Introduction
Over the past several decades, a multidisciplinary team approach to the evaluation and management of parapharyngeal space neoplasms has evolved. Over this timeframe, there has been a trend toward a more conservative approach to the management of these tumours. It is now clear that some patients can avoid significant morbidity with observation or non-surgical management. In addition, select patients with neurogenic neoplasms can be managed with nerve preservation strategies.
Imaging studies are the key to correct diagnosis of parapharyngeal space neoplasms, and cytopathology may provide additional diagnostic information for some tumours. Treatment should be individualised for a given patient, and management should minimise patient morbidity. Enhanced surgical access may be required for select patients with large or malignant neoplasms. Some sequelae that occur following resection of parapharyngeal space neoplasms require special approaches. This article will review new perspectives on the multidisciplinary team approach to parapharyngeal space neoplasms.
Anatomy
The parapharyngeal space is an inverted pyramidal shaped potential space with its base at the skull base and its apex at the greater cornu of the hyoid bone (Figure 1). Its three sides are made up of both immobile and distensible boundaries. The medial boundary is the superior pharyngeal constrictor muscle, and is thus distensible. The lateral boundary (the medial pterygoid muscle, mandibular ramus and deep lobe of the parotid gland) and the posterior boundary (the prevertebral fascia and the cervical vertebrae) are relatively immobile barriers. Tumours can thus grow medially and inferiorly within the potential space to present as a pharyngeal or neck mass.
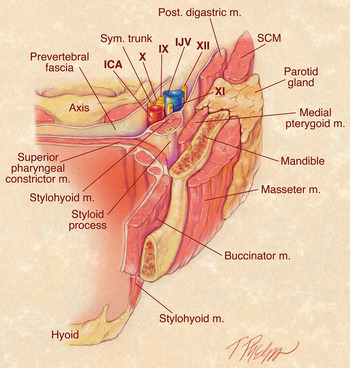
Fig. 1 Diagram of the parapharyngeal space demonstrating the pre-styloid and post-styloid compartments. Post = posterior; m = muscle; Sym = sympathetic; IJV = internal jugular vein; SCM = sternocleidomastoid; ICA = internal carotid artery
The parapharyngeal space can be divided into two compartments by the tensor veli palatini fascia which extends between the lateral pterygoid plate and the styloid process. These two compartments are the pre-styloid space, which is located antero-laterally, and the post-styloid space, positioned postero-medially. Knowledge of the content of these compartments is essential to the differential diagnosis of neoplasms that arise in the parapharyngeal space.
Most parapharyngeal space neoplasms are primary tumours that arise from the structures within the parapharyngeal space. Tumours arising in the pre-styloid space are most commonly of deep parotid gland origin. Adipose tissue is also present in this space. Post-styloid tumours, however, usually arise from neurovascular structures, including the carotid artery, internal jugular vein, cranial nerves IX to XII and the sympathetic chain. Also, some neoplasms (e.g. nasopharyngeal carcinoma) may extend into the parapharyngeal space from adjacent sites. In addition, malignant neoplasms may metastasise to poststyloid parapharyngeal space lymph nodes.
The majority of parapharyngeal space neoplasms (80 per cent) are benign. Salivary gland neoplasms comprise approximately 40 per cent of parapharyngeal space neoplasms, followed by neurogenic neoplasms (30 per cent).Reference Batsakis and Sneige1–Reference Shahab, Heliwell and Jones5 A variety of other tumour types make up the remainder.
Presentation
Parapharyngeal space neoplasms usually present as an asymptomatic pharyngeal mass or neck mass.
They are often found during routine physical examination or imaging performed for another clinical indication.Reference Carrau, Myers and Johnson6, Reference Farrag, Lin, Koch, Califano, Cummings and Farinola7 Neoplasm location, route of expansion, tumour size and nerve integrity determine a given patient's symptoms.
Some patients have symptoms related to tumour mass effect. Such symptoms can include voice change, nasal obstruction, foreign body sensation, dysphagia, snoring, airway obstruction, and hearing loss due to eustachian tube occlusion. Cranial nerve dysfunction can relate to the nerve of origin or to other, adjacent cranial nerves secondary to tumour pressure or invasion. Nerve deficits can be partial or complete. Patients may note hoarseness, dysphagia, dysarthria or cough.
