Introduction
Vestibular neuritis is one of the most common causes of acute vestibular syndrome.Reference Strupp and Brandt1 The acute phase is defined as severe rotatory vertigo with nausea and vomiting of variable duration where only vestibular function but not hearing is affected. An acute attack of vertigo is a challenge for clinicians who must differentiate a central problem from different peripheral problems. These problems cause severe attacks with vertigo and nystagmus, but the treatment, therapeutic progression, outcomes and prognosis for these two causes can be very different.
Recently, bedside ocular motor findings were collectively grouped into a three-step bedside examination called Head Impulse, Nystagmus, Test of Skew.Reference Newman-Toker, Kerber, Hsieh, Pula, Omron and Saber Tehrani2–Reference Kattah4 The Head Impulse, Nystagmus, Test of Skew protocol could help in differentiating peripheral from central causes of acute vestibular syndrome in the emergency department, but establishing vestibular organ damage and determining their possible functional recovery requires further examination. Furthermore, it is crucial to have information at the time of the attack (less than 72 hours from the onset of symptoms) and during the period following the acute phase.
Daily clinical use of the video head impulse test has provided evidence on the level of semicircular canal function, but this information alone is not sufficient to also test the function of the otolith system. The otolith system in the human vestibular system comprises approximately 33 000 receptors in each utricular macula and 18 000 in each saccular macula,Reference Lindeman5,Reference Gresty, Bronstein, Brandt and Dieterich6 and these can also be tested at the time of the attack.
Two different primary otolith afferents, irregular and regular neurons, originate from these receptors. They respond to linear accelerations, such as head tilts, in relation to gravity.Reference Manzari, Burgess, MacDougall and Curthoys7,Reference Rosengren, Colebatch, Straumann and Weber8 However, several studiesReference Curthoys, MacDougall, Vidal and de Waele9–Reference Weber, Rosengren, Michels, Sturm, Straumann and Landau13 have now outlined how these two types of neurons respond to different stimuli in the utricular macula and the saccular macula.
Irregular neurons have been defined as ‘transient’ and originate from the immediately adjacent region of the otolithic organs called striola; in essence they can be referred to as the neurons that perform ‘dynamic’ otolith function. Regular neurons have been defined as ‘sustained’ and originate from the remaining portion of the otolithic structures, the extra-striola regions; in essence they can be referred to the neurons that perform the ‘static’ otolith function.
In emergency department or tertiary clinical settings, the two otolith functions just mentioned can currently be instrumentally and objectively studied using ocular vestibular-evoked myogenic potentials and subjective visual vertical tests even at the time of the attack.Reference Manzari, Koch and Tramontano14 The ocular vestibular-evoked myogenic potential mainly tests for dynamic utricular function,Reference Curthoys, Vulovic and Manzari15 and the cervical vestibular-evoked myogenic potential mainly tests for dynamic saccular function.Reference Oh, Kim, Yang, Shin and Jeong16 However, the deviation of the subjective visual vertical from the vertical line is a clinical sign of static vestibular disfunction.Reference Faralli, Ricci, Manzari, Zambonini, Lapenna and Pettorossi17 Ipsilesional deviation of the subjective visual vertical indicates damage within the peripheral vestibular system,Reference Bohmer and Rikenmann18 and contralateral deviation can be a sign of damage to the central vestibular system.Reference Dieterich, Brandt, Tabak, Collewijn and Boumans19
Our hypothesis is that studying the two functions in a sample of patients suffering from unilateral vestibular neuritis in the acute phase (less than 72 hours from onset) and some days after the onset of symptoms using ocular vestibular-evoked myogenic potentials and subjective visual vertical could give different results. For this reason, the aim of this retrospective study was to investigate the clinical course of dynamic and static otolith function in patients with unilateral vestibular neuritis using ocular vestibular-evoked myogenic potentials compared with subjective visual vertical testing in acute and sub-acute stages.
Materials and methods
Study design
This was a retrospective study that aimed to investigate which is the most suitable instrumental test of utricular macula function in patients with unilateral vestibular neuritis during acute and sub-acute attack stages. The study was carried out according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the World Medical Association Declaration of Helsinki. All participants gave written consent to publish the results obtained from their clinical examinations and instrumental tests. The study was approved by MSA ENT Academy Center Institutional Review Board
Setting
Medical records of patients with unilateral vestibular neuritis who were evaluated in the first 72 hours after onset and with at least one follow up at the MSA ENT Academy Center Clinic, Cassino, Italy (a tertiary vestibular referral centre), were reviewed.
