Introduction
Nasopharyngeal carcinoma (NPC) is considered a distinct entity from other head and neck carcinomas because of its association with Epstein–Barr virus, its aggressive locoregional behaviour and the relatively higher risk of distant metastases.Reference Lee, Lin and Ng1 Given the anatomical location and radiosensitive behaviour of the tumour, radiotherapy (RT) has been the mainstay of local treatment.Reference You, Cao, Huang, Chen, Yang and Liu2 Since the publication of the Al-Saraff et al. study in 1998,Reference Al-Sarraf, LeBlanc, Giri, Fu, Cooper and Vuong3 concurrent chemoradiotherapy has gradually become the standard treatment for locoregionally advanced NPC.
Epidermal growth factor receptor, a member of the ErbB family of receptor tyrosine kinases, is expressed in many epithelial carcinomas. It has been reported that epidermal growth factor receptor is expressed in more than 85 per cent of NPC cases.Reference Putti, To, Hsu, Chan, Lai and Tse4,Reference Ma, Poon, To, Zee, Mo and Chan5 Studies have also suggested that the expression of epidermal growth factor receptor is independently associated with poor clinical outcomes.Reference Ma, Poon, To, Zee, Mo and Chan5,Reference Chua, Nicholls, Sham and Au6 Bonner et al. showed that cetuximab, an immunoglobulin G1 monoclonal antibody against the ligand-binding domain of epidermal growth factor receptor, is efficacious in squamous cell cancers of the head and neck region.Reference Bonner, Harari, Giralt, Cohen, Jones and Sur7 However, patients with primary NPC were not included in this phase III trial. Similarly, in recurrent and/or metastatic squamous cell head and neck cancer patients, the addition of cetuximab to chemotherapy was shown to increase response rate, progression-free survival and overall survival in comparison to chemotherapy alone.Reference Vermorken, Mesia, Rivera, Remenar, Kawecki and Rottey8 However, primary NPC was an exclusion criterion. High quality evidence for the use of cetuximab in NPC would appear lacking. We aimed to examine the available literature on the use of cetuximab in NPC.
Materials and methods
Published data for this review were identified by searching PubMed-Medline and Embase databases, and the Cochrane (reviews and economic evaluations) library, from 1997 to the present day (a 20-year period; the date of the search was 11th August 2017, with a re-run on 23rd August 2018). The Medical Subject Heading terms and keywords used in the search were: ‘nasopharyngeal neoplasm’, ‘cetuximab’ and ‘Erbitux’. A professional librarian conducted the literature search, and two authors (MSI and AT) analysed the list to identify suitable studies. All pertinent articles were retrieved, and selected studies were considered for this review. The references were also manually searched to identify other relevant studies. One author (MSI) collected the literature data and another author (AT) reviewed them for quality assurance. A flow chart of the search is shown in Figure 1.

Fig. 1. Flowchart of the literature search. NPC = nasopharyngeal carcinoma; EGFR = epidermal growth factor receptor
In this review, the selected studies were allocated either to locally advanced or to recurrent or metastatic nasopharyngeal carcinoma groups. For each group, the results are summarised in terms of subgroups based on the sequence of cetuximab use.
Results
The literature search identified 55 studies for review based on titles and abstracts. A manual search of references identified 14 further studies. Twenty studies were included in the final review (Figure 1).Reference Feng, Guo, Li, Zhong, Chen and Huang9–Reference Xu, Ou, Shen and Hu28 The inclusion and exclusion criteria are also shown in Figure 1. Given the heterogeneous nature of the studies, and the fact that many studies were available in abstract form only with variable reporting on outcomes, it was not possible to perform either a meta-analysis or statistical analyses of the pooled data.