Symptoms consistent with tumour invasion, such as pain, trismus or sensory loss, suggest malignancy. Paralysis of cranial nerves VII, IX, X, XI and XII can occur due to tumour invasion or compression. Secreting paraganglioma, although uncommon, may cause hypertension, flushing, sweats, nausea, palpitations or headaches.
Evaluation
Imaging studies are essential to the evaluation of parapharyngeal space neoplasms due to the limitations of physical examination in this anatomical area.Reference Gupta, Chazen and Phillips8 Imaging studies provide important information including location (i.e. pre- or post-styloid space) and neoplasm extent (i.e. size, invasion and presence of enlarged lymph nodes). In addition, the degree of vascularity can be determined with contrast administration. Tumour multicentricity may also be noted. In general, magnetic resonance imaging (MRI) with gadolinium is the preferred imaging study for parapharyngeal space neoplasms. Computed tomography (CT) scan with contrast is an acceptable alternative imaging study for select patients who cannot have an MRI.
Magnetic resonance imaging T1 sequences define normal anatomy and the tumour–fat interface, whereas T2 sequences show the tumour margin and the tumour–muscle interface. Magnetic resonance imaging is better than CT for demonstrating the neoplasm, the direction of adjacent structure displacement, and the tumour's relationship to the deep lobe of the parotid gland. Pre-styloid masses push carotid sheath structures posteriorly. Ectopic salivary neoplasms, which are uncommon, have a fat plane preserved between the neoplasm and the parotid gland.
Schwannomas are ovoid to fusiform in shape with smooth, sharp margins. They have variable signal intensity on T1 sequences, but have greater signal intensity than muscle on T2 sequences. In addition, schwannomas can contain intramural cysts. Schwannomas usually show uniform enhancement with contrast but are less vascular than paragangliomas and have no flow voids. Parapharyngeal space schwannomas typically arise from the vagus nerve or the cervical sympathetic chain. The nerve of origin can be determined with good success by observing how the tumour relates to the internal carotid artery (ICA) and the internal jugular vein (IJV). Vagus nerve schwannomas typically grow to effect a separation between the ICA and the IJV, whereas a cervical sympathetic schwannoma usually displaces the ICA and the IJV together without separation.Reference Furukawa, Furukawa, Katoh and Tsukuda9, Reference Saito, Glastonbury, El-Sayed and Eisele10 This information can be useful in patient counselling and management planning.
Paragangliomas are ovoid, lobulated and well marginated, with T1 signal intensity similar to muscle and with mildly hyperintense signals on T2 sequences. They can have serpentine or punctate flow voids, which are areas of loss of signal on T1 and T2 sequences. Paragangliomas have intense and rapid dynamic gadolinium enhancement.
At present, arteriography is performed infrequently for diagnostic purposes. Rather, it is used for the pre-operative embolisation of large vagus paragangliomas and carotid body tumours. Arteriography can also aid the search for multicentric tumours. In rare instances, it can be used to evaluate cervicocerebral collateral circulation in cases in which sacrifice of major blood vessels (e.g. the ICA) may be necessary.
If the diagnosis is unclear following imaging studies, or if the clinical information is concerning, then fine needle aspiration biopsy can be useful diagnostically.Reference Oliai, Sheth, Burroughs and Ali11 This can be done transorally or transcervically if the neoplasm is clinically apparent. Other neoplasms will require image guidance, usually via CT or ultrasound. An adequate cytological specimen and good clinicopathological correlation are important. Fine needle aspiration biopsy has excellent accuracy and, in particular, excellent sensitivity and specificity for detecting malignant neoplasms. Fine needle aspiration biopsy of the parapharyngeal space is very useful for the diagnosis of lymphoma, malignant neoplasms and metastatic malignant neoplasms, guiding further evaluation and correct management.
Transoral biopsy of a parapharyngeal space neoplasm is rarely indicated and can be hazardous. There are risks of injury to the ICA and the cranial nerves if they are displaced by the neoplasm or located in an aberrant location. In addition, transoral biopsy violates the neoplasm and creates an adhesion between the tumour and the pharyngeal structures.Reference Lau, Lam and Wei12 This sequela is particularly problematic for pleomorphic adenoma as it can make neoplasm removal via blunt finger dissection difficult without tumour rupture. Incisional biopsy of vascular tumours such as paragangliomas is to be avoided in all circumstances, especially transorally as bleeding may result in airway compromise.