Participants
All medical records of patients who were admitted to the MSA ENT Academy Center between 1 July 2015 and 31 July 2020 with an acute vestibular syndrome (vertigo, postural unsteadiness, nausea, vomiting or with spontaneous nystagmus suppressed by vision), thereby giving the appearance of a vestibular neuritis, were screened.
The inclusion criteria were: (1) diagnosis of unilateral vestibular neuritis in the acute phase (less than 72 hours since the acute vestibular syndrome onset); (2) at least two vestibular function evaluation sessions in the first month after onset or later; (3) absence of hearing loss on pure tone audiometry that could be related to other types of vestibular pathology (i.e. Ménière's disease) and abnormal findings on neurological examination; (4) patients who had not undergone pharmacological or rehabilitative treatment for unilateral vestibular neuritis; (5) presence of Head Impulse, Nystagmus, Test of Skew peripheral pattern.
We excluded the medical records of patients who showed one of the following exclusion criteria: (1) other vestibular diagnoses (e.g. unilateral vestibular neuritis less than 72 hours since the acute vestibular syndrome, Ménière's disease, bilateral vestibular loss, vestibular migraine, benign positional paroxysmal vertigo), somatic or psychiatric disorders; (2) presence of neurological diseases; and (3) any eye abnormalities that would prevent the use of the video head impulse test, goggles and camera. Demographic and clinical characteristics at baseline are reported in Table 1.
Table 1. Demographic and clinical characteristics at baseline

SD = standard deviation
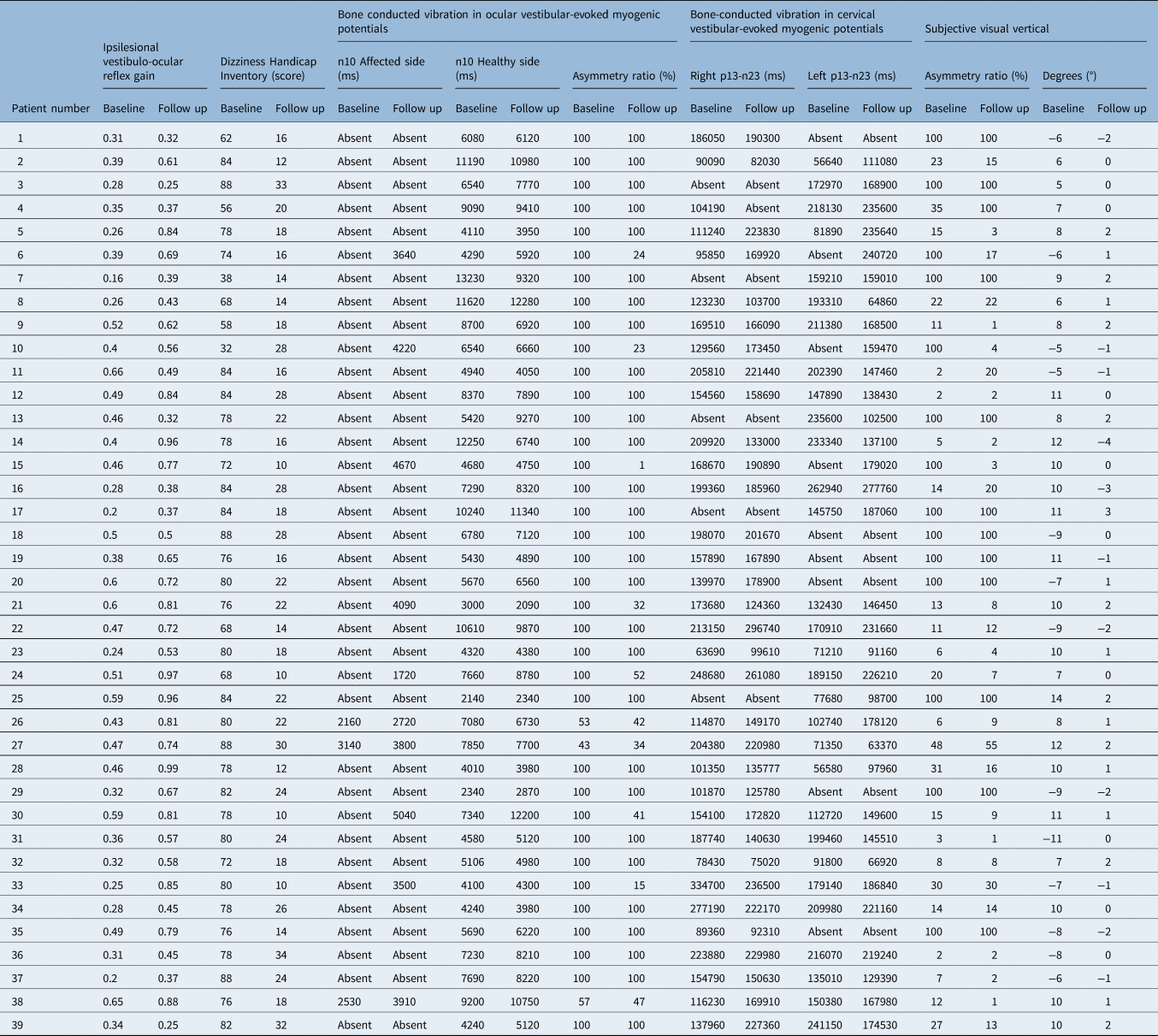
At the time of the first evaluation, all patients were instructed to return to normal daily activities as soon as possible. All patients admitted to MSA ENT Academy Center, Cassino (Italy) with a diagnosis of unilateral vestibular neuritis underwent a vestibular assessment that included a self-assessment inventory with the Dizziness Handicap Inventory, an assessment of horizontal and vertical semicircular canals with bedside head impulse test and video head impulse test, air-conducted sound and bone-conducted vibration tests, cervical and ocular vestibular-evoked myogenic potentials testing and subjective visual vertical testing. At baseline and follow up, vestibular evaluation data on horizontal vestibulo-ocular reflex gain, data on proportion of head impulses with covert saccades, bone-conducted vibration in response to ocular vestibular-evoked myogenic potentials, bone conducted vibration in response to cervical vestibular-evoked myogenic potentials, and subjective visual vertical tests were collected (clinical data are reported in Table 2).
