Introduction
Cochlear implants have created a paradigm shift in the treatment of sensorineural hearing loss. The contributions of scientists and doctors in the past decades have served as building blocks in laying the foundation of cochlear implant surgery, even in patients with inner-ear malformations.
Although most implanted patients have normal gross temporal bone anatomy, Jensen has estimated that 20 per cent of children with congenital sensorineural hearing loss (SNHL) will have some inner-ear abnormality.Reference Jensen1 One of the most common cochlear abnormalities seen in cochlear implant surgery patients is Mondini dysplasia, as described by Carlo Mondini in 1791.Reference Lo2 This inner-ear abnormality appears at about the seventh week of gestation. The auditory or vestibular function may range from normal to severely impaired.Reference Nomura and Nomura3 Silverstein and colleagues were the first to implant a multichannel cochlear implant in a patient with Mondini dysplasia, in a 31-year-old man.Reference Silverstein, Smouha and Morgan4
Although several studies have shown a clear benefit of cochlear implantation in patients with Mondini dysplasia, there are relatively few reports of the benefits over a long period.Reference Chen, Yan, Liu, Liu, Kong and Zheng5 This study aimed to: (1) evaluate the complications encountered intra-operatively in patients with Mondini dysplasia; (2) assess the development of auditory skills post-implantation in young children with Mondini dysplasia; and (3) compare the auditory skills of children with Mondini dysplasia and profoundly deaf children with radiologically normal inner ears.
Materials and methods
This is a retrospective study conducted on paediatric cochlear implant patients operated on at the ENT Department, Gujarat Medical Education and Research Society (‘GMERS’) Medical College and Hospital, Gandhinagar, India, from February 2015 to May 2017.
In that period, 338 patients were operated on, of which 82 had inner-ear anomalies. Of those 82 patients, 27 had classical Mondini dysplasia as confirmed by radiological examination.
For the study, patients were divided into two groups. Group A consisted of 27 patients with profound sensorineural hearing loss with radiologically normal ears, selected via random sample generating software. Group B consisted of 27 patients with Mondini dysplasia.
Ethical considerations
The study was approved by the central research committee and institutional ethics committee after thorough consideration.
Pre-operative evaluation
All young children underwent a thorough otorhinolaryngological examination to ensure they were free from any external and middle-ear pathologies. Audiometric tests were performed by experienced audiologists using behavioural audiometry and electrophysiological tests, including auditory brainstem response and distortion product otoacoustic emissions testing, and tympanometry.
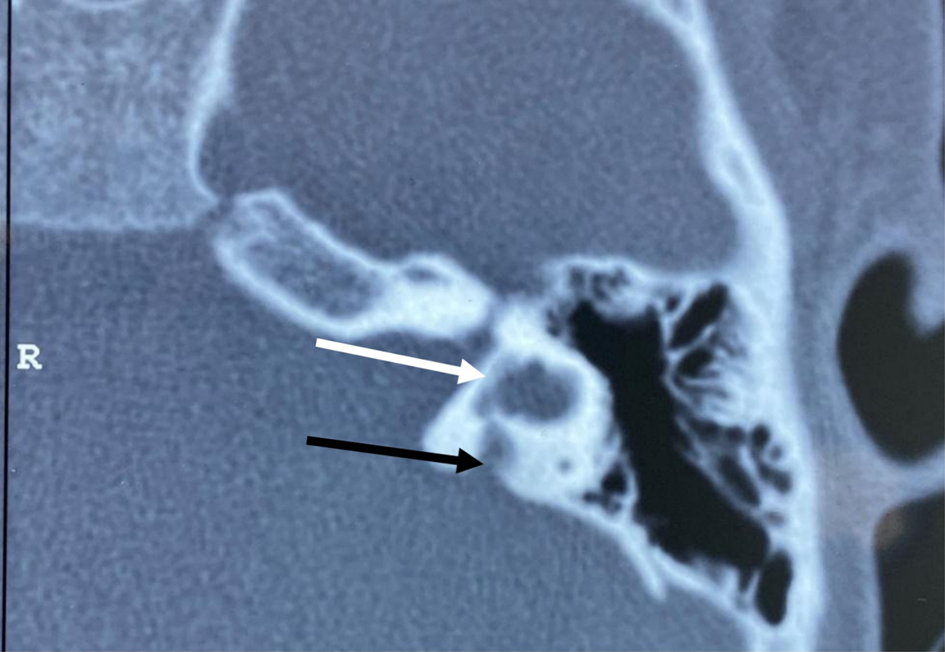
This was followed by radiological evaluation with high-resolution computed tomography (CT) of the temporal bone, and magnetic resonance imaging of the brain and membranous labyrinth, to rule out any inner-ear anomalies. The term Mondini dysplasia was used when the apical part of the modiolus and the corresponding interscalar septa were defective, giving the cochlear apex a cystic appearance because of the confluence of middle and apical turns, along with an enlarged vestibular aqueduct and dilated vestibule. The external dimensions of the cochlea were similar to those seen in normal cases. The enlarged vestibular aqueduct was diagnosed radiographically when its anteroposterior diameter exceeded 1.5 mm on CT scan of the temporal bone measured midway between its aperture and crus communes (Figures 1 and 2).