Management
The management of parapharyngeal space neoplasms is diagnosis-dependent and should be individualised for each patient. Patient factors such as age and comorbidity require consideration. In addition, cranial nerve functional status and tumour multifocality must be taken into account. It is important to understand that patient acceptance of surgery may be slow, especially for asymptomatic patients. Also, because most parapharyngeal space tumours are benign there is often no urgency for treatment. For these reasons, there has been an increasing trend for observation in select patients.
Management considerations include the fact that as neoplasm size increases there is a greater risk of development of cranial nerve deficits. Also, increased tumour size may make surgery more difficult and require extended access approaches with an increased risk of surgical complication. Natural cranial nerve deficits tend to occur gradually and are better tolerated than those that occur acutely after surgical resection. Elderly and frail patients do not compensate well for acute cranial nerve dysfunction, particularly if the deficits are multiple.
Surgical candidates include patients with primary malignant neoplasms for which surgery is appropriate therapy, select metastatic malignancies (e.g. papillary thyroid carcinoma), benign salivary gland neoplasms, neurogenic neoplasms with existing cranial nerve deficits, and neoplasms with mass effect symptoms.
The transoral approach is now considered to be acceptable for select small and accessible neoplasms. There are risks associated with this approach, however, which include neurovascular injury, tumour rupture and spillage, incomplete tumour resection, and salivary contamination of the parapharyngeal space. On the other hand, the advantages of this approach include direct access, decreased morbidity, early oral intake, avoidance of a cervical scar and shorter hospitalisation.Reference Ducic, Oxford and Pontius13
Recently, transoral endoscopic and robotic approaches to the parapharyngeal space have been described.Reference O'Malley, Quon, Leonhardt, Chalian and Weinstein14–Reference Chan, Li, Lim, Hinojosa and Boahene18 These are best suited to non-salivary gland neoplasms. The literature reports an unacceptably high rate of tumour rupture and recurrence for pleomorphic adenomas of the parapharyngeal space managed by transoral removal.Reference Lau, Lam and Wei12, Reference Goodwin and Chandler19–Reference Betka, Chovanec, Klozar, Taudy, Plzák and Kodetová21 Given this risk, the use of this approach for salivary gland neoplasms should be carefully considered.
The transcervical approach is the most commonly used surgical approach to the parapharyngeal space.Reference Chang, Goldenberg and Koch22, Reference Malone, Agrawal and Schuller23 It provides good access to parapharyngeal space neoplasms with good exposure of blood vessels and nerves. This approach also maintains sterility as there is no oral flora contamination. The transcervical approach uses a transverse incision in the upper neck. Exposure of the parapharyngeal space can be facilitated by a number of different methods including the use of nasotracheal intubation, submandibular gland mobilisation, division of the stylomandibular ligament, and the use of muscle relaxation when appropriate. Additional exposure can be achieved with division of the digastric and stylohyoid muscles. Video- and image-guided approaches may help to improve visualisation and tumour dissection beyond the usual transcervical line of sight.Reference Beswick, Vaezi, Caicedo-Granados and Duvvuri24
The transcervical–parotid approach is used for deep lobe parotid neoplasms that have expanded into the parapharyngeal space. This approach provides exposure and protection of the facial nerve. The common trunk of the facial nerve is identified and preserved while the parotid parenchyma is divided, so as to ensure complete tumour resection with a sufficient margin of normal gland but without tumour violation or inadvertent facial nerve injury.
The transcervical approach with mandibulotomy provides enhanced access to the parapharyngeal space when additional access may be warranted, for example in the case of malignant tumours, recurrent tumours, massive tumours, tumours adjacent to the skull base, and vascular tumours for which improved vascular control is needed. The literature shows a variable need for mandibulotomy, which ranges from 10 to 30 per cent and is series-dependent.Reference Shahab, Heliwell and Jones5, Reference Malone, Agrawal and Schuller23, Reference Bozza, Vigili, Ruscito, Marzetti and Marzetti25, Reference Zhi, Ren, Zhou, Wen and Zhang26 A number of mandibulotomy approaches have been described that preserve the integrity of the inferior alveolar nerve and avoid morbidity and are thus generally preferred.Reference Jungehuelsing, Guntinas-Lichius, Klussmann, Eckel and Stennert27–Reference Smith, Brennan, Webb and Ilankovan30 We have utilised a vertical parasymphyseal mandibulotomy anterior to the mental foramen, between the canine and the first premolar.Reference Kolokythas, Eisele, El-Sayed and Schmidt31 If further exposure is needed, an additional horizontal ascending ramus mandibulotomy above the lingula and thus the mandibular foramen can be performed to reflect the mandible segment superiorly and further expose the contents of the parapharyngeal space.Reference Kolokythas, Eisele, El-Sayed and Schmidt31
The key to any mandibulotomy approach is maintenance of proper occlusion with proper plating techniques. Also, the benefit of the additional exposure attained with mandibulotomy must be carefully weighed against its potential morbidity. A mandibulotomy may require a temporary tracheotomy to manage airway oedema, and patients should be appropriately counselled pre-operatively on all possible procedures.