Table 2. Vestibular assessment
Unilateral vestibular neuritis was diagnosed on the following criteria: (1) a history of acute onset of severe, prolonged, rotatory vertigo, nausea and postural imbalance; (2) on clinical examination the presence of horizontal spontaneous nystagmus with a rotational component toward the unaffected ear (fast phase) without evidence of a central vestibular lesion; (3) abnormal bedside head impulse test showing an ipsilateral deficit of the horizontal semicircular canal; (4) alterations in the vestibular-evoked myogenic potential results and absence of neurological signs; and (5) magnetic resonance imaging of the brain that showed no lesions that could account for any vestibular disturbance.
In addition, a total of 20 healthy participants were tested on one occasion (n = 20 (males n = 6; age range, 28–77; mean age, 45.5 years and females, n = 14; age range, 15–72; mean age, 44 years)). The healthy participants were patients’ partners. None of the healthy participants reported any auditory, vestibular, neurological or visual problems (apart from standard refractive errors). The horizontal, torsional and vertical components of the spontaneous nystagmus were measured in complete darkness using three-dimensional infrared video-oculography (50 Hz sampling; Torsio VNG Ulmer, Synapsys, Marseille, France). For this test and the subjective visual vertical test, the patient was seated with Reid's line (the line joining the inferior margin of the orbit and the middle of the external auditory meatus) approximately earth horizontal. Testing, in all cases, was conducted in complete darkness. The participants wore a mask and had a camera fixed in front of the left eye. The participants were instructed to keep their eyes open and to try to keep their gaze close to the centre. On the basis of the tentative diagnosis of unilateral utricular loss during unilateral vestibular neuritis, all of these patients were referred to a tertiary radiological centre for magnetic resonance imaging of the posterior cranial fossa using paramagnetic contrast enhancement; the whole brain was imaged to exclude other brain disorders.
