Introduction
Congenital aural atresia is characterised by hypoplasia or aplasia of the external auditory canal at birth. It may occur sporadically or as part of syndromes such as Goldenhar or Treacher Collins syndromes.Reference Chandrasekhar and De La Cruz1 The incidence of congenital aural atresia is 1 in 10 000 live births.Reference De la Cruz and Teufert2 The condition is often associated with microtia and middle-ear anomalies, and occasionally with inner-ear anomalies.Reference Schuknecht3 Twenty-five per cent of congenital aural atresia cases occur bilaterally.Reference De la Cruz and Teufert2
There are two main issues to be addressed in children with congenital aural atresia: the aesthetic issue and the functional problems. Affected patients will have conductive hearing loss due to the canal atresia, with or without middle-ear deformity. Bilateral cases need to be addressed more urgently than unilateral occurrences to restore normal hearing, in order to ensure proper speech and language development.
For cases younger than five years, bone conduction hearing aids should be fitted for hearing amplification before the child reaches a suitable age for various surgical treatments. In terms of audiological outcomes, Jovankovičová et al. reported that atresiaplasty or surgical reconstruction of an atretic ear showed an inconsistent functional outcome, regardless of the possible operative complications.Reference Jovankovičová, Staník, Kunzo, Majáková and Profant4 If one considers a 20–30 dB hearing threshold as a successful outcome, the reported success rates of atresiaplasty have varied from 12 per cent to 71 per cent in different studies.Reference Jovankovičová, Staník, Kunzo, Majáková and Profant4 Surgical success depends largely on the malformation severity.Reference Jahrsdoerfer, Yeakley, Aguilar, Cole and Gray5 Evans and Kazahaya reported that 93 per cent of patients still required a hearing aid or implant post-atresiaplasty.Reference Evans and Kazahaya6 Common complications encountered during canaloplasty include canal restenosis, skin graft lateralisation, and, less commonly, post-operative hearing deterioration and intra-operative facial nerve injury.Reference De la Cruz and Teufert2, Reference Jovankovičová, Staník, Kunzo, Majáková and Profant4
Bone conduction hearing implants offer a better treatment option for conductive hearing loss patients with aural atresia, especially those with high grade aural atresia, with higher acoustic gain and less risk of surgical complications.Reference Verhagen, Hol, Coppens-Shellekens, Snik and Cremers7 One study reported an average hearing threshold improvement of 37.45 dB in patients with a bone conduction implant, versus a gain of only 12.42 dB in post-atresiaplasty patients.Reference Jovankovičová, Staník, Kunzo, Majáková and Profant4
The implantable hearing device options for aural atresia patients with conductive hearing loss include: a percutaneous osseointegrated bone-anchored hearing aid (BAHA), a middle-ear implant system (e.g. Vibrant® Soundbridge™) and a transcutaneous bone conduction implant (e.g. Bonebridge).Reference Yu, Wong, Tsang and Tong8
A BAHA consists of a percutaneous vibration transducer, which is coupled to a titanium implant anchored in the skull bone to stimulate the inner ear transcranially. Despite promising functional gain, problems frequently arise from the junction of the skin and titanium. A meta-analysis by Kiringoda and Lustig reported an incidence of skin reactions in adult or mixed populations of 16–38 per cent, with an incidence as high as 78 per cent in children.Reference Kiringoda and Lustig9 The incidence of implant infections ranged from 1 per cent to 50 per cent, and implant loss rates ranged from 2 per cent to 17 per cent.Reference Kiringoda and Lustig9 Furthermore, osseointegration issues or head trauma resulted in higher fixture loss rates in children, with revision surgery rates of 17–44 per cent.Reference Baumgartner, Hamzavi, Böheim, Wolf-Magele, Schlögel and Riechelmann10 A retrospective study by Kraai et al., which involved 27 children with percutaneous osseointegrated bone conduction implants, reported that 89 per cent of the children experienced some form of complication post-implantation, and nearly half underwent revision surgery.Reference Kraai, Brown, Neeff and Fisher11 Siau et al. reported that 30 per cent of patients who were eligible for a BAHA rejected BAHA implantation because of cosmetic concerns, including the size of the abutment and subsequent hair loss.Reference Siau, Dhillon, Siau and Green12
