Introduction
Chronic kidney disease is a multisystem disorder characterised by renal damage that leads to a slow and progressive loss of kidney functions. The effects of such a disorder could be due to: the failure of uremic toxin excretion, prolonged haemodialysis (which affects almost every tissue in the body, including the auditory system) or the treatment itself.Reference Peyvandi and Roozbahany1 The incidence of chronic kidney disease has increased significantly in recent years because of the increased number of diabetic and hypertensive patients, who are considered at major risk of chronic kidney disease. According to the National Kidney Foundation,2 chronic kidney disease is classified into five stages according to the glomerular filtration rate, which is considered the best measure of kidney function. Stage V kidney disease includes patients with renal failure who undergo regular haemodialysis.
There are anatomical, physiological and pharmacological similarities between the nephron and the stria vascularis of the cochlea, in addition to an immunological connection between both organs where antibodies raised against the nephron can also deposit in the stria vascularis.Reference Quick, Fish and Brown3,Reference Gatland, Tucker, Chalstrey, Keene and Baker4 Several studies have reported progressive sensorineural hearing loss (SNHL) in renal patients, especially in long-term cases,Reference Yassin, Badry and Fatt-Hi5–Reference Adler and Ritz7 and some syndromes (including Alport syndrome) can affect the kidney and cochlea together. The severity of hearing loss increases with the duration of the disease, and may give an indication of the extent of damage to auditory function.Reference Thodi, Thodis, Danielides, Pasadakis and Vargemezis8
The vestibule is a sensitive organ that responds to metabolic derangements such as abnormal glucose metabolism, high blood pressure, hyperlipidaemia and mineral bone disorders. Chronic kidney disease is a well-known risk factor for the presence and accumulation of uremic toxins, which are increased in advanced stages of chronic kidney disease.Reference Soriano, Ojeda, Rodríguez, Almadén, Rodríguez and Martín-Malo9 These changes consequently induce microangiopathy and vascular calcification in multiple organs, including the vestibular system. Therefore, chronic kidney disease could be a triggering factor of vestibular dysfunction if vasculopathy occurs in the vestibular system. Patients with chronic kidney disease are at greater risk of vestibular dysfunction when compared to the normal population.Reference Jung, Lee, Do and Kang10
Aim
Given the impact of chronic kidney disease on different body systems that include the audiovestibular system, this study was designed to study the possible impact on both the auditory and vestibular pathways at different levels, taking into consideration the effect of haemodialysis. Most previous studies focused on cochlear impairment and there are fewer reports on vestibular dysfunction in patients with chronic kidney disease; this latter issue will be addressed in this work.
Materials and methods
This cross-sectional study was carried out at the Audiovestibular Medicine Unit, in collaboration with the Internal Medicine Department (Nephrology Unit) at Tanta University Hospitals, over a period of about one year starting from 1 January 2016.
Forty subjects participated in the study, with an age range of 20–60 years. The subjects were divided into 3 groups: group 1 consisted of 20 healthy control subjects; group 2 comprised 20 patients with chronic kidney disease receiving regular conservative treatment; and group 3 consisted of 20 patients with chronic kidney disease undergoing regular haemodialysis.
Informed written consent was obtained from all patients after a full explanation of the benefits and risks of the study. Privacy of all patients’ data was granted by allocating a special code number to every patient file that included all investigation findings. The study carried no risks to the participants as the investigations were non-invasive.
All patients included in this study (groups 2 and 3) had chronic kidney disease of different stages. The exclusion criteria included: a history of any hereditary or acquired hearing loss or other auditory diseases, a history of noise exposure, the use of ototoxic drugs, and the presence of any neurological or psychiatric problems.
The following data were collected: patients’ demographic details; any history of underlying or accompanying diseases; the time of onset of chronic kidney disease; drug history; details of any auditory or vestibular diseases including hearing loss, tinnitus or vertigo; and dialysis prescription details (e.g. number of sessions per week and duration of the sessions).
All patients were subjected to full audiological history taking, otoscopic examination and basic audiological evaluation. The latter included: pure tone audiometry at the frequencies of 0.25–8 kHz, in addition to extended high frequencies at 10, 12 and 16 kHz; and speech audiometry (speech reception threshold and speech discrimination scores (in percentages)). Both the pure tone and speech audiometry tests were conducted using a GSI-61 audiometer (Grason-Stadler, Eden Prairie, Minnesota, USA). Pure tones were delivered using TDH-39 headphones for a frequency range of 0.25–8 kHz, while circumaural headphones were used for the extended high-frequency testing. The basic audiological evaluation also included immittancemetry (including both tympanometry and acoustic reflex threshold measurements using an Interacoustics AT235 tympanometer).
Two types of otoacoustic emissions (OAEs) were measured for this study: transient evoked OAEs (TEOAEs) and distortion product OAEs (DPOAEs). The TEOAEs were elicited using non-linear click stimuli at a stimulus intensity of 80 dB SPL, of 80 μs duration, and at a rate of 19 per second within a time window of 20 ms. The TEOAEs were analysed by recording 260 sweeps in 1 session, and were averaged within 5 frequency bands centred at 1, 1.5, 2, 3 and 4 kHz. The differential non-linear test paradigm was used. The stimulus was characterised by a train of four clicks: three with the same amplitude and polarity, followed by the fourth one with a three-fold greater amplitude and opposite polarity. Responses were represented by an average of a maximum of 260 click stimuli trains (total of 1040 clicks) stored into 2 different buffers averaged separately (A and B), for a total of 2080 clicks. The gain factor was 2000 and the artefact rejection was 19.1.