The vestibular assessment
Ocular vestibular-evoked myogenic potentials
The ocular vestibular-evoked myogenic potential n10 is a small (5–10 μV) negative (excitatory), crossed vestibular-evoked myogenic potential of the stretched inferior oblique eye musclesReference Rosengren, Colebatch, Straumann and Weber8,Reference Curthoys, Vulovic and Manzari15 that can be recorded by surface electromyography on the skin beneath the eyes in response to stimulation by bone-conducted vibration delivered to the midline of the forehead at the hairline or by air-conducted sound. On the basis of evidence of utriculo-ocular projectionsReference Suzuki, Tokumasu and Goto20 and neural evidence of the preferential activation of irregular otolithic afferent neurons by 500 Hz bone-conducted vibration and air-conducted sound,Reference McCue and Guinan21–Reference Curthoys, Vulovic, Burgess, Cornell, Mezey and Macdougall26 the ocular vestibular-evoked myogenic potential (n10) to these stimuli is held to index mainly utricular function.
Subjective visual vertical methods
The subjective visual vertical test was carried out with the participant in a sitting position and the head erect. For determination of the subjective visual vertical, we used a dimly lit bar of light that was 40 cm long and 1 cm wide in a dark room at 1 metre from the participant and so subtending a visual angle of 22.6°. The bar was displayed on a computer screen, and the movement was controlled by the participant using a joystick. The participant wore a mask which occluded vision of screen edges and was instructed to set the bar to true gravitational vertical. Setting of the upper tip of the bar to the participant's left was scored negative. The angle in degrees was recorded with a resolution of 0.1°. A total of 6 measurements were made with the starting position of the bar alternating between +45° and –45° from vertical. The determination of the subjective visual vertical was also carried out in 20 healthy participants who did not have a history of vertiginous episodes or balance disorder in the past or present. In healthy participants, normal estimation of the vertical is when participants are able to indicate the vertical very accurately.Reference Böhmer and Rickenmann27 In our clinic, we define the normal range of the subjective visual vertical in the upright position as –2° to +2°.
In the event of a sudden loss of unilateral vestibular function as in the case of unilateral vestibular neuritis, the participant tilts the upper end of the bar toward the dysfunctional ear, shifting by several degrees with respect to the gravitational axis.Reference Faralli, Ricci, Molini, Longari, Altissimi and Frenguelli28 The error in perception could be a result of an ipsilesional ocular torsion deviation as an integral part of a postural static synkinesis known as ocular tilt reaction.Reference Brandt and Strupp29
Dizziness Handicap Inventory
The quality of life of all vestibular neuritis patients was assessed by the Dizziness Handicap Inventory. The Dizziness Handicap Inventory is a self-assessment inventory, including 25 questions to evaluate self-perceived activity limitation and restriction resulting from dizziness.Reference Jacobson and Newman30
Video head impulse test
The function of the semicircular canals was measured using video head impulse test (OtosuiteV®, GN Otometrics, Denmark) during head impulse paradigm testing. The criterion for a normal vestibulo-ocular reflex velocity gain was that it should be 0.68 or greater, based on head impulse test data from previously published trials in which the mean head impulse test velocity gain measured by search coils with identical apparatus and procedures to those used here was 0.81 (± 0.068 standard deviation (SD)), so that the mean 2 SD units incorporates 95 per cent of the population and yields a lower cut off of 0.68. A gain value of less than 0.76 was used to identify the affected side of unilateral vestibular neuritis.Reference MacDougall, Weber, McGarvie, Halmagyi and Curthoys31,Reference Weber, Aw, Todd, McGarvie, Curthoys and Halmagyi32
Results
Statistical analysis
The asymmetry ratio between the affected and healthy side was calculated and considered as pathological with an asymmetry ratio equal to or more than 40Reference Manzari, Burgess and Curthoys33 for the ocular vestibular-evoked myogenic potentials and equal to or more than 30Reference Curthoys, Burgess and Manzari34 for the cervical vestibular-evoked myogenic potentials.Reference Oh, Kim, Yang, Shin and Jeong16 The average horizontal slow phase eye velocity vestibulo-ocular reflex gain for each side was calculated at first and second vestibular evaluation as the sum of the vestibulo-ocular reflex gains for each trial.