The percutaneous complications of BAHA can be avoided with an active middle-ear implant and transcutaneous bone conduction implant. A middle-ear implant consists of a floating mass transducer, which is connected mostly to the stapes, and infrequently to the incus, round window or oval window, to stimulate the cochlea directly.Reference Célérier, Thierry, Coudert, Blanchard, Loundon and Garabédian13 The advantages of a middle-ear implant over a transcutaneous bone conduction implant include stimulation solely of one cochlea and greater power in the higher frequencies.Reference Riss, Arnoldner, Baumgartner, Blineder, Flak and Bachner14 However, middle-ear implant surgery involves manipulation of the ossicles, with possible risks of surgical trauma and permanent sensorineural hearing loss.Reference Yu, Wong, Tsang and Tong8 Other potential complications include post-operative implant displacement due to scar tissue development and taste disturbance as a result of chorda tympani nerve damage.Reference Yu, Wong, Tsang and Tong8 Transcutaneous bone conduction implant surgery is easier than middle-ear implant implantation.Reference Riss, Arnoldner, Baumgartner, Blineder, Flak and Bachner14 Furthermore, the Bonebridge transcutaneous bone conduction implant is magnetic resonance imaging (MRI) compatible up to 1.5 T, whereas the middle-ear implant is not MRI compatible.Reference Riss, Arnoldner, Baumgartner, Blineder, Flak and Bachner14
The Bonebridge (Med-El, Innsbruck, Austria) was launched onto the European Union market in September 2012 and was subsequently approved by the Communauté Européenne for implantation in children aged five years and above.Reference Sprinzl and Wolf-Magele15 In Malaysia, the first Bonebridge implantation was performed in 2012. The Bonebridge is an active transcutaneous bone conduction implant system that transmits sound waves through cranial bone directly to the inner ear.Reference Tang, Devesahayam and Narayanan16 It consists of an external part (audio processor) and internal implanted parts (bone conduction implant). The audio processor contains a microphone and a digital signal processor, powered by a standard hearing aid battery. The internal part consists of a demodulator that processes the signal, a receiver coil and an active electromagnetic bone conduction floating mass transducer that transforms the electrical signal into mechanical vibrations.
Bonebridge implantation is indicated in adults and children aged five years and above with conductive or mixed hearing loss, who can still benefit from sound amplification. The pure tone average bone conduction threshold (measured at 0.5, 1, 2, 3 and 4 kHz) should be 45 dB HL or less. Bonebridge implantation is also indicated in those with single-sided sensorineural deafness. The pure tone average air conduction threshold in the contralateral ear (measured at 0.5, 1, 2, 3 and 4 kHz) should be 20 dB HL or less.Reference Sprinzl and Wolf-Magele15
The absence of retrocochlear or central auditory disorders, and presence of suitable anatomy for bone conduction implant placement, must be confirmed via computed tomography (CT) prior to transcutaneous bone conduction implant surgery.
Bonebridge implantation has proved increasingly popular. Only a few studies have investigated this transcutaneous bone conduction implant in children. This study aimed to investigate the surgical and audiological outcomes of Bonebridge transcutaneous bone conduction implantation among children with congenital aural atresia.
Materials and methods
Study design
This study was conducted in a tertiary referral centre from January 2013 to December 2016, using a prospective, intra-subject repeated measures design in which each subject was his or her own control.
Patients
Six patients aged 11–18 years were enrolled into this study within the study period. Patient demographics and medical parameters are shown in Table 1.
Table 1. Demographic data and medical parameters

Pt no. = patient number; PTA4 = mean audiometric pure tone thresholds for frequencies 0.5, 1, 2 and 4 kHz; BC = bone conduction; AC = air conduction; HL = sound-field hearing level; F = female; L = left; CHL = conductive hearing loss; M = male; R = right
The patients were selected according to the following criteria: children aged 5–18 years; presence of congenital canal atresia; fulfilled criteria for transcutaneous bone conduction implant surgery, as described above (bone conduction threshold below 45 dB HL at frequencies between 0.5 kHz and 4 kHz); and benefit from a bone conduction hearing aid trial.
