Introduction
Autologous fat injections in the vocal folds were first reported in 1991 by Mikaelian et al.Reference Mikaelian, Lowry and Sataloff 1 Although this treatment has become common because of the absence of any reaction to foreign bodies, the issue of weakened effects due to injected fat being gradually absorbed is a problem.Reference McCulloch, Andrews, Hoffman, Graham, Karnell and Minnick 2 Basic fibroblast growth factor and hepatocyte growth factor injections have been used in animal experiments to control the absorption of autologous fat injections; however, as the effects have not been proven in humans, this technique has not been clinically adapted.Reference Tamura, Fukuda and Tabata 3 , Reference Umeno, Chitose, Murofushi, Kosai, Sato and Kawahara 4
Somatic stem cells are autologous stem cells that are multipotent and have replication competence, with few rejections and few ethical problems. Of these, mesenchymal stem cells have been shown to differentiate into cells of various types.Reference Pittenger, Mackay, Beck, Jaiswal, Douglas and Mosca 5 , Reference Umezawa, Maruyama, Segawa, Shadduck, Waheed and Hata 6 Adipose-derived stem cells are known to be similar to bone marrow mesenchymal stem cells that exist in fat tissue, which not only function as the precursors to fat cells but also have multipotency for bone and cartilage mesenchymal cells.Reference Zuk, Zhu, Mizuno, Huang, Futrell and Katz 7 – Reference Yamamoto, Gotoh, Kato, Majima, Toriyama and Kamei 13 Lo Cicero et al. used fat that had been injected and absorbed into the vocal folds to culture and analyse fat-derived stem cells, and reported that adipose-derived stem cells play an important role in the long-term engraftment of the transplanted fat tissue.Reference Lo Cicero, Montelatici, Cantarella, Mazzola, Sambataro and Rebulla 14
We used adipose-derived regenerative cells to develop a novel treatment for unilateral vocal fold paralysis without the need for cell culture. There have not been any previous reports of the effects of adipose-derived regenerative cells with regard to unilateral vocal fold paralysis in a large animal model. In this study, we extracted adipose-derived regenerative cells from autologous fat, created fat with a high concentration of adipose-derived regenerative cells and injected it into vocal folds, using a unilateral vocal fold paralysis model in large animals to investigate the effects and safety.
Materials and methods
Animals
Two adult (aged 29 and 21 months) micro mini-pigs (weighing 28.3 and 28.9 kg) (Fuji Micra, Shizuoka, Japan) were used in this study. The animal experiments were approved by the Institutional Animal Care and Use Committee (approval number: 2013–74). The experiments were conducted at the CIMIC Bioresearch Center (Yamanashi, Japan), an institution approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (identification number: 001182).
Study protocol and surgical procedures
We employed two pigs to verify the effectiveness and safety of a high concentration of adipose-derived regenerative cells added to autologous fat injection therapy. Our study protocol is shown in Figure 1.

Fig. 1 Injection protocol. RLN = recurrent laryngeal nerve; ADRCs = adipose-derived regenerative cells; CT = computed tomography
The injection was performed 30 days after making the unilateral vocal fold paralysis model (described below) and the two pigs were observed for 90 days in the same manner as reported by Kruschewsky et al.Reference Kruschewsky Lde, de Mello-Filho, dos Santos and Rosen 15 All examinations and surgical procedures were conducted under general anaesthesia by head and neck surgeons and plastic and reconstructive surgeons. The two micro pigs were anaesthetised via ketamine and atropine (30 mg/kg and 0.05 mg/kg) (induction), and were mechanically ventilated via a mask using isoflurane (2.0 per cent) in order to maintain anaesthesia. The laryngeal structures and the mobility of the vocal fold were observed by means of a 3.7 mm laryngoscope (Flexible CMOS Video Rhino-Laryngoscope series 11101 CM with Monitor 8402 ZX; Karl Storz Endoscope, Tuttlingen, Germany). All surgical procedures conducted inside the larynx were performed under flexible fibrescopy.