Statistical analysis was performed with SPSS® statistical analysis software. Data were reported in terms of means and standard deviations. The Wilcoxon signed rank test was used for the within-participant comparison. The Mann–Whitney U test was used to compare Dizziness Handicap Inventory and vestibulo-ocular reflex gain data between sub-groups (superior branch of the vestibular nerve vs the nerve in toto) with variable time (at baseline and follow up) and considering significant results as p < 0.05. The Pearson's correlation coefficient was calculated at the baseline and follow up, between Dizziness Handicap Inventory score, affected vestibulo-ocular reflex gain, asymmetry ratio, the time (days) between the first and second visit, the subjective visual vertical, the patients’ age and considering significant correlation with p < 0.05. In order to test the impact of confounding factors on the Dizziness Handicap Inventory score at follow up, multivariate regression analysis with Dizziness Handicap Inventory score as dependent variable and vestibulo-ocular reflex gain at follow up, asymmetry ratio, time (days) between the first and second visit, subjective visual vertical and patients’ age as independent variables were performed.
A total of 3525 medical records of patients who were referred for vertigo were reviewed, including 1504 patients with acute vestibular syndrome who received an instrumental assessment within 72 hours. A total of 39 patients suffering from unilateral vestibular neuritis (mean age, 57.21 ± 15.80; 18 female patients; 21 patients with the left side affected; 21 patients where only the superior branch of the vestibular nerve was affected; and 18 patients with unilateral vestibular neuritis in toto) were enrolled.
At baseline, all patients showed a significant alteration (asymmetry ratio more than 40 per cent) for ocular vestibular-evoked myogenic potentials whereas at follow up normal values (asymmetry ratio less than 40 per cent) were found in 6 of 39 patients for ocular vestibular-evoked myogenic potentials as shown in Figure 1. Altered values were found for all patients at baseline for the subjective visual vertical test (more than −2° to more than +2°) whereas at follow up a normal range of the subjective visual vertical was found in 36 of 39 patients (less than −2° to less than +2°) as reported in Figure 2.
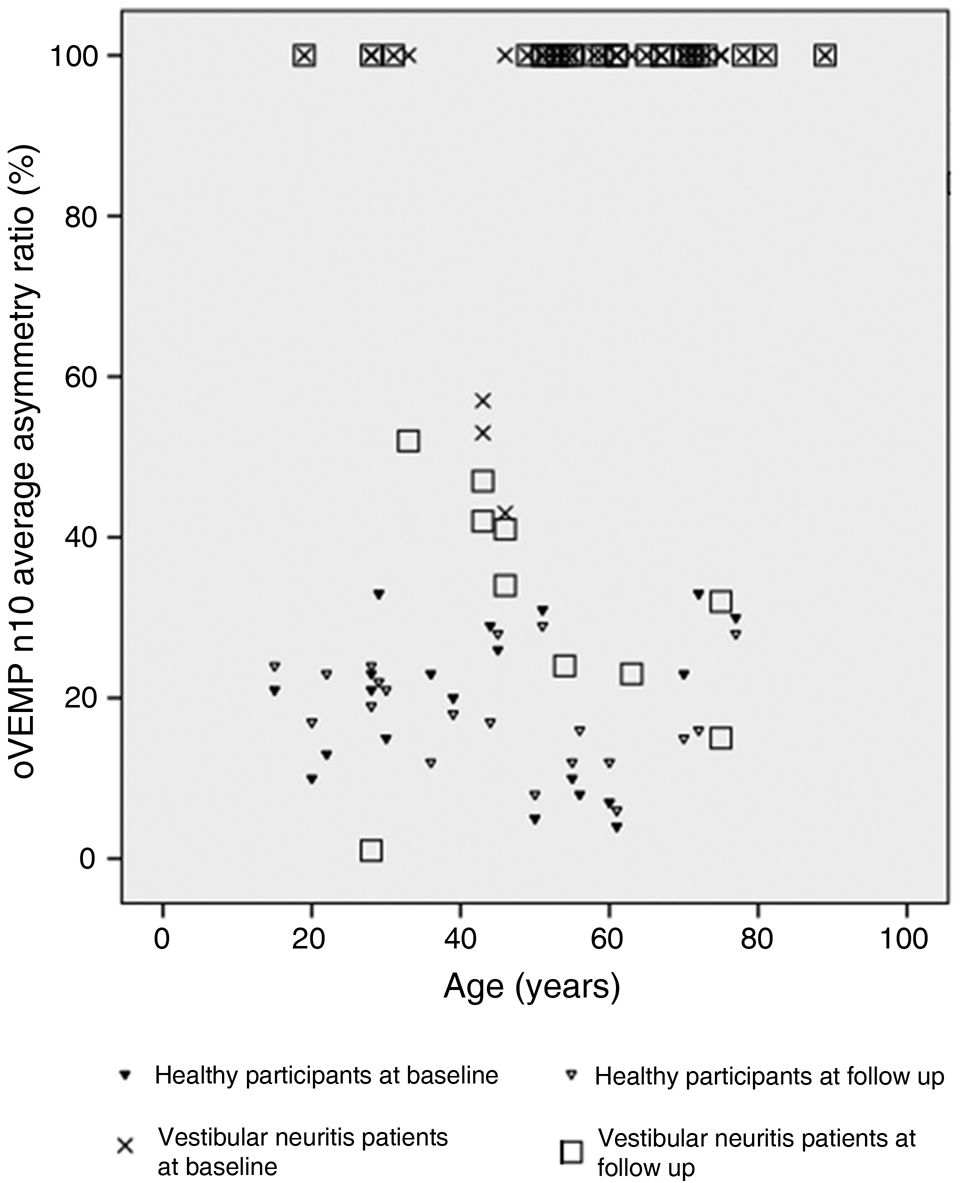
Fig. 1. The graph shows ocular vestibular-evoked myogenic potentials (oVEMP) asymmetry ratio percentage of all 39 patients with vestibular neuritis (superior division involvement + in toto involvement) at baseline (crosses) and follow up (squares), as well as 20 healthy participants at baseline (filled triangles) and at follow up (open triangles). Both groups, patients and healthy participants, were tested at baseline and at follow up.

