Introduction
Tuberculous otitis media is rare in the UK, accounting for only 0.04 per cent of chronic suppurative otitis media cases.Reference Weiner, O'Connell and Pahor1 For this reason, it is often diagnosed late.Reference Vaamonde, Castro, García-Soto, Labella and Lozano2
Tuberculous otitis media may occur at any age, and commonly presents with features of chronic otitis media, including otorrhoea and hearing loss, with or without facial palsy.Reference Vital, Printza and Zaraboukas3 Complications include fistulae, facial nerve palsies, labyrinthitis, tuberculous osteomyelitis, mastoiditis, hearing loss and spread of infection to the central nervous system.Reference Wallmer4 It is therefore imperative that this condition is diagnosed and treated early.
Computed tomography (CT) is the imaging modality of choice for the investigation of chronic middle-ear disease, and is highly sensitive in detecting soft tissue disease and bony erosion.Reference Rhoa, Kimb, Kima, Sunga, Kwona and Leea5 The radiological signs of tuberculous otitis media include soft tissue filling the entire middle-ear cavity, preservation of mastoid air cells without bony damage, soft tissue extension, and mucosal thickening of the external auditory canal.Reference Rhoa, Kimb, Kima, Sunga, Kwona and Leea5 While these changes are characteristic of tuberculous otitis media they are not diagnostic, and accurate diagnosis depends on tissue histology or microbiological culture.
The mainstay of treatment for tuberculous otitis media is quadruple therapy with the antituberculous drugs isoniazid, rifampicin, pyrazinamide and ethambutol.Reference Hoca, Ögretensoy, Dayioglu and Akbafl6 The involvement of a multidisciplinary team is essential, in order to investigate for extra-aural foci of infection and to trace patient contacts, given the high incidence (up to 93 per cent) of pulmonary tuberculosis (TB) in patients presenting with tuberculous otitis media.Reference Kirsch, Wehner, Jensen, Kagawa and Campagna7
Case report
A 40-year-old Eastern European woman presented to the ENT department with a two-month history of unilateral, right-sided otalgia and otorrhoea. She was systemically well. Otoscopy revealed debris in the external auditory canal and oedema of the canal wall.
A diagnosis of otitis externa was made and the patient treated with topical steroid-antibiotic drops.
However, her symptoms failed to resolve, and over the course of the next three months she developed moderate to severe right-sided hearing loss, vertigo, tinnitus and a right-sided facial palsy (House–Brackmann grade V).
Examination of the ear with an operating microscope showed a large posterior perforation, with pale debris in the middle ear and attic. At this stage, the diagnosis was revised to probable cholesteatoma. A CT scan of the temporal bones showed soft tissue material filling the middle-ear cleft, but no bony erosion (Figure 1).

Fig. 1 Axial computed tomography scan demonstrating soft tissue reaction in the right middle-ear cleft. A = anterior; R = right; L = left; P = posterior
The patient was admitted for examination of the right ear under general anaesthesia. During this procedure, the tympanic membrane was found to be absent, and pale granulation tissue was seen in the middle-ear cleft (Figure 2). An intra-operative culture swab taken from the middle-ear cleft grew Pseudomonas aeruginosa, causing the diagnosis to be revised again, to necrotising malignant otitis externa.
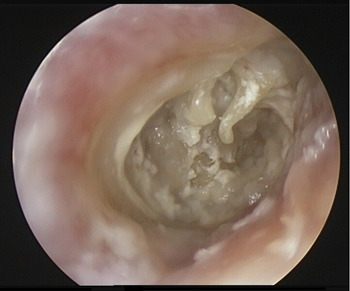
Fig. 2 Endoscopic image of the right ear showing a large central perforation. The handle of the malleus, long process of the incus and pale granulation tissue in the middle-ear cleft can all be clearly seen. (0° endoscope, Karl Storz).
A tissue biopsy from the middle ear, taken during the procedure, showed pale granulation tissue but no epithelial cells, malignant cells or granulomata. Staining of the biopsy material for acid-fast bacilli showed no evidence of mycobacteria. However, subsequent culture of biopsy tissue grew mycobacterium species, identified as Mycobacterium tuberculosis on polymerase chain reaction. This allowed the diagnosis of tuberculous otitis media, and the patient was commenced on quadruple therapy (i.e. rifampicin, pyrazinamide, pyridoxine and ethambutol) for 12 months.
Due to poor compliance, antituberculous medication was only taken for six months, after which time the patient's right-sided otorrhoea and otalgia had settled and her facial palsy had partially recovered (to House–Brackmann grade III).
Discussion
Two valuable learning points can be derived from this case.
Firstly, this case exemplifies the fact that tuberculous otitis media closely mimics other, more common otological and systemic disorders. For this reason, a high index of clinical suspicion is required to make the diagnosis. As in our patient, the diagnosis may be confused with otitis externa, cholesteatoma and necrotising otitis externa. The differential diagnosis also includes squamous cell carcinoma of the middle-ear cleft and fungal infection.
Secondly, this case highlights the difficulty that may be encountered in obtaining microbiological confirmation of M tuberculosis infection. In our patient, an ear swab taken directly from the middle-ear cleft yielded only P aeruginosa, further adding to the diagnostic confusion. Histological examination of biopsied granulation tissue did not identify any classical features of TB. Moreover, acid-fast bacilli testing on both the tissue biopsy and the ear swab was negative. The diagnosis was only made after mycobacterium species were cultured from tissue samples. The rarity of tuberculous otitis media, combined with the difficulty in gaining microbiological confirmation of the disease, may delay the correct diagnosis.
• Tuberculous otitis media is rare (representing 0.04 per cent of chronic suppurative otitis media cases)
• Symptoms may mimic other diseases of the external and middle ear
• Obtaining microbiological confirmation of disease may be difficult
• Diagnosis is often made late
• Polymerase chain reaction testing is often necessary to identify Mycobacterium tuberculosis
Previous studies of extrapulmonary TB have emphasised the challenge of obtaining positive microbiological evidence of TB.Reference Hoca, Ögretensoy, Dayioglu and Akbafl6 Mycobacterial counts are often low, and other micro-organisms may interfere with culture (e.g. staphylococcus, pseudomonas, klebsiella, proteus and streptococcal species).Reference Lee and Drysdale8 The incidence of positive acid-fast bacilli testing in tissue samples has been reported to be in the region of 27%, while the incidence of positive M tuberculosis culture from tissue samples may be as low as 70%, again demonstrating the difficulty of achieving a positive diagnosis.Reference Cleary and Batsakis9, Reference Williams and Douglas-Jones10
Conclusion
This case highlights the diagnostic challenge presented by tuberculous otitis media, and the high index of clinical suspicion needed for its diagnosis. Furthermore, it reinforces the fact that testing for acid-fast bacilli cannot in isolation be relied upon to make the diagnosis, and that culture together with polymerase chain reaction testing are also necessary.
A combination of clinical vigilance and appropriate diagnostic investigations will assist the early identification of cases of tuberculous otitis media. This may reduce the risk of developing potentially significant complications of the disease.




