Introduction
Chronic rhinosinusitis is a common nasal disorder in children. A recent epidemiology study in China showed that the prevalence of chronic rhinosinusitis is 6.37 per cent in children aged up to 14 years.Reference Shi, Fu, Zhang, Cheng, Wang and Zhu 1 The presentation of chronic rhinosinusitis is different in children compared to adults; mucosal hyperplasia and polyps are uncommon in children with chronic rhinosinusitis. Infection is also relatively easier to manage in children than in adults.Reference Shi, Fu, Zhang, Cheng, Wang and Zhu 1 , Reference Fokkens, Lund, Mullol, Bachert, Alobid and Baroody 2 Despite this, however, the comparatively underdeveloped immune system in children means that they are more susceptible to chronic rhinosinusitis recurrence. It is this recurring nature of chronic rhinosinusitis that makes its management and treatment challenging in children.Reference Fokkens, Lund, Mullol, Bachert, Alobid and Baroody 2 – Reference Albegger 4
Broncho-Vaxom®, a lysate of 21 strains of 8 bacteria (Staphylococcus aureus, Haemophilus influenzae, Streptococcus pyogenes, Moraxella catarrhalis, Klebsiella pneumoniae, Klebsiella ozaenae, Streptococcus viridans and Diplococcus pneumoniae), has been shown to boost immunological response.Reference De Benedetto and Sevieri 5 Studies have also shown that bacterial lysate is efficacious in preventing and treating recurrent respiratory tract infections in children and adults.Reference Ahrens 6 , Reference Carmona-Ramirez, Alvarez-Gomez and Berber 7 However, its efficacy, especially in the long-term, in the prevention of chronic rhinosinusitis recurrence in children requires further investigation.Reference Heintz, Schlenter, Kirsten and Nelson 8 , Reference Zagar and Löfler-Badzek 9 In this study, the bacterial lysate was administered during the remission period in children with chronic rhinosinusitis to assess its efficacy in the prevention of chronic rhinosinusitis recurrence, and to determine how that affected antibiotic use over a period of one year.
Materials and methods
All procedures contributing to this work complied with the ethical standards of the relevant national and institutional guidelines on human experimentation (register number: ChiCTR-OPN-15006592) and with the Helsinki Declaration of 1975, as revised in 2008.
Patients
Children aged 4–12 years were recruited from April 2013 to October 2013. The children presented mainly with nasal obstruction, purulent nasal discharge and/or cough. Diagnosis was based on the European Position Paper on Rhinosinusitis and Nasal Polyps (‘EPOS’) 2012.Reference Fokkens, Lund, Mullol, Bachert, Alobid and Baroody 2 Inclusion criteria included a chronic rhinosinusitis history of at least three months and the presence of purulent secretion in the middle nasal meatus as confirmed by nasal endoscopy.
In order to reduce bias in the results, nasal endoscopy was conducted to exclude patients with breathing difficulties due to adenoid hyperplasia. Allergen skin prick tests were also performed to exclude patients with allergies (tested allergens included dust mites, mould combinations, cat fur, dog fur, cockroaches, spring pollen combinations, mugwort, ragweed and others). Additionally, nasal secretion smears were carried out to exclude patients with eosinophil-dominated inflammation (eosinophils account for less than 10 per cent of the white cells in secretions).
Patient history and details were provided by the patient's carer, and included: gender, age, disease course, overall visual analogue scale (VAS) score for rhinosinusitis in the previous month (0–10, with 0 indicating that the symptoms did not disturb daily life at all, and 10 indicating that the symptoms disturbed daily life most seriously), nasal obstruction and discharge scores (0 = asymptomatic, 1 = mild, 2 = moderate and 3 = severe), average number of days with rhinosinusitis attacks in the previous month, and number of acute attacks of rhinosinusitis in the previous year.
Treatments
At study entry, patients were treated for two to six weeks with: oral antibiotics (amoxicillin and clavulanate potassium or clarithromycin), decongestant intranasal sprays, intranasal steroid sprays, saline intranasal spray, mucolytic agents and other medications at the physicians’ discretion. The treatment was continued until the total score of nasal obstruction and nasal discharge was ≤1 for at least one week, and the nasal meatus and nasal cavity were both clear, as assessed by nasal endoscopy (defined as the remission period).
At the start of the remission period, patients were randomised into two groups: a prevention group, in which 3.5 mg/d bacterial lysate was given over 10 days per month for 3 months, together with intermittent spraying of intranasal saline; and a control group, which received only intermittent spraying of intranasal saline. Intranasal steroid and other preventative medications were not given during the remission period.