Fig. 1. Pre-operative high-resolution computed tomography (axial view) of the temporal bone. The cochlear apex showing a cystic appearance due to the confluence of middle and apical turns (black arrow). R = right

Fig. 2. Pre-operative high-resolution computed tomography (axial view) of the temporal bone showing a slightly dilated vestibule (white arrow) and large vestibular aqueduct (single black arrow). R = right
All patients had been fitted with a hearing aid, with no significant benefit.
Peri-operative details
All patients were operated on using the transmastoid posterior tympanotomy approach. Nucleus 22 straight electrode arrays were used (this being a government funded programme). Intra-operative neural response telemetry and impedance measurements, and intra-operative ‘C’ arm imaging in Stenvers view, were conducted to confirm electrode placement.
Post-operative evaluation
Audiological and speech assessments were conducted using the Categories of Auditory Perception scale, Speech Intelligibility Rating scale and Meaningful Auditory Integration Scale. The assessments were performed pre-implantation (with a hearing aid), and at three months, six months, nine months, one year, two years and three years after implantation. All patients underwent regular auditory verbal training, and speech and language therapy.
Results
From February 2015 to May 2017, 338 patients were implanted; 82 (24.26 per cent) presented with inner-ear anomalies on high-resolution CT. Of those 82 patients, 27 (7.99 per cent of anomaly cases) had classical Mondini dysplasia.
In group B (Mondini dysplasia), 14 patients had a ‘gusher’ or ooze; 12 cases had a gentle flow of cerebrospinal fluid (CSF) (ooze), while 2 patients had a CSF gusher. The patient's head was immediately elevated about 30 degrees to reduce the intracranial pressure. After waiting 20 minutes, the CSF started pulsating. A small piece of fascia, about 1–2 cm in diameter, was harvested. Because of difficulty in inserting pieces of fascia around the electrode inside the cochleostomy, we preferred a larger cochleostomy. The electrode was passed through the hole made in the centre of the fascia (Figure 3). There was constant suction of the bone at the edge of the cochleostomy, to clear the area of the fluid. The electrode was introduced into the scala tympani and the cochleostomy was firmly packed with the fascia (with two-thirds of it being placed inside and one-third outside the cochleostomy site).

Fig. 3. Fascia harvested (black arrow), and electrode seen passing through it for sealing of the cochleostomy.
Initially, the leak was believed to be adequately controlled in both cases, but in one case, 24 hours after the initial operation, exploration was required because of a profuse CSF leak (Figure 4). Partial extrusion of the electrode was present during exploration; the electrode was re-inserted and the cochleostomy site was firmly packed with fascia lata. Tisseel® fibrin glue was applied to strengthen the seal. Along with measures like head end elevation and absolute bed rest, acetazolamide (20 mg/kg/day) was given in tablet form to reduce CSF pressure. Lumbar drain placement was not required. The post-operative period was uneventful.

Fig. 4. Post-operative high-resolution computed tomography (axial view) showing fluid in the middle ear and mastoid, with the electrode array in the cochlea.
In order to assess auditory outcomes, we statistically analysed the scores for the Categories of Auditory Perception scale, Speech Intelligibility Rating scale and Meaningful Auditory Integration Scale in groups A (profound sensorineural hearing loss with radiologically normal ears) and B (Mondini dysplasia), at pre-implantation, and at 3, 6, 9, 12, 24 and 36 months post-implantation (Figures 5–7). There were no differences between the groups in terms of auditory performance at pre-implantation and post-implantation at 12, 24 and 36 months. There was a significant difference between the groups at three, six and nine months post-implantation (p < 0.005; Table 1). The overall results for auditory performance showed greater differences between the groups at, and prior to, nine months post-implantation.