The combined transoral and transcervical approach is useful for the resection of neoplasms that require resection of part of the pharynx in order to achieve complete resection. In addition, it can be used for tumours that have been previously violated by incisional biopsy. As discussed above, removal of such a neoplasm with a margin of the pharynx around the prior biopsy site will reduce the risk of tumour spillage, with the associated risk of multifocal tumour recurrence within the parapharyngeal space. With this approach, the pharyngeal defect can usually be closed primarily. Larger pharyngeal defects require tissue augmentation, usually free tissue transfer, for closure.
The combined transcervical and lateral skull base approach is required for neoplasms that involve the skull base or extend intracranially. This approach requires careful planning with skull base surgeons or neurosurgeons, as appropriate.
Neurogenic neoplasms
The parapharyngeal space is the most common location of non-vestibular schwannomas of the head and neck. As described above, these neoplasms usually arise from the vagus nerve or the cervical sympathetic chain, although other nerves of origin can occur. The decision to resect a schwannoma should be based on the patient's age and comorbidity. Symptoms, mass effect on adjacent nerves, and patient preference require consideration. The consequences of resection must be carefully weighed. Preserving nerve integrity during schwannoma resection can be challenging, and even those nerves that remain intact tend to be non-functional for extended periods of time. Since most patients present without cranial nerve deficits, the resection of a schwannoma can be expected to result in an immediate neuropathy which may be poorly tolerated by the patient. A malignant schwannoma is unusual.Reference Valentino, Boggess, Ellis, Hester and Jones32 Most of these tumours tend to grow slowly or remain stable, although their natural history is poorly defined in the literature. Thus, observation with serial imaging studies is reasonable for most patients with schwannomas.
Intracapsular tumour excision is a method of schwannoma resection which enables preservation of nerve integrity and function.Reference Kang, Soo and Lim33–Reference Kim, Kim, Kim, Lee and Choi35 With this method, the epineurium is opened longitudinally and the tumour enucleated. This approach allows for the preservation of nerve continuity in most cases. Although there is always some degree of temporary nerve dysfunction, in a majority of cases gradual, partial or complete recovery occurs over time. However, structural nerve preservation may not guarantee preservation of nerve function.
Paragangliomas of the parapharyngeal space usually arise from the vagus nerve. Also, large carotid body tumours can extend superiorly into the parapharyngeal space. Paragangliomas differ from schwannomas in several respects. Vagus paragangliomas are intimately related to the vagus nerve, such that resection without nerve sacrifice is not feasible. In addition, a vagus nerve functional deficit is present in 20 to 50 per cent of patients at the time of initial presentation.Reference Urquhart, Johnson, Myers and Schechter36
Paragangliomas tend to exhibit slow, progressive growth. Growth rates have been studied and are estimated to be approximately 1–2 mm/year.Reference Jansen, van den Berg, Kuiper, van der Mey, Zwinderman and Cornelisse37–Reference Langerman, Athavale, Rangarajan, Sinard and Netterville39 With increased growth, these tumours can cause secondary nerve deficits. Also, they have the potential for intracranial extension. In addition, vagus nerve paragangliomas have the highest malignant potential of all head and neck paragangliomas, approximately 15 per cent.Reference Kahn40 It is important to note that a benign paraganglioma is indistinguishable from a malignant one histopathologically. Rather, the presence of nodal or other metastases guides the diagnosis of malignancy.