One research nurse conducted the follow up via a social media platform called WeChat (similar to Facebook) to minimise dropout rates. Patients were strongly encouraged to contact the research nurse first if they experienced any nasal discomfort. The follow-up period was one year; telephone interviews were conducted at three months and one year (Figure 1). Acute occurrences of nasal problems during the course of follow up were managed and treated according to the guidelines.Reference Fokkens, Lund, Mullol, Bachert, Alobid and Baroody 2

Fig. 1 Follow-up schedule of children with chronic rhinosinusitis.
Outcomes
Outcomes included: completion of three-month bacterial lysate treatment (prevention group only), overall VAS score for rhinosinusitis in the previous month, nasal obstruction and discharge scores, average number of days with rhinosinusitis attacks in the previous month, number of days with antibiotic use in the previous month, number of acute rhinosinusitis attacks in the previous year, and subjective assessment of immune system improvement over one year (worsening, no change, a little improvement, intermediate improvement or marked improvement).
Statistics
All statistical analyses were carried out using SPSS version 19.0 software (SPSS, Chicago, Illinois, USA). Data pertaining to age, disease course, number of days with rhinitis attacks, number of days with antibiotic use, number of acute rhinitis attacks, proportion of neutrophils in nasal secretion, nasal symptoms and related symptoms in each group were expressed as means ± standard deviations. The Wilcoxon signed rank test was used to detect whether data were normally distributed. The two-sample equal variance t-test was used for intergroup comparison. Differences were considered statistically significant when the p value was less than 0.05.
Results
Patient data
A total of 96 patients were recruited, with 48 in each group. In the prevention group, one patient complained of gastric discomfort and another complained of skin rash after using the bacterial lysate; these patients did not complete their course of medication. All other patients successfully completed the three-month prophylactic immunomodulation and follow up. In the control group, all patients completed follow up except one who could not be contacted because of change of residence and contact telephone number (Table I).
Table I Baseline characteristics of patients in prevention and control groups

*Defined as the number of days with rhinosinusitis symptoms within the month preceding the hospital visit for treatment. †Defined as the number of acute attacks of rhinosinusitis within the year prior to the hospital visit for treatment. SD = standard deviation; VAS = visual analogue scale
Bacterial lysate effects
Following use of the bacterial lysate for three months, the nasal obstruction score significantly decreased in the prevention group versus the control group (p = 0.03). At one year, the VAS score (p = 0.023), the nasal obstruction score (p = 0.04) and the nasal discharge score (p = 0.04) were all significantly lower in the prevention group than in the control group (Figure 2).

Fig. 2 Effect of bacteria lysate on (a) overall visual analogue scale (VAS) scores, (b) nasal obstruction scores and (c) nasal discharge scores, at each assessment period. *p < 0.05.
After use of the bacterial lysate for three months and at one year, the number of days with rhinitis attacks per month (p = 0.038 at three months, p = 0.022 at one year) and the number of days with use of antibiotics per month (p < 0.01 at three months and one year) were both significantly lower in the prevention group compared to the control group. The number of acute nasal syndrome attacks over one year was also significantly lower in the prevention group versus the control group (p < 0.01) (Figure 3).

Fig. 3 Effects of bacterial lysate on (a) rhinosinusitis attacks, (b) use of antibiotics and (c) acute nasal syndrome attacks. No. = number; *p < 0.05; **p < 0.01.
After use of the bacterial lysate for 3 months and at 1 year of follow up in the prevention group, only 4 patients (8.7 per cent) reported that their immunity had not changed, and 42 (91.3 per cent) reported improvement in immunity to different degrees (a little to markedly improved). In the control group, 26 patients (55.3 per cent) reported that their immunity had not changed or had worsened, and 21 (44.7 per cent) reported improvement in immunity (Figure 4).
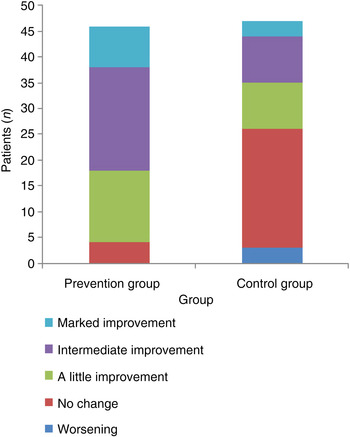
Fig. 4 Subjective immunity improvement over one year of observation following use of the bacterial lysate.
