Introduction
After sinus surgery, disease sometimes persists despite appropriate medical therapy and post-operative debridement, until the underlying bone is removed. Additional studies of this bone process could significantly improve our knowledge of the pathogenesis of chronic sinusitis. If the underlying bone does indeed play an active role in the disease process of chronic sinusitis, this may in part explain the recalcitrance of late-stage disease.Reference Perloff, Gannon, Bolger, Montone, Orlandi and Kennedy1, Reference Tos and Mogensen2 Obliteration of the haversian system and inflammation of the bone would presumably make antibiotic penetration difficult. These processes would also explain the clinical observation that removal of the underlying bone tends to result in disease resolution, whereas mucosal removal alone results in disease persistence.Reference Khalid, Hunt, Perloff and Kennedy3–Reference Lund6
The aims of this study were to explore the pathogenic role of bone in sinus surgery, and to determine whether mucosal disease after sinus surgery may be induced by underlying disease within the bone.
Material and methods
Animals
Twenty-five white, female New Zealand rabbits were divided into five groups of five animals each.
The animals were anaesthetised with an intramuscular injection of ketamine hydrochloride (70 mg/kg). The animals breathed spontaneously throughout the surgical procedure.
All procedures carried out on the animals were conducted in compliance with national and local regulations and institutional guidelines for the humane use of animals.
Surgery
In group one, wide surgical removal of the right maxillary sinus mucosa and creation of a nasoantral window were performed. In group two, only nasoantral window creation was performed. In group three, the mucosa of the anterior maxillary sinus was removed. In group four, a selective strip of sinus mucosa around the ostium was removed. Group five was used as a control group; the maxillary sinus was opened without further surgical manipulation. Figure 1 illustrates the various surgical procedures performed.

Fig. 1 Schematic drawing of the right maxillary sinus cavity, seen from the medial aspect, for treatments undergone by groups one (a), two (b), three (c), four (d) and five (e). The hatched area indicates the region of sinus mucosa removed. A = anterior; P = posterior; OST = ostium (indicated by arrow); EM = extensive mucosal removed; VNA = nasoantral window; RTL = strp of mucosa removed
In all animals, the areas over the bridge of the nose and maxillary sinuses were shaved and a midline incision made through the skin and periosteum. The periosteum was lifted over the right maxillary sinus, and a 5 × 5 mm window was opened on the superior-anterior wall using a small hammer and drill.
Animal analysis
The animals were observed for three months post-procedure. Every two days, the animals were examined for nasal symptoms. The rabbits were not given prophylactic antibiotics.
After three months, the animals were sedated with an intramuscular injection of ketamine hydrochloride (100 mg/kg) and tissue fixation was performed by intra-arterial perfusion. The animals were then painlessly sacrificed. In all animals, specimens of the mucosa were removed from the same sites around the ostium. Fixation was done by perfusion with 2.5 per cent phosphate-buffered glutaraldehyde, followed by processing for scanning (Hitachi S-2300 scanning electron microscope, Tokyo, Japan).
In addition, at three months, the left maxillary sinus was opened to assess signs of infection, such as pus or hyperaemia of the maxillary sinus mucosa. The mucosa of these contralateral sinuses was also studied using electron microscopy. Bacteriological samples of the right maxillary sinus cavities were taken, using transport swabs EUROTUBO (I.A.S.A., Barcelona, Spain) with AMIES transport medium, and cultured using blood agar, McConkey agar and haematin agar.
Results
During the three months post-procedure, the animals were in good health, and none exhibited nasal symptoms such as rhinorrhoea, sneezing or eyelid tumefaction. After three months, every animal in groups one and three was found to have purulent secretions in the right maxillary sinus.
At the beginning of the study, every animal was found to have Pasteurella multocida in the right maxillary sinus. After three months, P multocida was again found in all maxillary sinus in every animal; however, flavobacterium and bordetella species were also found in two animals of group one, and moraxella species in one animal of group three.
After three months, the following macroscopic findings were noted. In those groups in which the mucosa had been widely removed, the maxillary sinuses demonstrated retraction of the medial wall of the right sinus (Figure 2). In group two, the nasoantral window was open (Figure 3). However, in group one (wide removal of mucosa plus nasoantral window), all the windows were closed. In group two (nasoantral window only), the windows were open and no inflammatory signs were observed in the mucosal and bone structures in the vicinity of the windows.

Fig. 2 Retraction of bone wall of the maxillary sinus, in group one animal.

Fig. 3 Open nasoantral windows in the right maxillary sinus, in group two animal.
After three months, the following electron microscopic findings were noted. In groups one and three, the underlying bone of the entire maxillary sinus was disturbed, showing inflammation, fibrosis and osteoclastic resorption (Figure 4). In addition, increased of osteoclast within the haversian system (Figure 5) and increased vascularity were observed. In the groups without radical removal (two and four), the mucosa and bone of the right sinus were similar to those in the control group, with no signs of infection (Figure 6). In groups two, four and five, the mucosal cilia were normal and no immature bone was observed. Also in these groups, no increase in osteoblast or osteoclast numbers was observed, and no haversian system alterations were found (Figure 7).

Fig. 4 Scanning electron micrograph showing underlying bone alteration in group one animal (×2300 Å).

Fig. 5 Scanning electron micrograph showing osteoclastic resorption in group one animal (×6000 Å).
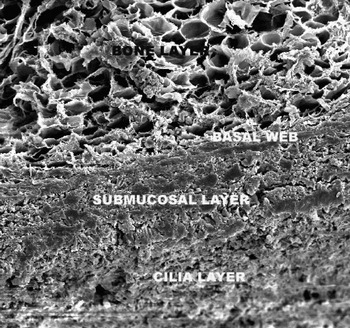
Fig. 6 Scanning electron micrograph showing normal underlying bone in group four animal (×2600 Å).