Fig. 5. The mean Categories of Auditory Perception (CAP) scores for groups A (profoundly deaf with radiologically normal inner ears) and B (Mondini dysplasia) at pre-implantation, and at 3, 6, 9, 12, 24 and 36 months after implantation. There were greater differences between groups A and B pre-implantation, and at three, six and nine months after implantation. Categories of Auditory Perception scores were improved at 12, 24 and 36 months, with less difference between the groups.

Fig. 6. The mean Speech Intelligibility Rating (SIR) scores for groups A (profoundly deaf with radiologically normal inner ears) and B (Mondini dysplasia) at pre-implantation, and at 3, 6, 9, 12, 24 and 36 months after implantation. There were greater differences between groups A and B at three and nine months after implantation. Speech Intelligibility Rating scores were improved at 12, 24 and 36 months after implantation, with less difference between the groups.

Fig. 7. The mean Meaningful Auditory Integration Scale (MAIS) scores for groups A (profoundly deaf with radiologically normal inner ears) and B (Mondini dysplasia) at pre-implantation, and at 3, 6, 9, 12, 24 and 36 months after implantation. There were greater differences between groups A and B at three, six and nine months after implantation. Meaningful Auditory Integration Scale scores were improved at 12, 24 and 36 months, with less difference between the groups.
Table 1. Statistical analysis comparing CAP, SIR and MAIS scores for group A versus B at different time points*

