukuro, Tsukiyama and Ishii15 It remains pragmatic, however, for definitive treatment of a newly diagnosed tumour to occur soon after diagnosis, and clinicians should consider including the core needle track in any planned surgical resection or radiation field.
Conclusion
Freehand core biopsy is a valuable tool in the diagnosis of unknown head and neck masses. It rapidly provides high quality tissue specimens. It is safe and well tolerated by patients. While the technique does not completely replace open biopsy, it reduces the operative workload to a small number of cases for which particular lymphoma subtypes need to be excluded. Core-needle biopsy should therefore be considered an intermediary step (after FNAC) in many patients being considered for nodal excision.