There were no randomised controlled phase III trials. Nine phase II trials were identified.Reference Feng, Guo, Li, Zhong, Chen and Huang9–Reference Chen, Zhao, Gao, Lang, Pan and Hu11,Reference Ma, Kam, Leung, Hui, King and Chan15,Reference Zhu, Liu, Guan, Hu and Li18,Reference Xu, Liu, Dou, Li, Guan and Zhu20,Reference Ng, Ngan, Kwong, Tung, Yuen and Kam24–Reference Kerboua, Tahrat and Bouzid26 Of 20 selected studies, 5 were presented in the form of abstracts only. The findings of the selected studies that describe the use of cetuximab in nasopharyngeal carcinoma (NPC) are summarised in Tables 1–8.Reference Feng, Guo, Li, Zhong, Chen and Huang9–Reference Xu, Ou, Shen and Hu28
Table 1. Cetuximab combined with chemoradiotherapy for locally advanced NPC

*Standard dose of cetuximab: initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. NPC = nasopharyngeal carcinoma; RT = radiotherapy; TNM = tumour–node–metastasis; PS = performance status; wk = week; f/u = follow up; mth = months; CI = confidence interval; y = years; PFS = progression-free survival; OS = overall survival; IMRT = intensity-modulated radiotherapy; DFS = disease-free survival; RFS = relapse-free survival; DMFS = distant metastasis-free survival; CRT = chemoradiotherapy; TP = docetaxel plus cisplatin (without 5-fluorouracil); 2D = two-dimensional; WHO = World Health Organization; NG = nasogastric; EGFR = epidermal growth factor receptor; IQR = interquartile range; HR = hazard ratio
Table 2. Cetuximab plus RT compared with chemoradiotherapy for locally advanced NPC

*Standard dose of cetuximab: initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. RT = radiotherapy; NPC = nasopharyngeal carcinoma; CRT = chemoradiotherapy; ERT = Erbitux® plus radiotherapy; IMRT = intensity-modulated radiotherapy; f/u = follow up; mth = months; y = years; PFS = progression-free survival; BRT = bio-radiotherapy; PS = performance status; TPF = docetaxel, cisplatin and 5-fluorouracil; wk = week; OS = overall survival; DFS = disease-free survival; MFS = metastasis-free survival; RFS = relapse-free survival
Table 3. Cetuximab with RT, with or without chemotherapy, for locally advanced NPC

*Standard dose of cetuximab: initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. RT = radiotherapy; NPC = nasopharyngeal carcinoma; IMRT = intensity-modulated radiotherapy; WHO = World Health Organization; PS = performance status; CRT = chemoradiotherapy; mth = months; f/u = follow up; y = years; PFS = progression-free survival; DMFS = distant metastasis-free survival; OS = overall survival; CI = confidence interval
Table 4. Cetuximab with induction chemotherapy followed by RT for locally advanced NPC

*Standard dose of cetuximab: initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. RT = radiotherapy; NPC = nasopharyngeal carcinoma; PS = performance status; wk = week; IMRT = intensity-modulated radiotherapy; f/u = follow up; mth = months; y = years; DMFS = distant metastasis-free survival; PFS = progression-free survival; OS = overall survival; EBV = Epstein–Barr virus