Fig. 2. Long-term subjective visual vertical measurements of all 39 patients with vestibular neuritis (superior division involvement (grey) + in toto involvement (black)). Measurements were performed between the 72 hours (baseline) after the onset of the disease to six months after the first evaluation (follow up).
Four of 18 patients who showed a significant alteration (asymmetry ratio more than 30 per cent) for the cervical vestibular-evoked myogenic potentials at baseline showed normal (asymmetry ratio less than 30 per cent) values at follow up. At baseline, ocular vestibular-evoked myogenic potentials and subjective visual vertical were 100 per cent sensitive for the presence of unilateral vestibular neuritis, and at follow up the subjective visual vertical was 8 per cent sensitive for the presence of unilateral vestibular neuritis, giving a positive likelihood ratio less than 2 and a negative likelihood ratio more than 1.
Significant differences were found in the within-subject analysis for Dizziness Handicap Inventory score at baseline (75.33 ± 12.19) versus follow up (19.92 ± 6.78; p = 0.000) and in the ipsilesional horizontal vestibulo-ocular reflex gain at baseline (0.40 ± 0.13) versus follow up (0.62 ± 0.22; p = 0.000). Patients were divided into two sub-groups according to the interested branch nerve (superior or in toto). No differences between groups were found at baseline and follow up for Dizziness Handicap Inventory and vestibulo-ocular reflex gain (p > 0.05). The correlation analysis showed significant results between Dizziness Handicap Inventory score and affected vestibulo-ocular reflex gain (p < 0.01). Multivariate regression model showed that the vestibulo-ocular reflex gain at follow up had a significant effect (p < 0.01) greater than age (p < 0.05) on Dizziness Handicap Inventory score at follow up.
Discussion
The aim of this study was to explore the clinical course of dynamic and static otolith function in patients with unilateral vestibular neuritis using ocular vestibular-evoked myogenic potentials compared with subjective visual vertical tests in acute and sub-acute stages. For this purpose, in the first 72 hours from the onset of the symptoms, we simultaneously used both video head impulse test (for studying the function of the semicircular canals) and instrumental tests for the study of otolith function (bone-conducted vibration in response to ocular vestibular-evoked myogenic potentials, bone conducted vibration in response to cervical vestibular-evoked myogenic potentials and subjective visual vertical testing).
It is our belief that this type of dual approach is of fundamental importance especially in view of understanding both the functional recovery of the peripheral end organ and the symptomatic evolution of the patient's clinical conditions in light of objective measurements of vestibular function. This is also to verify what role, in the current state of our knowledge, the semicircular system and the otolith system plays in the patient's post-unilateral vestibular neuritis well-being.