Unilateral vocal fold paralysis model
At day 0, two pigs underwent right recurrent laryngeal nerve (RLN) transection with complete removal of a 1 cm nerve segment. Subsequently, the proximal and distal edges of the right RLN were ligated using a non-absorbable surgical suture, to prevent the elongation and regeneration of the RLN. After wound closure, with the animal still under anaesthesia, unilateral vocal fold motion immobility was confirmed via visualisation through flexible fibrescopy.
Tissue harvesting and cell isolation
At day 30, 50 ml of adipose tissue was harvested from the anterior abdominal wall by making two 3 mm incisions. Ringer's lactate was first infused in the subcutaneous layer, and the adipose tissue was harvested. The suctioned adipose tissue was placed in saline, and allowed to stand for settling of the blood and cellular debris; adipose tissue floated at the top of the mixture.
Adipose-derived regenerative cells were isolated from the harvested adipose tissue using the Celution System (Cytori Therapeutics, San Diego, California, USA),Reference Lin, Matsubara, Masuda, Togashi, Ohno and Tamura 16 which is a commercially available kit designed to isolate adipose-derived regenerative cells from human adipose tissue in a short time. This instrument allows the isolation of therapeutic doses of autologous adipose-derived regenerative cells after liposuction without the need for culture. The nucleated cell composition of adipose-derived regenerative cells includes approximately 0.6–1.6 per cent of adipose-derived stem cells, as well as: mature and progenitor endothelial and smooth muscle cells; CD45+ haematopoietic cells, and resident tissue macrophages and monocytes; pericytes; pre-adipocytes; and other less well characterised stromal fibroblastic cell populations.Reference Lin, Matsubara, Masuda, Togashi, Ohno and Tamura 16
The final concentrated cell output was measured using a NucleoCounter (Chemometec, Allerod, Denmark), which exclusively detected nucleated cells. Using the Celution System, we were eventually able to obtain 5 ml of solution containing concentrated adipose-derived regenerative cells.
Vocal fold injections
At day 30, in the same manner as with clinical surgery in humans, 0.5 ml adipose-derived regenerative cells mixed with 1 ml autologous fat was injected into the right vocal fold of one pig (adipose-derived regenerative cells pig), with the other pig receiving 0.5 ml Ringer's solution (Lactec, Otsuka Pharmaceutical) mixed with 1 ml autologous fat as a sham control condition (control pig). The oral injection was performed using a syringe with an 18-gauge needle. After the injections, we pressed the vocal fold and confirmed no leakage of the fat.
Larynx removal
At day 120, both pigs were sacrificed using an overdose of sodium pentobarbital. The larynges were dissected out and stored in 10 per cent formalin neutral buffer solution. After fixation in 10 per cent formalin neutral buffer solution, the vocal folds were removed from the larynges and cut into thick sections. The sections were stained using haematoxylin and eosin and Masson's trichrome for histological analysis.
Assessment
We used a non-contact laser Doppler flowmeter (model ALF21N; Advance, Tokyo, Japan) to measure and record blood flow in the left and right vocal folds while the animals were alive. Blood flow, measured by the same person, was recorded after confirming a stable value for 30 seconds. The average value was calculated from five measurements, representing the blood flow at that time.
Computed tomography (CT) of the neck was conducted, with 1 mm slice thickness, using the non-ionic, low-osmolar contrast agent iohexol (Omnipaque; Daiichi-Sankyo, Tokyo, Japan) at 2 ml/kg. The Aplio 300 TUS-A300 ultrasonography system (Toshiba Medical Systems, Tokyo, Japan) was used to observe the entire neck, and its elasticity was evaluated with elastography. A fluorescence microscope (model BZ-9000; Keyence, Osaka, Japan) was employed to analyse pathological findings.
The voice data of two pigs were recorded with pulse-code modulation digital recording (at 2304 kilobits per second) using a 90-degree directional microphone. The recorded voice was analysed by sonography and sound wave pattern analysis was conducted using Praat sonography software (version 5.3.51). 17
Results
Liposuction was carried out in the abdomen without significant morbidity, and 50 ml of adipose tissue was harvested in two pigs. The isolated adipose tissue solution contained 2.0 × 106 and 2.0 × 106 adipose-derived regenerative cells (1.8 × 106 and 1.8 × 106 viable cells). We checked the blood sample, skin, brain, lung, heart, bowels, liver and kidney. The two pigs maintained their body weight, and no signs of side effects from the treatments were noted during the observation period.