Table 5. Cetuximab with induction chemotherapy followed by chemoradiotherapy, or induction chemotherapy followed by cetuximab and RT, for locally advanced NPC

*Standard dose of cetuximab: initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. RT = radiotherapy; NPC = nasopharyngeal carcinoma; CRT = chemoradiotherapy; y = years; DFS = disease-free survival; OS = overall survival; DMFS = distant metastasis-free survival; RFS = relapse-free survival
Table 6. Induction chemotherapy followed by bio-chemoradiotherapy for locally advanced, recurrent NPC

*Standard dose of cetuximab: initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. NPC = nasopharyngeal carcinoma; CRT = chemoradiotherapy; TPF = docetaxel, cisplatin and 5-fluorouracil; RT = radiotherapy; y = years; PFS = progression-free survival; OS = overall survival
Table 7. Cetuximab plus chemotherapy for recurrent and/or metastatic NPC

*Standard dose of cetuximab: initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. NPC = nasopharyngeal carcinoma; EGFR = epidermal growth factor receptor; mth = months; PS = performance status; wk = week; CI = confidence interval; OS = overall survival
Table 8. Cetuximab plus chemoradiotherapy for recurrent and/or metastatic NPC
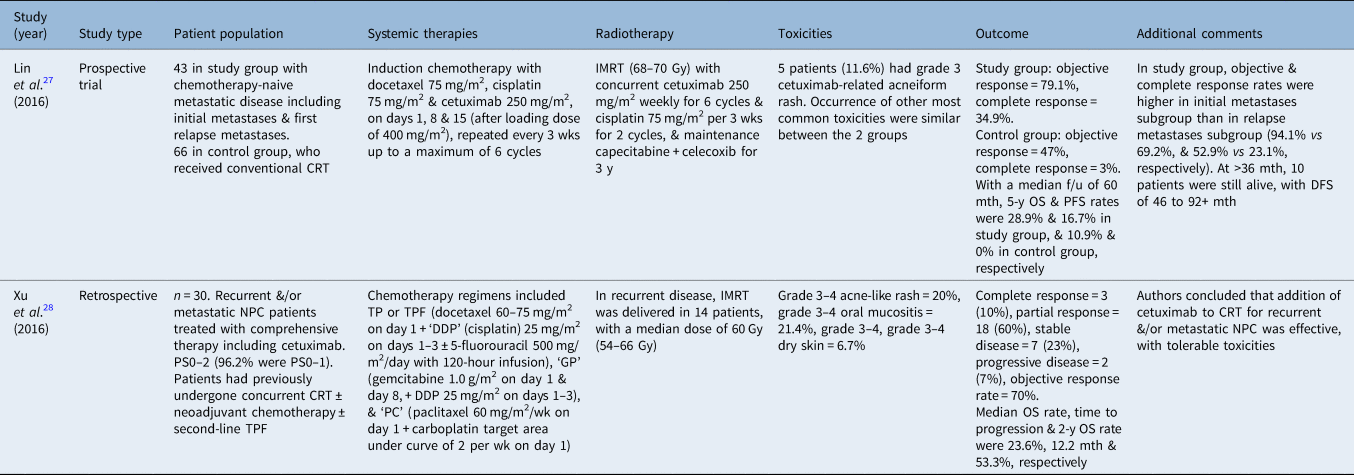
NPC = nasopharyngeal carcinoma; CRT = chemoradiotherapy; wk = week; IMRT = intensity-modulated radiotherapy; y = years; f/u = follow up; OS = overall survival; PFS = progression-free survival; mth = months; DFS = disease-free survival; PS = performance status; TPF = docetaxel, cisplatin and 5-fluorouracil; TP = docetaxel plus cisplatin (without 5-fluorouracil)
Locally advanced nasopharyngeal carcinoma
Cetuximab with chemoradiotherapy
Four phase II trials,Reference Feng, Guo, Li, Zhong, Chen and Huang9–Reference Chen, Zhao, Gao, Lang, Pan and Hu11,Reference Ma, Kam, Leung, Hui, King and Chan15 one prospective studyReference He, Xu, Guo, Jiang, Wang and Zong14 and four retrospective studiesReference Wu, Zhu, Xu, Jiang, Yin and Jiang12,Reference Li, Chen, Tang, Liu, Guo and Guo13,Reference Xia, Liang, Lv, Wang, Qian and Ye16,Reference You, Hua, Liu, Yang, Zhang and Li17 assessed the effects of adding cetuximab to concurrent chemoradiotherapy. In the 4 phase II trials, the number of patients enrolled ranged from 30 to 100. A standard dose of cetuximab (initial loading dose of 400 mg/m2 followed by 250 mg/m2 on a weekly basis) was added to cisplatin-based concurrent chemoradiotherapy. In two of these phase II studies, a variable number of patients also received neo-adjuvant or adjuvant chemotherapy. Grade 3 or 4 mucositis was the most common toxicity, with incidence ranging from 71 per cent to 87 per cent. The two-year overall survival rate ranged from 89.9 per cent to 93 per cent. Only one of these four phase II trials reported a five-year overall survival rate (of 82.1 per cent).