From a clinical point of view, it is important to follow the evolution of a nosological event from the first hours of pathology for two reasons: (1) to set the correct differential diagnosis between peripheral and central damage, which is absolutely fundamental especially in the first hours from the onset of symptoms regarding the prognostic judgment and the choice of complementary tests to be performed (i.e. magnetic resonance imaging);Reference Newman-Toker, Kattah, Alvernia and Wang35 and (2) to monitor the possible functional recovery of the neurogenic damage in all the components of the VIIIth pair of cranial nerves.
With this in mind, we aimed to focus our interest on the comparison between the results of two tests of the otolith system (ocular vestibular-evoked myogenic potentials vs subjective visual vertical) that mainly explore the utricular macula function. We were starting from the assumption that these tests analyse, in light of recent physiological evidence,Reference Curthoys, MacDougall, Vidal and de Waele9,Reference Curthoys, Grant, Pastras, Brown, Burgess and Brichta11,Reference Curthoys and Dlugaiczyk12 the two different characteristics of the otolithic organ: the ‘transient’ system, which is tested using ocular vestibular-evoked myogenic potentials and the ‘sustained’ system, which is tested using the subjective visual vertical.
In the labyrinth, the utricular and saccular macula with their afferents form two complementary otolithic systems: the sustained system concerned with signalling low frequency linear accelerations and the transient system which is activated by high frequency stimuli such as sounds and vibration.Reference Curthoys, MacDougall, Vidal and de Waele9,Reference Curthoys and Vulovic25 Our results showed that in the acute phase (less than 72 hours from symptom onset), ocular vestibular-evoked myogenic potentials and subjective visual vertical testing had a high sensitivity for the presence of unilateral vestibular neuritis, but they differed significantly in the sub-acute phase assessment. In fact, subjective visual vertical testing showed very low sensitivity and specificity for the presence of unilateral vestibular neuritis at follow up.
At rest in a healthy patient, the medial portion of the utricular maculae send the same resting signal to the central otolithic neuronal in the vestibular nuclei. When an inflammatory process intervenes, as in the case of a vestibular neuritis, this balance of resting signals is altered, causing both eyes to adopt a maintained rolled eye positionReference Curthoys, Dai and Halmagyi36 and alteration of the spatial orientation relative to gravity. This loss of otolithic type I and type II neurons on the lesioned side contrasts with otolithic type I and type II neuron activity on the intact side, which presumably has normal resting activity. Such an imbalance corresponds to the utricular response to roll head tilt to the healthy side, which causes a small ocular counter rolling towards the opposite (lesioned) ear. This is a phenomenon that determines and is defined as visual bias and is responsible for the subjective visual vertical altered response at the time of the attack because it simulates a large roll-tilt, which drives the head and eyes to roll towards the lesioned side and to maintain this rolled position. For this reason, subjective visual vertical is considered as a simple useful clinical indicator of asymmetric sustained otolithic function.
According to Vibert et al.,Reference Vibert, Häusler and Safran37 this visual bias disappears at follow up with normalisation of subjective visual vertical results, confirming that these graviceptive sensory signals forwarded to brainstem, cerebellar, thalamic and eventually cortical areas are probably centrally compensated.Reference Herdman38–Reference Schmid-Priscoveanu, Straumann, Böhmer and Obzina39 Our hypothesis is that the subjective visual vertical test would not be suitable for diagnosing unilateral vestibular neuritis in the non-acute phase.
We found a significant persistence of utricular macula damage with ocular vestibular-evoked myogenic potentials at follow up, confirming that extraocular myogenic responses triggered by the high frequency activation of the near striola (type I) receptors, a main measure of transient responses, do show unilateral otolithic loss because of inflammatory insult in both acute and chronic stages. Our findings underpin the suitability of ocular vestibular-evoked myogenic potentials to detect unilateral otolith loss and confirm that these otolith tests are, to date, the best clinical instrumental objective measurement of unilateral otolith function after vestibular neuritis. This clinical finding underlines the usefulness of performing ocular vestibular-evoked myogenic potentials both in the acute phase and in the follow-up phase.