Fig. 7 Scanning electron micrograph showing normal haversian system in group five animal (×6200 Å).
Discussion
The rabbit maxillary sinus is considered to be a good experimental model for regeneration following sinus surgery.Reference Melgarejo-Moreno, Ribera-Cortada and Hellin-Meseguer7–Reference Yang, Liu, Wang, Zhang and Tao11 After surgical removal, epithelial regeneration is considered to be complete by approximately one month post-operatively. Following selective removal, re-epithelialisation with ciliated epithelium has been observed at 10 and 14 days post-operatively.Reference Gilbert, Kirker, Gray, Ward, Szakacs and Prestwich12 In the lamina propia, fibrosis as well as a rich microcirculation and local angiogenesis has been observed during the first day after surgery of the maxillary sinus in rabbits.Reference Forsgren, Otori, Stierna and Kumlien13 Reinnervation of the mucosa has been observed between four and eight weeks after experimental surgery in maxillary sinus.Reference Forsgren, Jung, Stierna and Rivero14
There is ample literature on tissue morphology following radical maxillary sinus surgery. Some of these authors have noted that bone may play a significant role in experimentally induced sinusitis. Following radical maxillary sinus surgery, alterations in bone architecture include coordinated osteoclasis and appositional bone formation adjacent to the infected sinuses, followed by intramembranous bone remodelling.Reference Maccabee, Trune and Hwang15, Reference Forsgren, Stierna, Kumlien and Carlsöö16 If bone does play an active part in the disease process of chronic sinusitis, this may in part explain the recalcitrance of late-stage disease. It would also explain the clinical observation that removal of the underlying bone tends to result in disease resolution, whereas mucosal removal alone results in disease persistence. Obliteration of the haversian system and inflammation of the bone would presumably make antibiotic penetration difficult.Reference Perloff, Gannon, Bolger, Montone, Orlandi and Kennedy1
Clinically, within the spectrum of acute to chronic sinusitis, nasal endoscopy and computed tomography studies have shown that bone undergoes resorption followed by subsequent hyperostosis.Reference Kennedy, Senior, Gannon, Montone, Hwang and Lanza17, Reference Forsgren, Westrin, Fukami and Stierna18 Using histomorphometry and tetracycline labelling techniques, ethmoid bone affected by chronic sinusitis has been shown to undergo rapid remodelling that is histologically identical to that seen in osteomyelitis.Reference Hwang, Montone, Gannon, Senior, Lanza and Kennedy19
It is well known that infection may spread through bony structures, as may be shown in daily practice facing, e.g. sinugen orbital cellulitis frontal osteomyelitis. The present experimental study in rabbits adds further evidence to the hypothesis that chronic infection after sinus surgery can spread through bony structures. If the potential for inflammation to spread through the bone is also present in patients, it may well explain some of the clinical findings seen in chronic sinusitis. Clearly, bony ethmoid septa undergo demineralisation early in sinusitis, followed by significant, irregular bony thickening later in the disease process. In addition, inflammation may spread across an inter-sinus septum from one frontal sinus or sphenoid sinus to the other.Reference Perloff, Gannon, Bolger, Montone, Orlandi and Kennedy1, Reference Khalid, Hunt, Perloff and Kennedy3 A variety of other clinical phenomena could also potentially be explained by bone involvement. These include the recalcitrance of severe disease, the tendency for disease to persist in localised areas until the underlying bone is removed, and the potential for ethmoid disease to spread medially to the middle turbinate, thus involving both the rima olfactoria and the adjacent nasal septum.Reference Kern20
However, it is possible that entering the maxillary sinus via an external approach (e.g. during dental procedures or trauma) may in itself introduce organisms, leading to secondary infection. A previous study demonstrated the absence of infection after three months in rabbits in which the maxillary sinus had been exposed, using the same approach as in the present study, but no procedure had been performed on the maxillary sinus mucosa.Reference Melgarejo-Moreno, Ribera-Cortada and Sarroca-Capell21
• This study aimed to explore the pathogenic role of bone in sinus surgery, and to determine whether mucosal disease after sinus surgery may be induced by underlying disease within the bone
• The rabbit was used as an animal model for sinusitis
• After extensive sinus mucosa removal was performed, changes occurred in the bony structures of the maxillary sinus
• Changes in the underlying bone may contribute to the development of chronic sinusitis
However, significant care must be taken in extrapolating changes seen in this animal model to disease in patients. The rabbit is not an ideal model for the study of rhinosinusitis. Rabbits are likely to be different to humans with regards to bone tissue reactions. The rabbit was chosen for this study because it has been the most commonly studied animal model for sinusitis. The absence of sinus infection in groups two, four and five is surprising, since in rabbits a certain incidence of spontaneous sinusitis would be expected. It is possible that some infections occurred during the time of observation but resolved spontaneously and were not present at re-examination. Pasturella multocida, in particular, has been reported as a major airway pathogen, carried by more than 90 per cent of one US rabbit population examined. In the majority of cases, the infection is subclinical, but when there is interference with the sinus mucociliary transport (for example, following radical surgery), the infection can progress.Reference Perko and Karin22, Reference Friedman and Tourini23
Conclusions
Despite its limitations, the New Zealand white rabbit was found to be a technically viable model for studying osseus changes following sinus surgery. Following extensive sinus mucosa removal, changes can occur in the bony structures of the maxillary sinus. These mucosal and bone changes may develop into chronic sinusitis. It also appears that inflammation can spread through the widened haversian canal system within the bone. However, further studies are necessary.
Acknowledgements
We thank Miss Ashlei Darr, medical student of the Ear & Eye Infirmary, New York, USA, for her assistance with use of the English language.