In a retrospective propensity score analysis, Xia et al.Reference Xia, Liang, Lv, Wang, Qian and Ye16 compared 96 patients who received concurrent cisplatin chemoradiotherapy plus cetuximab against 3126 patients who had received concurrent chemoradiotherapy alone. The median follow-up period was 5.17 years for the concurrent chemoradiotherapy plus cetuximab group and 5.24 years for the concurrent chemoradiotherapy group; there was a statistically significant difference in 5-year distant metastasis-free survival. The locoregional relapse-free survival, disease-free survival and overall survival rates were similar. A subgroup analysis showed a significant distant metastasis-free survival benefit with concurrent chemoradiotherapy plus cetuximab in patients with nodal N2–3 stage disease, compared with N2–3 patients receiving concurrent chemoradiotherapy alone (87.9 per cent vs 66.2 per cent, respectively; p = 0.045). Grade 3–4 mucositis was not significantly more common in the concurrent chemoradiotherapy plus cetuximab group (47.9 per cent vs 37.5 per cent; p = 0.479), but grade 3–4 skin rash was (16.7 per cent vs 0 per cent; p < 0.0001).Reference Xia, Liang, Lv, Wang, Qian and Ye16
In another retrospective study, by You et al.,Reference You, Hua, Liu, Yang, Zhang and Li17 a propensity score-matched comparative analysis was carried out in patients with stage II–IVB NPC. The patients were treated with cisplatin-based chemoradiotherapy alone, or chemoradiotherapy in combination with biotherapy, either cetuximab or nimotuzumab. The survival outcomes appeared superior in the cetuximab/nimotuzumab group (three-year disease-free survival rate of 93.5 per cent vs 86.9 per cent (p = 0.028); three-year distant metastasis-free survival rate of 94.6 per cent vs 89.3 per cent (p = 0.03); and three-year overall survival rate of 96.6 per cent vs 92.9 per cent (p = 0.015)), but the treatment was associated with higher rates of grade 3 skin rash and grade 3–4 mucositis.Reference You, Hua, Liu, Yang, Zhang and Li17
In a recently published case–control retrospective study by Li et al.,Reference Li, Chen, Tang, Liu, Guo and Guo13 which compared the addition of cetuximab to chemoradiotherapy, there was no significant difference in five-year overall survival rates (89.7 per cent with cetuximab and 90.7 per cent without cetuximab; p = 0.386). However, there was a significant difference in grade 3 and 4 toxicities with the addition of cetuximab (grade 3–4 mucositis in 51.6 per cent of patients treated with cetuximab and 23.4 per cent in the group without cetuximab; p < 0.001).
Induction chemotherapy, followed by cetuximab plus radiotherapy or chemoradiotherapy
In a phase II trial conducted by Xu et al.,Reference Xu, Liu, Dou, Li, Guan and Zhu20 two cycles of induction chemotherapy (cisplatin and docetaxel) followed by either cisplatin-based chemoradiotherapy or cetuximab-RT (Erbitux® plus RT) were evaluated. Although there were no significant differences in the outcome, the study was closed ahead of schedule because of the higher rates of grade 3–4 mucositis in the Erbitux plus RT arm (80.9 per cent in the Erbitux plus RT arm, vs 47.8 per cent in the chemoradiotherapy arm; p = 0.023). The rate of grade 3–4 acneiform rash was 33.3 per cent in the Erbitux plus RT arm, versus 0 per cent in the chemoradiotherapy arm (p = 0.009).Reference Xu, Liu, Dou, Li, Guan and Zhu20
In a retrospective, matched case–control study that compared the safety and efficacy of concurrent cetuximab-based bio-radiotherapy and cisplatin-based chemoradiotherapy in the treatment of locally advanced NPC, patients received two cycles of docetaxel, cisplatin and 5-fluorouracil (‘TPF’) induction chemotherapy, followed by either bio-radiotherapy or chemoradiotherapy.Reference Wu, Huang, Liu, Li, Li and Zhang19 Survival outcomes were similar (five-year overall survival rate of 79.5 per cent for bio-radiotherapy and 79.3 per cent for chemoradiotherapy; p = 0.797). There was a higher incidence of grade 3–4 haematological toxicity and severe vomiting with chemoradiotherapy. The bio-radiotherapy patients experienced more severe rashes and mucositis.Reference Wu, Huang, Liu, Li, Li and Zhang19