Discussion
The incidence of chronic rhinosinusitis is relatively high in children because of the relative underdevelopment of their upper respiratory tract; this also explains the frequent recurrence.Reference Shi, Fu, Zhang, Cheng, Wang and Zhu 1 , Reference Fokkens, Lund, Mullol, Bachert, Alobid and Baroody 2 The disease, although not life-threatening, can compromise quality of sleep and daily life. If the symptoms are not adequately controlled, the condition may cause otitis media, tonsillitis and lower respiratory tract diseases (e.g. bronchitis).Reference Albegger 4
The bacterial lysate consists of antigens obtained after the lysis of 21 strains of 8 bacteria (S aureus, H influenzae, S pyogenes, M catarrhalis, K pneumoniae, K ozaenae, S viridans and D pneumoniae). It essentially includes all common bacteria for sinusitis, and can activate inherent immunity (e.g. activating macrophages, natural killer cells, dendritic cells) and enhance adaptive immunity (e.g. triggering specific T cell immunity and activating specific B cells to produce immunoglobulin A and immunoglobulin G). Clinical studies have demonstrated that it can effectively prevent recurrent respiratory tract infection in children and adultsReference Ahrens 6 , Reference Carmona-Ramirez, Alvarez-Gomez and Berber 7 and reduce attacks of bronchitis.Reference Cvoriscec, Ustar, Pardon, Palecek, Stipic-Markovic and Zimic 10 – Reference Pan, Jiang, Guo, Tian and Liu 12
In a placebo-controlled, double-blind study, Heintz et al. demonstrated the efficacy of Broncho-Vaxom in treating and preventing chronic rhinosinusitis in adults.Reference Heintz, Schlenter, Kirsten and Nelson 8 Nasal symptom scores were significantly decreased following the use of Broncho-Vaxom versus a placebo in the first month. Cough was also statistically significantly reduced with Broncho-Vaxom than with the placebo following use for a period of 10 days per month for 3 months.Reference Heintz, Schlenter, Kirsten and Nelson 8
Zagar et al. investigated the efficacy of Broncho-Vaxom in treating and preventing rhinosinusitis in children aged 4–12 years.Reference Zagar and Löfler-Badzek 9 The bacterial lysate was used in the acute phase, and was administered over 10 days per month for 6 months. Symptoms that included nasal obstruction, nasal discharge and cough were significantly reduced with Broncho-Vaxom than with the placebo. The clinical response correlated positively with a significantly higher serum immunoglobulin A level in the treatment group than in the placebo group.Reference Zagar and Löfler-Badzek 9
-
• This study assessed the efficacy of bacterial lysate on the prevention of chronic rhinosinusitis recurrence in children
-
• Three-months’ bacterial lysate use significantly decreased nasal symptoms scores, rhinitis attacks and antibiotic use
-
• Visual analogue scale scores, nasal symptoms scores and acute nasal syndrome attacks were decreased at one year follow up
-
• Bacterial lysate used in the rhinosinusitis remission period in children provided long-term prophylaxis
The current study aimed to assess the efficacy of the bacterial lysate in long-term prevention, not in treatment. Therefore, with respect to the overall therapeutic regimen, standard drugs for treating sinusitis were first administered to reduce local inflammation to the lowest level. Once the disease had entered the remission period, immunomodulation was applied, and changes in occurrence and intensity of nasal symptoms were assessed.
Patients with adenoid hyperplasia and hypersensitivity factors were excluded. It is inevitable that these conditions occur concomitantly with chronic rhinosinusitis in some patients.Reference Fokkens, Lund, Mullol, Bachert, Alobid and Baroody 2 , Reference Sedaghat, Phipatanakul and Cunningham 13 Nasal obstruction during sleep associated with adenoid hyperplasia cannot be expected to improve with treatment, nor would nasal symptoms associated with allergic rhinitis. In these situations, parents or carers may consider the treatment with Broncho-Vaxom to be ineffective. In a global assessment, these factors may lead to incorrect assessment and affect evaluation of the effects. Therefore, patients with these conditions were excluded.
Following use of the bacterial lysate for three months, the number of days with rhinitis attacks (p = 0.038) and the number of days with use of antibiotics (p < 0.01) both significantly decreased in the prevention group versus the control group, and the nasal obstruction symptom score was significantly improved (p = 0.03). After withdrawal of the bacterial lysate for nine months, improvements in the number of days with rhinitis attacks (p = 0.022), use of antibiotics (p < 0.01) and nasal symptoms persisted in the prevention group versus the control group. The number of acute rhinosinusitis attacks over one year, after use of the bacterial lysate, significantly decreased in the prevention group versus the control group (p < 0.01). These study results correspond to the findings of Zagar et al., although the observation period in this study was longer, and thus demonstrate the persistent prophylactic efficacy of the bacterial lysate.
Conclusion
The bacterial lysate used in the remission period of chronic rhinosinusitis in children was shown to provide long-term prophylactic efficacy. Bacterial lysate can effectively reduce the frequency of rhinosinusitis attacks and ameliorate symptoms.
Acknowledgements
The authors would like to thank Xu Yunjiao and Jiang Jing for conducting the telephone interviews. This work was supported by the State Natural Sciences Fund of China (JC grant number 81570898) and the 12th 5-year science and technology support programme (WK grant number 2014BAI07B04).