Fibrescopy findings
Figure 2a shows both vocal folds of the control pig. The experimental vocal fold was fixed in a paramedian position on days 30, 60 and 120. The vocal fold showed signs of atrophy and a gap in the vocal fold remained when vocalising.

Fig. 2 The experimental vocal folds of both pigs were fixed in a paramedian position on days 30, 60 and 120 (arrows). (a) In the control pig, the vocal fold itself showed atrophy, and a gap in the vocal fold remained when the pig was vocalising. (b) In the adipose-derived regenerative cell pig, an increase in volume was found in the experimental vocal fold as well as a decreased gap in the vocal fold. Continued on following page.
Figure 2b shows both vocal folds of the adipose-derived regenerative cell pig. The experimental vocal fold was fixed in a paramedian position on days 30, 60 and 120. An increase in volume was found in the experimental vocal fold, with a decreased gap in the vocal fold.
Laser Doppler flowmeter
The non-contact laser Doppler flowmeter results for both vocal folds of the two pigs are shown in Figure 3. There was no particular change in blood flow during the observation period in the control vocal folds of either pig. However, on day 30, in the control pig, a temporary decline in the blood flow meter value was observed. On day 60, this value was equivalent to the initial value, and there was no change thereafter. In the experimental vocal fold of the adipose-derived regenerative cell pig, a similar temporary decline in blood flow was observed on day 30. However, by day 60, the blood flow had actually increased. On day 120, the value was equivalent to the initial measurement, and there was no difference between both vocal folds.

Fig. 3 Changes in vocal fold blood flow in (a) control pig and (b) adipose-derived regenerative cell pig, measured using a non-contact laser Doppler flowmeter (ALF21N; Advance).
Computed tomography and ultrasonography
Results for both pigs are shown in Figure 4. The CT conducted on day 60 showed that both the control pig and adipose-derived regenerative cell pig had subcutaneous fat, with similar low intensity in the experimental vocal fold and thyroid cartilage interval, suggesting that the transplanted fat tissue was surviving. According to the CT conducted on day 120, the volume of transplanted fat in the adipose-derived regenerative cell pig seemed to be larger than that in the control pig.

Fig. 4 On days 60 and 120, axial computed tomography scans of both (a) control pig and (b) adipose-derived regenerative cell pig revealed a low-density region around the experimental vocal fold area, indicating that the injected fat remained (arrows). In ultrasonic elastography conducted after larynx removal (see images on the right), the experimental vocal folds of the adipose-derived regenerative cell pig were found to still have elasticity.
Regarding the neck, no new neoplasms or cervical lymph node swellings were identified on CT or ultrasonography. The ultrasound elastography conducted after laryngectomy revealed that experimental vocal fold elasticity had decreased in the control pig, but had improved in the adipose-derived regenerative cell pig.
Histological assessment
Haematoxylin and eosin stained images of both vocal folds of the two pigs are shown in Figure 5. In both pigs, there was no atrophy of the muscle fibre in the control thyroarytenoid muscle, and muscle fibres were densely arranged.
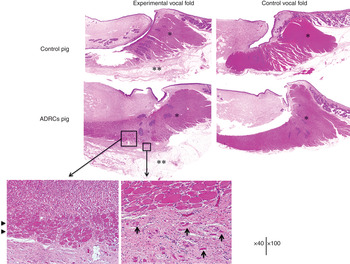
Fig. 5 Haematoxylin and eosin staining showed overall atrophy in the experimental thyroarytenoid muscle. In the experimental vocal fold of the adipose-derived regenerative cell pig, in the area surrounding the injection, the thyroarytenoid muscle fibre had clear hypertrophy compared to other parts (arrowheads). In addition, on the outside of the thyroarytenoid muscle, muscle fibre patches were scattered in accordance with the injection site (arrows). *Indicates thyroarytenoid muscle; **indicates right vocal fold injection site. ADRCs = adipose-derived regenerative cells
In the control pig, there were no findings indicating muscle hypertrophy, but overall atrophy was observed in the experimental thyroarytenoid muscle, with muscle fibre thinning. Survival of the injected fat was confirmed, but the volume was low and there was no clear fibrosis surrounding it.