Cetuximab with radiotherapy, with or without chemotherapy
In a retrospective study, Niu et al.Reference Niu, Hu and Kong21 evaluated the safety and efficacy of cetuximab plus intensity-modulated RT, with or without chemotherapy, for locally advanced NPC (n = 33). The majority of patients (91 per cent) received platinum-based neoadjuvant, concurrent or adjuvant chemotherapy. The three-year progression-free survival and overall survival rates were 70.5 per cent and 90.9 per cent, respectively. For the cetuximab plus intensity-modulated RT group, the grade 3–4 stomatitis rate was 84.9 per cent. Temporal lobe necrosis was observed in seven patients (21 per cent). The authors concluded that cetuximab plus intensity-modulated RT, with or without chemotherapy, for locally advanced NPC was effective and tolerable.Reference Niu, Hu and Kong21
Cetuximab with induction chemotherapy, followed by radiotherapy alone
At the European Society for Therapeutic Radiology and Oncology annual meeting in 2016, Lin et al.Reference Lin, Wang, Liu and Lin22 presented results from a case series of patients treated with an induction bio-chemotherapy regimen (cisplatin, 5-fluorouracil and leucovorin, with or without docetaxel or gemcitabine, and weekly cetuximab) followed by RT (70–76.4 Gy) in 42 patients with stage III/IV NPC. Each patient received a mean of 11 weeks of cetuximab treatment. After induction bio-chemotherapy, all patients responded (50 per cent complete response and 50 per cent partial response). The three-year progression-free survival and overall survival rates were 79.9 per cent and 92.1 per cent respectively. The rates of grade 3–4 toxicities were: skin rash, 50 per cent; leucopoenia, 11.9 per cent; anaemia, 9.5 per cent; thrombocytopenia, 2.4 per cent; and mucositis, 2.4 per cent. Basal plasma Epstein–Barr virus DNA levels were the most important prognostic factor.Reference Lin, Wang, Liu and Lin22
Cetuximab with induction chemotherapy followed by chemoradiotherapy, or induction chemotherapy followed by cetuximab and radiotherapy
In a recently published retrospective analysis by Peng et al.,Reference Peng, Tang, Liu, Chen, Li and Mao23 a cohort of patients who received cetuximab or nimotuzumab in combination with induction chemotherapy followed by chemoradiotherapy (investigational arm) was compared against those who received induction chemotherapy followed by RT plus cetuximab or nimotuzumab (control arm). Three-year overall survival rates were similar (94 per cent vs 92.1 per cent; p = 0.673); however, the three-year disease-free survival rate was higher in the investigational arm (84.3 per cent vs 74.3 per cent; p = 0.027). In the investigational arm, the rates of grade 3–4 skin reaction (15.4 per cent vs 2 per cent; p < 0.001) and grade 3–4 mucositis (10.1 per cent vs 3.4 per cent; p = 0.02) were higher during the induction phase.Reference Peng, Tang, Liu, Chen, Li and Mao23
Recurrent and/or metastatic nasopharyngeal carcinoma
Induction chemotherapy followed by bio-chemoradiotherapy
A phase II trial, published by Ng et al. in 2018,Reference Ng, Ngan, Kwong, Tung, Yuen and Kam24 evaluated three cycles of docetaxel, cisplatin and 5-fluorouracil, followed by weekly docetaxel and cetuximab concurrently with RT, in locally advanced recurrent NPC. Although complete response was achieved in 30.8 per cent of cases, and three-year progression-free and overall survival rates were 35.7 per cent and 63.8 per cent respectively, the regimen was very toxic (temporal lobe necrosis, 24 per cent; grade 3 or greater hearing loss, 30.8 per cent; grade 3 or greater trismus, 19.2 per cent; and grade 3 or greater soft tissue necrosis, 15.4 per cent). Overall, 5 out of 33 patients died owing to treatment-related complications.Reference Ng, Ngan, Kwong, Tung, Yuen and Kam24