Data represent p-values. The patients were profoundly deaf young children with radiologically normal inner ears (group A) or children with Mondini dysplasia (group B). CAP = Categories of Auditory Perception; SIR = Speech Intelligibility Rating; MAIS = Meaningful Auditory Integration Scale; N/A = not applicable
Discussion
Since Carlo Mondini first presented his findings of combined membranous and bony dysplasia of the inner ear based on anatomical dissection, many investigators have documented a variety of inner-ear malformations.Reference Fishman, Holliday, Waltzman and Cohen6 The present study recorded complications; a CSF ‘gusher’ was the commonest. It also showed that the auditory skills of young children with Mondini dysplasia develop at a similar pace in the first three years of life to those of profoundly deaf children with radiologically normal ears. In our study, the incidence of cochlear malformations in the form of Mondini dysplasia was 7.99 per cent. Similar incidence rates have been reported in other studies.Reference Papsin7–Reference Buchman, Copeland, Yu, Brown, Carrasco and Pillsbury10
The major problems that can be encountered in patients with congenitally malformed cochlea are facial nerve injury and a CSF gusher at the time of implantation.Reference Sennaroglu11 Evaluation of the pre-operative CT scan to assess the facial nerve course, and facial nerve monitoring in the surgery, are useful to prevent injury. In our cases, the facial nerve course was normal. We use facial nerve monitoring routinely. Intra-operative facial nerve monitoring is strongly advised in all patients with a congenitally malformed ear.
A previous histopathological study demonstrated that modiolar defects may be because of high CSF pressure transmission into the inner ear as a result of an enlarged vestibular aqueduct.Reference Sennaroglu12 Depending on the severity of the insult, it may cause the transmission of CSF pressure into the cochlea. The CSF oozing and gusher sometimes observed in Mondini dysplasia are due to modiolar defects occurring as a result of high CSF pressure transmission.Reference Sennaroglu and Bajin13
Graham et al. reported three Mondini deformity cases with a CSF gusher during surgery.Reference Graham, Phelps and Michaels14 Papsin, in their extensive review, reported a gusher in 6.7 per cent of 103 patients with malformations.Reference Papsin7 They pointed out the importance of distinguishing between two types of CSF outflow, so as not to overestimate the incidence of a gusher. Most of the papers reported the incidence of CSF gushers to be between 40 and 50 per cent in patients with inner-ear malformations.Reference Jackler, Luxford and House15,Reference Paparella16 A CSF leak is much more common in cochlear implant surgery patients with congenital anomalies of the cochlea, and patients with a known Mondini malformation should be considered as high-risk candidates for the development of CSF leaks.Reference Ohlms, Edwards, Mason, Igarashi, Alford and Smith17,Reference Phelps, King and Michaels18
The size of the cochleostomy one should use is also controversial. Weber et al. recommended cochleostomy to be small so that the electrode cable can partly block the flow of the gusher,Reference Weber, Dillo, Dietrich, Maneke, Bertram and Lenarz19 while Graham et al. preferred a large cochleostomy that allows easy insertion of the electrode array.Reference Graham, Phelps and Michaels14 In both studies, muscle tissue was used for packing the electrode array. Sealing of the cochleostomy is an important step. The packing should be strong enough to prevent the breakdown of the cochleostomy seal after the operation. We preferred a larger cochleostomy because of the ease of packing.
• Mondini dysplasia is a triad of: confluence of middle and apical turns with a normal basal turn, minimally dilated vestibule, and large vestibular aqueduct
• Cerebrospinal (CSF) ooze or ‘gusher’, sometimes observed in Mondini dysplasia, is due to modiolar defects resulting from high CSF pressure transmission
• Precise pre-operative imaging is emphasised, as Mondini dysplasia is associated with increased CSF gusher incidence
• Post-operative rehabilitation programmes need to be individually tailored
• Auditory skills of young children with Mondini dysplasia develop similar to those of profoundly deaf children with radiologically normal ears
A permanent CSF leak can lead to meningitis. Page and Eby reported recurrent meningitis occurring 19 months after cochlear implantation in a Mondini deformity case.Reference Page and Eby20 No incidences of facial nerve damage, skin necrosis, post-operative meningitis, haemorrhage or device failure requiring explantation were reported in our study.
We used the Categories of Auditory Perception scale, Speech Intelligibility Rating scale and Meaningful Auditory Integration Scale for assessing outcomes, because these tests are well recognised in the international literature.Reference Nikolopoulos, Archbold and Gregory21
There are reported to be 36 000 spiral ganglion cells in subjects with a normal cochlea, with no fewer than 3000 spiral ganglion cells located in the apical 10 mm of the organ of Corti, which helps in speech discrimination.Reference Otte, Schuknecht and Kerr22 Schmidt demonstrated that the number of spiral ganglion cells in Mondini dysplasia patients may range from 7677 to 16 110.Reference Schmidt23 The number of spiral ganglion cells required for electrical stimulation is not known, but the spiral ganglion cells in Mondini dysplasia patients are large enough to trigger a neural response from electrical stimulation of cochlear implants.Reference Linthicum and Fayad24 This may explain why the auditory outcome scores are not significantly different between the groups 12 months after implantation in our study.
Buchman et al. reported similar findings in their study.Reference Buchman, Copeland, Yu, Brown, Carrasco and Pillsbury10 One investigation compared contour with straight electrodes in 18 Mondini dysplasia cases, and found no significant differences in terms of post-operative hearing and speech scores.Reference Wang, Cao, Wang and Li25 As the basal turn is normal in Mondini dysplasia, and research has shown that the majority of spiral ganglion cells reside in the basal turn, all types of electrodes should provide sufficient stimulation.Reference Sennaroglu11 Another study, by Bille et al., compared the results of cochlear implantation in children with a normal inner ear with those with inner-ear malformations, using Categories of Auditory Perception and Speech Intelligibility Rating scales, and found no statistical differences between the groups.Reference Bille, Fink-Jensen and Ovesen26 Chen et al., in a study of 545 patients wherein 31 patients had Mondini dysplasia, reported similar findings, with no significant difference between the groups at two years.Reference Chen, Yan, Liu, Liu, Kong and Zheng5 A study by Munro et al. suggested similar outcomes for five Mondini dysplasia patients who were implanted, as compared to children with no developmental defects.Reference Munro, George and Haacke27 Luntz et al. reported that the hearing results in patients with inner-ear malformations other than a common cavity are comparable to those of other profoundly deaf children.Reference Luntz, Balkany, Hodges and Telischi8
Conclusion
The findings of this study confirm that cochlear implantation is an effective intervention for young children with Mondini dysplasia when the amplification of optimal hearing aids is insufficient. These findings further show that young children with Mondini dysplasia require a slightly longer time to attain appropriate auditory skills than those with radiologically normal inner ears, and thus suggest that more attention should be paid to their auditory verbal training during the first year post-implantation. Complications like a CSF ‘gusher’ or ooze should be anticipated in patients with Mondini dysplasia and should be managed appropriately. The study highlights the importance of early intervention, with more attention required for post-implantation rehabilitation in the first two years of life. Follow-up education and auditory verbal training must be tailored according to each patient's individual needs and situation.
Competing interests
None declared