Aside from these results, considering the data collected in this cohort of patients, other points can be considered. According to our previous studies,Reference Manzari and Tramontano40,Reference Manzari, Graziano and Tramontano41 a significant difference in horizontal vestibulo-ocular reflex gain values were found at follow up, suggesting that the horizontal vestibulo-ocular reflex gain values can change during the clinical course of vestibular neuritis. These clinical data are important because they represent an important indicator of lesion evolution in semicircular canal dynamic function, especially at the time of the attack. Testing patients in the acute phase and after this period provides information on increase in the horizontal vestibulo-ocular reflex gain as previously described but in a longer observation period.Reference Manzari and Tramontano40,Reference Manzari, Graziano and Tramontano41 Furthermore, correlating the horizontal vestibulo-ocular reflex gain with the Dizziness Handicap Inventory score in the different phases showed that the subjective disability is significantly lower at follow up as reported in Table 2. The data could therefore also correlate with the modifications in vestibulo-ocular reflex gain values (increase), which in the early stages is strongly and suddenly compromised if compared with the contralesional side.
• The utricular and saccular macula can be tested with vestibular-evoked myogenic potentials
• The otolithic organs perform the ‘dynamic’ otolith function
• Static and dynamic otolith functions can be tested in early-stage vestibular neuritis with vestibular-evoked myogenic potentials and subjective visual vertical
• After the acute phase of vestibular neuritis, vestibular-evoked myogenic potentials and subjective visual vertical could give different results
• Ocular vestibular-evoked myogenic potentials are the most suitable test to evaluate the dynamic otolith functions in different stages of vestibular neuritis
A possible explanation for this subjective trend of vestibular compensation may lie in the disappearance of symptoms related to semicircular canal dysfunction, nystagmus and vertigo, and in the reduction of postural symptoms and vestibular neuritis induced ocular torsion. This seems to be, in this kind of patient, more important than chronic transient otolith deficit for the happiness of the patient suffering the effects of vestibular neuritis. There is a clear dissociation between the vestibulo-ocular reflex gain, the semicircular horizontal canal, tested with video head impulse test, and the transient-dynamic otolith function, tested with ocular vestibular-evoked myogenic potentials in the follow up. Both are objective measures of dynamic vestibular activity, but the functional recovery of the two systems does not proceed simultaneously. Vestibulo-ocular reflex gain at follow up in our vestibular neuritis cohort of patients improves significantly in comparison with asymmetry ratio percentage to ocular vestibular-evoked myogenic potentials. No differences were found in the two subgroups in terms of Dizziness Handicap Inventory improvements and horizontal vestibulo-ocular reflex gain recovery at follow up, suggesting that the damage of superior branch or the nerve in toto does not affect the perceived disability of the patients.
This study presents some limitations that should be mentioned. Firstly, this is a retrospective study, with the inherent potential bias. Secondly, we did not use ocular cycloposition by optical coherence tomography in the clinical examination. Another limitation is the lack of a long-term follow up; the evaluation of the ‘dynamic’ and ‘transient’ vestibular otolith sensory function could remain substantially unchanged or could improve if performed later in time (i.e. over six months). This evolution in the recovery of otolith function could determine a possible fluctuation in the Dizziness Handicap Inventory values and obviously affect the therapeutic destiny of these patients (i.e. rehabilitation of the otolithic function). Testing the static and sustained vestibular-otolith sensory function with subjective visual vertical and the dynamic and transient vestibular otolith sensory function with ocular vestibular-evoked myogenic potentials simultaneously at the time of the attack (baseline) and during the follow up in patients with vestibular neuritis showed how both functions of the utricular macula are affected by the inflammatory process at very early stages of the disease. If the instrumental protocol is not used in this way, the results obtained (i.e. when the patient is tested in the post-acute phase) can lead to the false conclusion that vestibular neuritis can affect the two otolith functions differently. In reality, the two otolith components in the post-acute phase behave differently with regards to recovery.
Conclusion
Testing the sustained (static) otolith system with subjective visual vertical could be useful in the very early stages of unilateral vestibular neuritis (less than 72 hours) but do not show sub-acute unilateral loss, whereas testing the transient (dynamic) otolith function with ocular vestibular-evoked myogenic potentials is the best way to diagnose unilateral otolithic loss after unilateral vestibular neuritis in the acute and sub-acute phase. Further studies will be needed to confirm our results in the chronic or recovery stage.
Competing interests
None declared