In the adipose-derived regenerative cell pig, a sufficient quantity of remaining fat tissue was found in the injected area, and fibrosis and hyperplasia of collagen fibres were also observed. In the area surrounding the injection site, the thyroarytenoid muscle fibres displayed clear hypertrophy compared to other parts. On the outside of the experimental thyroarytenoid muscle, sporadic patches of muscle fibre and microvasculature hyperplasia (as was found for the injection area) were observed, indicating the regeneration of muscle fibre. Consistent with this, Masson's trichrome staining demonstrated hypertrophic areas of thyroarytenoid muscle fibre at the injection area and in sporadic muscle fibres externally.
Vocal function evaluation
Sonogram and voice waveform
Figure 6 shows the sonogram results and voice waveform data. Sonograms exhibited the central 1.0 second of a stable voice; the lowest band shows the fundamental frequency. The right RLN was severed in both pigs. This resulted in decreased formant continuity and stability, as shown in the lowest fundamental frequency. Moreover, the high frequency band, which indicated resonance of the pharyngeal cavity, never stabilised. In the adipose-derived regenerative cell pig, the formant formation was clear and voice continuity was maintained better than in the control pig, suggesting that it was essentially able to regain the same voice it had before the right RLN was severed.

Fig. 6 The sonogram showed a clear formant formation in the adipose-derived regenerative cell pig, while the high frequency appeared to be stable, and the pig was able to regain voice without disturbance in amplitude. The sonogram shows the central 1.0 second of a stable voice; the lowest band shows the fundamental frequency. The voice waveform is shown at a width of 0.1 second. ADRCs = adipose-derived regenerative cells
The voice waveform is shown at a width of 0.1 second. In both pigs, the amplitudes of voice signal waves were smaller and the rhythm was disturbed after the RLN was severed. In the control pig, the intervals between repeated waveforms widened compared with those before the procedure, and the waveforms did not stabilise. In the adipose-derived regenerative cell pig, a more concentrated waveform was observed and the high frequency band was stable, indicating that the animal was able to regain a voice without disturbance in amplitude.
Voice analysis
At day 30, the standard frequency in both the adipose-derived regenerative cell pig and the control pig had halved. At day 60, the frequency in the control pig temporarily showed the same values as before the severing of the right RLN; however, it subsequently dropped and remained fixed at half the frequency. The voice of the adipose-derived regenerative cell pig returned to its original frequency and that level was maintained throughout the observation period.
Discussion
In the treatment of vocal fold scarring, several cell-based therapies, such as autologous and non-autologous bone marrow mesenchymal stem cells,Reference Kanemaru, Nakamura, Omori, Kojima, Magrufov and Hiratsuka 18 – Reference Kanemaru, Nakamura, Yamashita, Magrufov, Kita and Tamaki 21 autologous adipose-derived stem cells,Reference Lee, Wang, Lee, Jung, Bae and Jeong 22 autologous fibroblasts,Reference Chhetri, Head, Revazova, Hart, Bhuta and Berke 23 and embryonic stem cells,Reference Cedervall, Ahrlund-Richter, Svensson, Forsgren, Maurer and Vidovska 24 have been reported. However, only a few studies have reported using cell-based therapies to treat unilateral vocal fold paralysis.