Chemotherapy plus cetuximab
In two phase II trials, the toxicity and efficacy of cetuximab in combination with carboplatinReference Chan, Hsu, Goh, Hui, Liu and Millward25 or cisplatinReference Kerboua, Tahrat and Bouzid26 in recurrent and/or metastatic NPC patients, in whom disease had progressed at or within 12 months following completion of platinum-based chemotherapy, were evaluated. The overall response rates (complete or partial response, and stable disease) were 60 per cent with carboplatin and 78 per cent with cisplatin. Median overall survival was 7.7 months with carboplatin and 22 months with cisplatin. The toxicity profile in both studies was acceptable. The authors of both studies concluded that the regimens were clinically effective, with acceptable safety profiles.Reference Chan, Hsu, Goh, Hui, Liu and Millward25,Reference Kerboua, Tahrat and Bouzid26
Chemoradiotherapy plus cetuximab
As presented in a poster at the European Society of Medical Oncology annual meeting in 2016, Lin et al.Reference Lin, Zhang, Huang, Li, Huang and Chen27 explored the efficacy of first-line cetuximab plus platinum and taxane as induction chemotherapy followed by chemoradiotherapy and a subsequent three-year maintenance treatment regime for patients with chemotherapy-naive distant metastatic NPC. In the study group, 43 patients (17 with newly diagnosed initial metastases, and 26 with first relapse metastases) received induction chemotherapy consisting of cetuximab, cisplatin and docetaxel followed by chemoradiotherapy concurrently with cetuximab and cisplatin, followed by maintenance capecitabine and celecoxib for three years. In the control group, patients received platinum-based induction chemotherapy followed by conventional chemoradiotherapy (n = 66). After induction chemotherapy, the objective response and complete response rates were 79.1 per cent and 34.9 per cent for the study group, and 47 per cent and 3 per cent for the control group, respectively. With a median follow up of 60 months, 5-year overall survival and progression-free survival rates were 28.9 per cent and 16.7 per cent in the study group, and 10.9 per cent and 0 per cent in the control group, respectively. The rate of grade 3 cetuximab-related acneiform rash was 11.6 per cent. The authors concluded that ‘the cetuximab-containing induction and consolidation chemoradiotherapy patients with chemotherapy-naive metastatic NPC resulted in excellent long-term disease-free survival and safety, indicating that metastatic NPC is potentially curable, especially in patients with IM [initial metastases]’.Reference Lin, Zhang, Huang, Li, Huang and Chen27
In a similar retrospective study, Xu et al.Reference Xu, Ou, Shen and Hu28 reported the efficacy and safety of cetuximab plus chemotherapy, using three different regimens (i.e. docetaxel and cisplatin plus 5-fluorouracil; gemcitabine plus cisplatin; or paclitaxel plus carboplatin). Each of these chemotherapy and cetuximab regimens was added to intensity-modulated RT in the treatment of 30 patients with recurrent and/or metastatic NPC. Twenty-one patients (70 per cent) achieved a response (3 complete responses and 18 partial responses). The median survival time was 23.6 months and the 2-year overall survival rate was 53.3 per cent. The toxicity profile was acceptable according to the authors.Reference Xu, Ou, Shen and Hu28
Ongoing study – cetuximab plus chemotherapy or chemoradiotherapy
A randomised, controlled, multicentre, phase III trial comparing cetuximab, cisplatin and docetaxel induction chemotherapy followed by concurrent chemoradiotherapy with cisplatin plus docetaxel in untreated metastatic NPC is currently ongoing. The estimated date of completion for this study (trial identifier: NCT02633176) is January 2023.