Surgical technique
The surgical technique has been extensively described elsewhere.Reference Tang, Devesahayam and Narayanan16 The transcutaneous bone conduction implant surgery was carried out under general anaesthesia. A pre-operative CT scan was performed to analyse the thickness and consistency of the temporal bone, sigmoid sinus and dura, so as to determine the optimum location for the bone conduction floating mass transducer and screws. The bone conduction floating mass transducer is normally placed at a sinodural angle, which has the least interference with the sigmoid sinus and dura. In cases of an under-pneumatised mastoid or prior mastoidectomy, the bone conduction floating mass transducer can be placed in the retrosigmoid region or above the temporal line, in view of limited space at the sinodural angle.
Device fitting
The first fitting of the audio processor was initiated as soon as the wound had completely healed, at about three to five weeks after implantation. The audio processor was programmed with Connexx 6.5 fitting software (Siemens Hearing Instruments, Piscataway, New Jersey, USA) in conjunction with Symfit 6.1 software (Med-El), using a programming cable connected to a Hi-Pro Box hearing aid programmer (GN Otometrics, Taastrup, Denmark). The target gain was evaluated using the bone conduction thresholds of the implanted ear.
Data collection and statistics
Patients were monitored for any surgery-related complications for up to six months post-implantation.
Patients were tested pre-operatively (unaided) and at six months post-operatively (aided). Audiometric pure tone thresholds for air conduction (through headphones) and bone conduction (through a bone conduction vibrator) were evaluated at 0.25–8 kHz. Sound-field tests were conducted through a loudspeaker placed 1 metre in front of the patient at 0.25–8 kHz, with the contralateral ear covered with earmuffs.
The data were analysed with SPSS® statistics software version 22. Paired sample t-tests were utilised to evaluate pre-operative and six-month post-operative differences in terms of mean air and bone audiometric thresholds and mean sound-field thresholds.
Patients’ satisfaction was evaluated six months post-operatively with the Hearing Device Satisfaction Scale. The answers were transformed into percentage scores, which ranged from 0 per cent (not satisfied) to 100 per cent (very satisfied).
Results
Six children (four males and two females) with conductive hearing loss due to canal atresia, aged 11–18 years, were included in the study. Four of the patents (66.7 per cent) had bilateral canal atresia.