In this study, the injection of adipose-derived regenerative cell concentrated fat led to increased blood flow and vocal improvement in unilateral vocal fold paralysis. We reproduced the real clinical situation of vocal fold paralysis in a similar manner to Kruschewsky et al., who investigated the rate of autologous fat graft absorption in 24 paralysed canine vocal folds 12 weeks after injection.Reference Kruschewsky Lde, de Mello-Filho, dos Santos and Rosen 15 The blood flow of the vocal fold mucous membrane decreased temporarily on day 30 because of voice atrophy associated with recurrent nerve paralysis; however, in the adipose-derived regenerative cell pig, our findings suggest that vocal fold blood flow increased as a result of fat injections containing concentrated adipose-derived regenerative cells. The pathological findings suggest that the injection of fat containing concentrated adipose-derived regenerative cells controls thyroarytenoid muscle atrophy and regenerates new muscle fibres. By injecting adipose-derived regenerative cells along with fat, the remaining fat volume can be improved as a result of an increase in microvasculature in the surrounding area, and muscle atrophy can be avoided.
The mechanism of action for fat tissue mixed with adipose-derived regenerative cells appears to be as follows: (1) adipose-derived regenerative cells in the tissue undertake metabolism as pre-adipocytes; (2) adipose-derived regenerative cells branch to vascular endothelial cells and stimulate vascularisation; (3) adipose-derived regenerative cells emit pH blood vessel growth factors such as hepatocyte growth factor, basic fibroblast growth factor and vascular endothelial growth factor, thereby stimulating vascularisation; and (4) branching to muscle fibre and other tissues can therefore be expected.
Voice analysis revealed that the pigs had dysphonia plicae ventricularis following severing of the right RLN. In the adipose-derived regenerative cell pig, the voice returned, whereas in the control pig dysphonia plicae ventricularis persisted.
No previous studies have investigated the effects of adipose-derived regenerative cell therapy with a unilateral vocal fold paralysis model in large animals in a clinically matched manner. We selected a porcine model because of pigs’ anatomical and neurophysiological resemblance to humans and similarities in wound healing.Reference Goding, Richardson and Trachy 25 , Reference Woodson 26 Examination of collagen and elastin distribution in larynx tissue has revealed that the pig larynx is most similar to that of humans.Reference Jiang, Raviv and Hanson 27 – Reference Hahn, Kobler, Starcher, Zeitels and Langer 29 Alipour and colleagues found that out of pigs, sheep and cows, the pig's voice has the highest and widest frequency, and they concluded that the pig larynx is the most appropriate for investigating vocal fold elasticity characteristics in animal experiments.Reference Alipour and Jaiswal 30 , Reference Alipour, Jaiswal and Vigmostad 31
The injection of fat containing adipose-derived regenerative cells into paralysed vocal folds is postulated to improve the treatment outcome for unilateral vocal fold paralysis in the following ways: (1) by aiding the long-term maintenance of injected fat volume; (2) by increasing blood flow to the mucous membrane of vocal folds; and (3) by stabilising the voice.
-
• Autologous fat injection in vocal folds is popular because of the absence of any reaction to foreign bodies
-
• However, weakened effects due to injected fat being gradually absorbed is a problem
-
• Adipose-derived regenerative cells were used in a novel unilateral vocal fold paralysis treatment, without the need for cell culture
-
• The effectiveness and safety of a high concentration of adipose-derived regenerative cells added to autologous fat injection therapy was verified in two pigs
-
• This method has potential to improve treatment outcomes for unilateral vocal fold paralysis patients
Our study was based on a pre-existing model, and has been applied to large animals evaluated using clinical methods, making its application in humans a possibility in the future. Furthermore, because our method enables non-culture adipose-derived regenerative cells to be extracted and takes less time than cell culture, it could be applied to the current fat injection method immediately after liposuction. In our study, only two animals were used. Further investigations with more animals are needed to determine whether our results can be replicated and to validate the hypothesis generated from our analyses.
Conclusion
We used a unilateral vocal fold paralysis model in large animals and extracted adipose-derived regenerative cells from subcutaneous fat to create concentrated adipose-derived regenerative cell fat, which was then endoscopically injected into the vocal fold muscle. By injecting fat with a higher concentration of adipose-derived regenerative cells than is used in normal liposuction into the vocal folds, the injected volume was maintained for a longer amount of time, which could lead to better voice quality.