Familial paragangliomas have been reported to occur in patients with the following genetic mutations: succinate dehydrogenase complex subunit D (also termed ‘SDHD’) mutation (paraganglioma 1 (also termed ‘PGL-1’) syndrome), succinate dehydrogenase complex subunit C mutation (paraganglioma 3 syndrome) and succinate dehydrogenase complex subunit B mutation (paraganglioma 4 syndrome). These patients tend to be younger and have a higher incidence of multifocal tumours, compared with patients with sporadic tumours.
The presence of tumour bilaterality or a pre-existing contralateral vagus nerve deficit will affect treatment decision-making, as bilateral vagus nerve paralysis causes significant morbidity. Low recurrence rates have been reported following vagal paraganglioma resection.
Radiation therapy is an alternative to surgical resection for paragangliomas. Both conventional fractionated radiation therapy and stereotactic radiation therapy have been shown to be effective.Reference Hinerman, Amdur, Morris, Kirwan and Mendenhall41, Reference Foote, Pollock, Gorman, Schomberg, Stafford and Link42 Responses tend to comprise stabilisation or a modest (10–20 per cent) decrease in tumour size. Low patient morbidity is reported. Radiation therapy should be considered as an alternative to surgical resection, especially in patients with bilateral neoplasms or those with an existing contralateral vagus nerve deficit.
Complications and sequelae
The various surgical approaches to parapharyngeal space tumour resection have many potential complications. Major complications include nerve injury, blood vessel injury, infection, airway obstruction and tumour recurrence. These complications, and their avoidance and management, are described in detail elsewhere.Reference Olsen43, Reference Moore, Olsen, Eisele and Smith44 In general, complications can be avoided with proper surgical planning and careful technique.
Sequelae of parapharyngeal space tumour resection should be differentiated from surgical complications. Vagus nerve paralysis is a major anticipated sequela of resection of vagus nerve schwannoma and paraganglioma. Vagus nerve deficit causes significant dysphagia and voice impairment. It has a worse impact on older patients than younger patients. Also, it is better tolerated if an existing dysfunction was present pre-operatively, as the patient may have gradually become habituated to the deficit.
Immediate vocal fold medialisation is helpful in cases in which the vagus nerve is rendered dysfunctional. Speech and language pathology consultation is recommended to evaluate the patient and to institute safe swallowing therapy. Placement of a temporary nasogastric feeding tube is sometimes necessary to ensure safe and adequate nutritional intake. Velopharyngeal incompetency can be another sequela. This can be managed with a palatal lift prosthesis or a palatal adhesion procedure.Reference Netterville and Vrabec45
Another sequela of resection of some parapharyngeal space neoplasms is first bite syndrome.Reference Gardner and Abdullah46, Reference Haubrich47 This is characterised by recurrent, severe pain in the parotid region with initial oral intake. The pain subsides with successive bites. The pathophysiology of first bite syndrome is thought to be related to interruption of the sympathetic innervation of the parotid gland with subsequent denervation supersensitivity of myoepithelial cell receptors.Reference Netterville, Jackson, Miller, Wanamaker and Glasscock48, Reference Chiu, Cohen, Burningham, Andersen and Davidson49 This occurs with sacrifice of the sympathetic chain or during deep lobe parotid tumour extirpation. The severity of first bite syndrome gradually reduces over time.Reference Albasri, Eley and Saeed50 Severe symptoms can be managed medically with drugs such as gabapentin or pregabalin. Recently, injection of the parotid gland with botulinum toxin has proven to be effective therapy for the symptoms of first bite syndrome.Reference Ali, Orloff, Lustig and Eisele51, Reference Lee, Lee, Lee, Wang and Kim52
Conclusion
The comprehensive evaluation and management of parapharyngeal space tumours requires a multidisciplinary approach involving the expertise of head and neck surgeons, radiologists, cytopathologists, pathologists, speech and language pathologists, and radiation oncologists, and also occasionally neurosurgeons, oral surgeons, endocrinologists and interventional radiologists. Imaging studies are the key to diagnosis, with MRI with gadolinium contrast being the favoured imaging technique. Cytopathology may provide additional diagnostic information. Open biopsy is rarely necessary and is contraindicated for vascular tumours. Treatment should be individualised with regard to recommendations for surgery and specific surgical approach. Complications are best avoided with careful surgical planning, and the expected sequelae of surgery, which may result in significant patient morbidity, require full patient disclosure. In lieu of surgery, some patients are best observed with serial imaging studies. Select patients who are not favourable surgical candidates may be considered for radiation therapy.