Discussion
The cornerstone of treatment for locoregionally advanced nasopharyngeal carcinoma (NPC) is RT. Additional chemotherapy given in the concurrent setting is associated with improved outcomes, but at the expense of increased toxicity, especially radiation-induced mucositis. The meta-analysis by Blanchard et al.Reference Blanchard, Lee, Marguet, Leclercq, Ng and Ma29 confirmed that the addition of concomitant chemotherapy to RT significantly improves overall survival in NPC (hazard ratio = 0.79, 95 per cent confidence interval (CI) = 0.73–0.86 (p < 0.0001); absolute benefit at five years = 6.3 per cent, 95 per cent CI = 3.5–9.1). The addition of chemotherapy, either adjuvant or induction, alongside concomitant chemoradiotherapy is gaining popularity, although the most effective sequence has not been determined. In an individual patient data network meta-analysis, the addition of adjuvant chemotherapy to concomitant chemoradiotherapy achieved the highest survival outcome,Reference Ribassin-Majed, Marguet, Lee, Ng, Ma and Chan30 while another network meta-analysis showed that induction chemotherapy followed by concurrent chemoradiotherapy was the most effective regimen.Reference You, Cao, Huang, Chen, Yang and Liu2
Concurrent cetuximab with RT has been widely used in the treatment of head and neck cancer;Reference Bonner, Harari, Giralt, Cohen, Jones and Sur7 however, in NPC, level 1 evidence is lacking. In the management of primary NPC, there has only been one phase II trial comparing cetuximab and RT versus cisplatin and RT, and both these regimens were given after initial induction chemotherapy with docetaxel and cisplatin.Reference Xu, Liu, Dou, Li, Guan and Zhu20 The study closed early because of the much higher incidence of mucositis in the cetuximab arm (80.9 per cent) compared with the cisplatin arm (47.8 per cent). Other morbidities were also higher in the cetuximab group (Table 2). This study comprised only 44 patients: 23 in the cisplatin-RT arm and 21 in the cetuximab-RT arm. The final sample size was therefore too low to draw any firm conclusions regarding survival outcomes.Reference Xu, Liu, Dou, Li, Guan and Zhu20
Given the heterogeneity of the studies presented in this narrative review, and the inherent selection bias evident in the non-randomised trials, meta-analysis of the results was not appropriate. While some series have presented encouraging survival outcomes, no single trial has offered level 1 evidence or irrefutable evidence to demonstrate the clinical effectiveness of adding cetuximab to standard chemoradiotherapy. However, there appears to be a trend towards greater toxicity (especially in regard to skin reactions and mucositis) reported in those patient groups treated with additional cetuximab compared to standard chemoradiotherapy regimens. There is also some evidence that the addition of cetuximab to RT following induction chemotherapy may lead to unacceptably high toxicity.Reference Wu, Huang, Liu, Li, Li and Zhang19,Reference Xu, Liu, Dou, Li, Guan and Zhu20
Lin et al.Reference Lin, Wang, Liu and Lin22 claimed that induction bio-chemotherapy with cetuximab, followed by intensity-modulated RT, was a ‘highly effective protocol’. However, the authors of the current review feel that the results of Lin and colleagues’ study should be interpreted with caution. The results have not yet been published in full, and there were a limited number of patients in the study. In addition, a recent randomised phase II European Organisation for Research and Treatment of Cancer (‘EORTC’) trial, of docetaxel, cisplatin and 5-fluorouracil plus cetuximab induction chemotherapy followed by bio-chemoradiotherapy, with weekly cetuximab plus weekly cisplatin or carboplatin, in head and neck squamous cell carcinoma patients, showed unacceptable complications that led to the study closing prematurely.Reference Specenier, Remenar, Buter, Schrijvers, Bergamini and Licitra31
A series of retrospective studies compared cetuximab use in NPC against matched historic chemoradiotherapy data.Reference Xia, Liang, Lv, Wang, Qian and Ye16,Reference You, Hua, Liu, Yang, Zhang and Li17,Reference Wu, Huang, Liu, Li, Li and Zhang19,Reference Niu, Hu and Kong21 These showed a potential benefit with the addition of cetuximab, either in generalReference You, Hua, Liu, Yang, Zhang and Li17,Reference Wu, Huang, Liu, Li, Li and Zhang19,Reference Niu, Hu and Kong21 or in some subgroups.Reference Xia, Liang, Lv, Wang, Qian and Ye16 These retrospective studies, which comprised a small number of patients, can also be criticised for inappropriate subset analyses when the studies were originally not set up to answer such questions. All the studies showed increased morbidity in patients who received cetuximab, including one study with a 21 per cent incidence of temporal lobe necrosis.Reference Niu, Hu and Kong21 A wide range of historic treatments, including neoadjuvant, concurrent and adjuvant chemotherapy, was used in these comparative studies, and so the benefit of using cetuximab alone or in combination with platinum-based chemotherapy is difficult to determine.