The bone conduction floating mass transducer was placed at the sinodural angle in five cases (86.7 per cent) and at the retrosigmoid region in one case (13.3 per cent).
No major complications were reported. One patient (13.3 per cent) had mild infection at the surgical site; this was treated with local and oral antibiotics, and the patient recovered within one week.
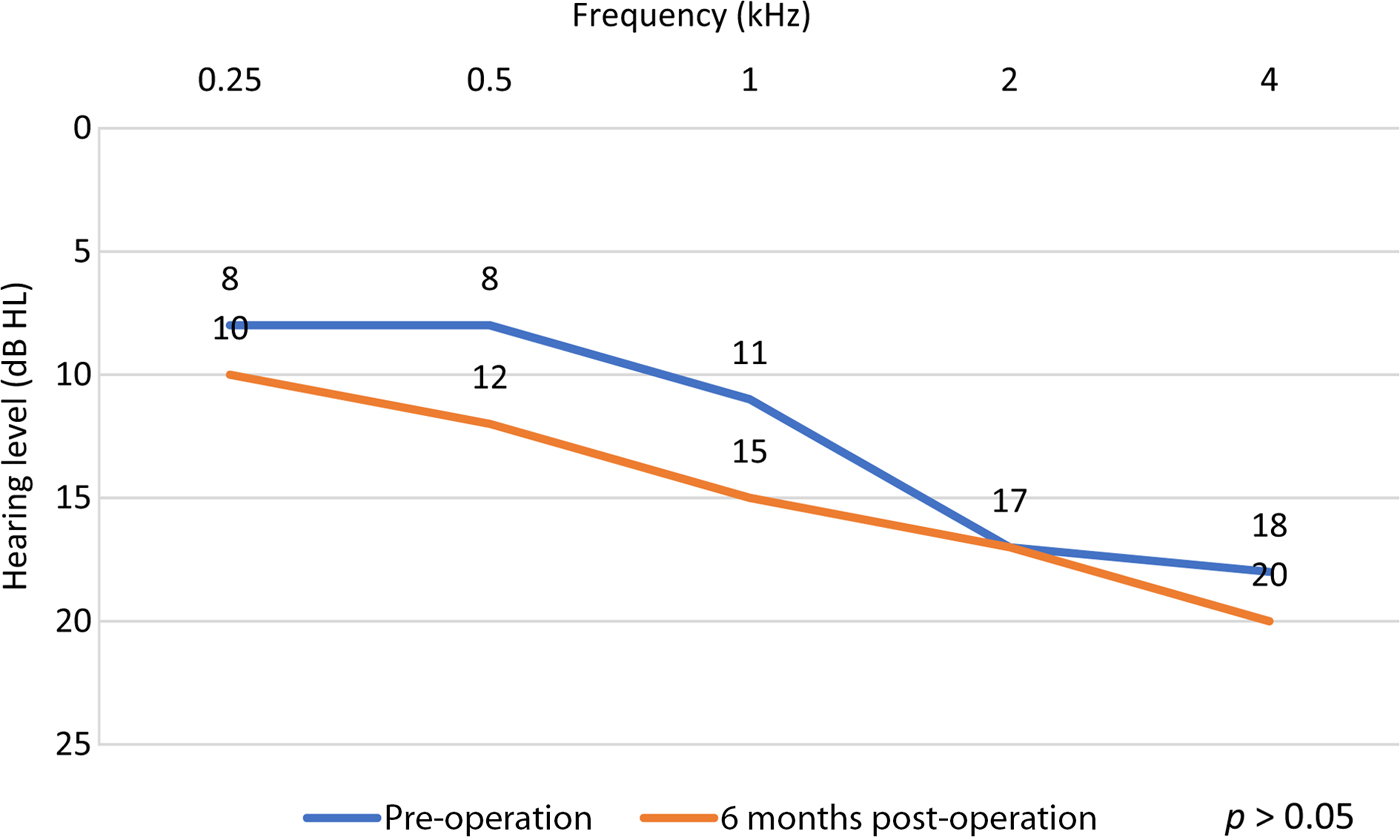
Sound-field testing showed significant changes at six months post-operatively (compared to pre-operatively; p < 0.05) for 0.5–4 kHz, with a functional gain ranging from 31 dB to 61 dB, and a mean hearing threshold of 46.3 dB (Figures 1 and 2). Mean audiometric thresholds for bone conduction (Figure 3) and air conduction (Figure 4) showed no significant changes at six months post-operatively (compared to pre-operatively; p > 0.05) for 0.5–4 kHz (Figures 3 and 4).

Fig. 1. Hearing level (audiometric pure tone thresholds for frequencies 0.5, 1, 2 and 4 kHz (PTA4)) pre-operatively (air conduction (AC) and bone conduction (BC)) and post-operatively (sound-field hearing level (HL)) for the implanted ear in the six patients.

Fig. 2. Mean sound-field thresholds for the implanted ear, pre-operation (unaided) and six months post-operation (aided).

Fig. 3. Mean bone conduction thresholds for the implanted ear, pre-operation (unaided) and six months post-operation (aided).

Fig. 4. Mean air conduction thresholds for the implanted ear, pre-operation (unaided) and six months post-operation (aided).
Patient device satisfaction ranged from 91 per cent to 98 per cent (Figure 5).

Fig. 5. Hearing Device Satisfaction Scale (HDSS) scores for the six patients (0 per cent = not satisfied; 100 per cent = very satisfied).
Discussion
Transcutaneous bone conduction implant surgery is indicated in children with conductive or mixed hearing loss due to pinna abnormalities and canal atresia, either bilateral or unilateral. Compared to a percutaneous bone conduction implant, a transcutaneous bone conduction implant leaves the skin intact and does not require long-term skin care. Children are able to participate in activities such as swimming without the risk of skin infection at the implant site. Furthermore, the audio processor can be easily worn and handled by children, reducing the parental burden. The audio processor has a streamlined design and can be hidden under the hair, with no cosmetic concerns. Moreover, children with a transcutaneous bone conduction implant can keep up to date with the latest technology as the audio processor is replaceable.