Evidence for the use of cetuximab in the management of recurrent or metastatic NPC is limited by the small number of studies, all of which contain relatively small numbers of patients. The use of cetuximab in combination with chemotherapy was tested in two phase II trials (the total number of patients in these 2 trials was only 114).Reference Chan, Hsu, Goh, Hui, Liu and Millward25,Reference Kerboua, Tahrat and Bouzid26 The authors of both studies concluded that the regimens were clinically effective, with acceptable safety profiles. However, there has been criticism of these findings. Both studies enrolled patients with progressive disease within 12 months of completing platinum-based chemotherapy, which means they are likely to have included patients who were partially cisplatin-sensitive. Indeed, the authors themselves found that prolonged overall survival was dependent on the interval (over 90 days vs under 90 days) between completing platinum-based previous chemotherapy and platinum-based second-line chemotherapy.Reference Chan, Hsu, Goh, Hui, Liu and Millward25 Therefore, the use of cetuximab would be better explored in combination with other chemotherapeutic agents in a population of patients who are strictly defined as platinum-resistant.Reference Licitra, Bossi, Locati and Bergamini32
The results of a small study of cetuximab with induction chemotherapy, followed by chemoradiotherapy with concomitant cisplatin and cetuximab, and then followed by maintenance capecitabine and celecoxib, in chemotherapy-naive distant metastatic NPC patients, seem promising.Reference Lin, Zhang, Huang, Li, Huang and Chen27 However, this has only been presented as an abstract (no information is available regarding the inclusion criterion for patients enrolled in the controlled group), and a high rate of ‘cure’ in metastatic disease seems unlikely. For such intense treatment regimes, good patient selection is key (i.e. oligometastatic disease in fit patients). However, the very small numbers of patients in the subgroups do not allow firm deductions to be made.Reference Lin, Zhang, Huang, Li, Huang and Chen27
In the locoregional setting, there is consistently no significant benefit from the addition of cetuximab, and it comes with an increase in toxicity. However, the use of cetuximab in the metastatic or locally recurrent situation may have more promise, either for more effective palliation alongside other chemotherapy regimens, or as part of an induction regimen prior to salvage treatment.
This review has drawn together published studies focusing on NPC that have used cetuximab in combination with standard, evidence-based treatments. It shows that there has been great interest and endeavour in trying to improve the outcome for NPC patients, and a wide variety of treatment strategies have been utilised. The reason why cetuximab does not seem to add benefit to the treatment of locoregional disease may be because the outcome is already very good for the majority of patients, and the side effects from conventional chemoradiotherapy, although tolerable, are significant. Therefore, if future trials focus on NPC patients with poorer prognosis, they are likely to produce more convincing evidence for a beneficial role of cetuximab.
Conclusion
At this point in time, there is no evidence supporting the addition of cetuximab to standard management protocols for nasopharyngeal carcinoma. There is more promise for its use in the metastatic or locally recurrent setting, and this could be a focus for future investigation.
Competing interests
None declared