Safe surgery
Our study showed that Bonebridge transcutaneous bone conduction implantation has an acceptable level of safety in terms of the surgical techniques and complications, with little risk of major intra-operative or post-operative complications. In a systematic review by Sprinzl and Wolf-Magele, which included 12 studies with a total of 117 patients, no major complications were reported.Reference Sprinzl and Wolf-Magele15 The rate of minor adverse events after transcutaneous bone conduction implant surgery was 5.12 per cent and the rate of revision surgery was 0.85 per cent.Reference Sprinzl and Wolf-Magele15
Another systematic review, by Zernotti and Sarasty, also found no reports of severe complications in transcutaneous bone conduction implant cases, and most of the complications reported could be prevented with refined technique and good pre-operative planning.Reference Zernotti and Sarasty17 The flap necrosis or infection risk was similar to that in other implantation surgery (e.g. cochlear implant surgery), and could be minimised by performing the double flap with good vascularisation and minimal incisions.Reference Zernotti and Sarasty17 Risk of injury to the meninges or sigmoid sinus was generally avoidable with meticulous surgery.Reference Zernotti and Sarasty17
Lassaletta et al. investigated post-operative pain in patients who underwent transcutaneous bone conduction implant surgery; they reported that implantation did not cause any significant post-operative pain, irrespective of sinus or dura compression.Reference Lassaletta, Calvino, Zernotti and Gavilán18
Audiological outcome
This study showed promising functional gain: the mean aided sound-field thresholds improved by more than 30 dB for 0.5–4 kHz, which is comparable with other studies. A systematic review of 7 studies with 58 subjects reported a functional gain ranging from 24 dB to 37 dB.Reference Sprinzl and Wolf-Magele15 Another systematic review of 5 studies with 20 patients reported a functional gain of 24 dB to 43 dB.Reference Zernotti and Sarasty17 Baumgartner et al. reported a significant improvement in aided thresholds post-operatively, with improvement in speech perception, as measured by word recognition scores and speech reception thresholds for 50 per cent word intelligibility in sentences, of approximately 67.6 per cent and 27.5 per cent respectively.Reference Baumgartner, Hamzavi, Böheim, Wolf-Magele, Schlögel and Riechelmann10 Rahne et al. stated that transcutaneous bone conduction implant surgery also resulted in a significant improvement in speech recognition in noisy environments and sound localisation.Reference Rahne, Seiwerth, Götze, Heider, Radetzki and Herzog19
Hearing preservation
Our study showed that mean unaided air conduction and bone conduction thresholds pre-operatively and six months post-operatively differed by less than 5 dB for 0.5–4 kHz, which was within the test–retest variability range.Reference Stuart, Stenstrom, Tompkins and Vandenhoff20 These non-significant changes post-implantation confirm that patients’ residual unaided hearing was not damaged by the treatment. The aforementioned study by Baumgartner et al., which investigated the short-term safety of transcutaneous bone conduction implantation in children, also reported that patients’ residual unaided hearing did not deteriorate with the treatment.Reference Baumgartner, Hamzavi, Böheim, Wolf-Magele, Schlögel and Riechelmann10
Patient satisfaction
All six patients in our study were very satisfied with the implant. The Hearing Device Satisfaction Scale scores ranged from 91 per cent to 98 per cent, with a mean score of 95.5 per cent. In interviews, patients revealed that they were satisfied with the aided hearing threshold improvements and considered the cosmetic appearance of the audio processor acceptable. The study by Baumgartner et al, which comprised 12 children, reported a mean Hearing Device Satisfaction Scale score of 88 per cent.Reference Baumgartner, Hamzavi, Böheim, Wolf-Magele, Schlögel and Riechelmann10
• The Bonebridge transcutaneous bone conduction implant provides an alternative audiological rehabilitative option for children with conductive hearing loss due to congenital aural atresia
• This transcutaneous bone conduction implant surgery is safe and effective
• Proper pre-operative planning and good techniques are crucial for successful surgical and audiological outcomes
Conclusion
In conclusion, the Bonebridge transcutaneous bone conduction implant is safe and effective in children with conductive hearing loss due to congenital aural atresia. Proper pre-operative planning and good techniques ensure a safe procedure without major complications and a significant audiological benefit post-operatively.
Competing interests
None declared