The twoƒ1-ƒ2 DPOAEs were measured two pure tones, ƒ1 and ƒ2, presented at L1 and L2 intensity respectively, where L1 = 65 dB SPL and L2 = 55 dB SPL. The DPOAEs were recorded at different ƒ2 frequencies, ranging approximately from 500 Hz to 8800 Hz in half-octave steps. The ƒ1/ƒ2 ratio was 1.22. At each distortion product frequency, both distortion product amplitude and signal-to-noise ratio were measured using the SmartEP platform (Intelligent Hearing Systems, Miami, Florida, USA). The measurement was conducted in two blocks; in each block, DPOAEs were recorded at 10 frequencies per octave. An ear tip transducer was securely positioned in the external auditory canal, for each ear. Patients were instructed to remain still and quiet during testing, which took place in a quiet room.
The oculomotor test battery of the videonystagmography test was conducted in both study groups (groups 2 and 3) using the ICS™ Chartr system. This battery included: a tracking test in the horizontal plane (in the frequency range of 0.2–0.7 Hz, divided into low-frequency (less than 0.3 Hz), mid-frequency (0.3 Hz to less than 0.5 Hz) and high-frequency (0.5 Hz or more)), and saccade, gaze and optokinetic tests.
Cervical and ocular vestibular-evoked myogenic potentials were simultaneously recorded, as per Chou et al.Reference Chou, Wang and Young11 Nine electrodes were used to record the combined vestibular-evoked myogenic potentials (five electrodes for each stimulation). For the recording of cervical vestibular-evoked myogenic potentials, two active electrodes were placed on the middle-third of the contracted sternocleidomastoid muscle of the neck on each side. Two reference electrodes were placed on the middle-third of both clavicles. For the recording of ocular vestibular-evoked myogenic potentials, two active electrodes were placed just inferior to each eye, about 1 cm below the centre of the lower eyelid. Two reference electrodes were placed about 1–2 cm below the corresponding active electrodes, below each eye. One ground electrode was placed over the forehead.
The subjects were asked to rotate their head to the opposite side of recording, flexing the head approximately 30 degrees forward to contract the sternocleidomastoid muscle, while looking upward at a distant target in the subject's visual midline. The eye position was measured as a vertical visual line angled at approximately 30–35 degrees above a horizontal line. Stimulation of the right ear results in right cervical vestibular-evoked myogenic potentials (ipsilateral) and left ocular vestibular-evoked myogenic potentials (contralateral), and vice versa for left ear stimulation. Click stimuli were delivered through insert phones at an intensity of 95 dB nHL, with 128 sweeps at a rate of 5 per second. The filter setting was 0.03–3 kHz, with a gain of 50 000. For all recorded traces, the positive and the negative peaks were identified according to their latencies; this was followed by measurement of the peak-to-peak amplitude of the waves. At least two consecutive averages were recorded from each side to verify reproducibility.
Results
The collected data were organised and statistically analysed using SPSS® statistical software for Windows, version 22. For quantitative data, the Shapiro–Wilk test for normality was performed. For normally distributed data, values were expressed as means ± standard deviations (SDs), and the independent sample t-test or one-way analysis of variance test were used for comparison. For data that were not normally distributed, median and interquartile range (expressed as 25th to 75th percentiles) were calculated, and Mann–Whitney U or Kruskal–Wallis tests were used for comparison between groups. Spearman's rank order correlation was used to determine the relationship between two numerical variables. For qualitative data, the Pearson's chi-square test or Fisher's exact test were conducted. The significance level adopted for the interpretation of test results was p < 0.05.Reference Dawson-Saunders and Trapp12
Sixty subjects were recruited to participate in this study. They were divided into three groups: group 1 consisted of 20 healthy control subjects (5 females (25 per cent) and 15 males (75 per cent); mean age (± SD) of 38.2 ± 7.4 years); group 2 comprised 20 patients with chronic kidney disease receiving conservative treatment without haemodialysis (11 females (55 per cent) and 9 males (45 per cent); mean age of 42.8 ± 14.3 years); and group 3 consisted of 20 patients with chronic kidney disease undergoing regular haemodialysis (8 females (40 per cent) and 12 males (60 per cent); mean age of 42.3 ± 13 years). No statistically significant differences were found between the three groups regarding sex or age (p > 0.01).
The aetiology and stages of chronic kidney disease, and co-morbidities of the patients, are listed in Table 1. None of the patients in groups 2 or 3 had a history of ototoxic drug use, and only two patients in group 2 complained of hearing loss. A statistical difference was observed regarding chronic kidney disease stage: all group 3 patients had stage V disease, whereas the five stages of chronic kidney disease were distributed in group 2 patients (p = 0.013).
Table 1. Comparison of chronic kidney disease stage, aetiology and co-morbidities
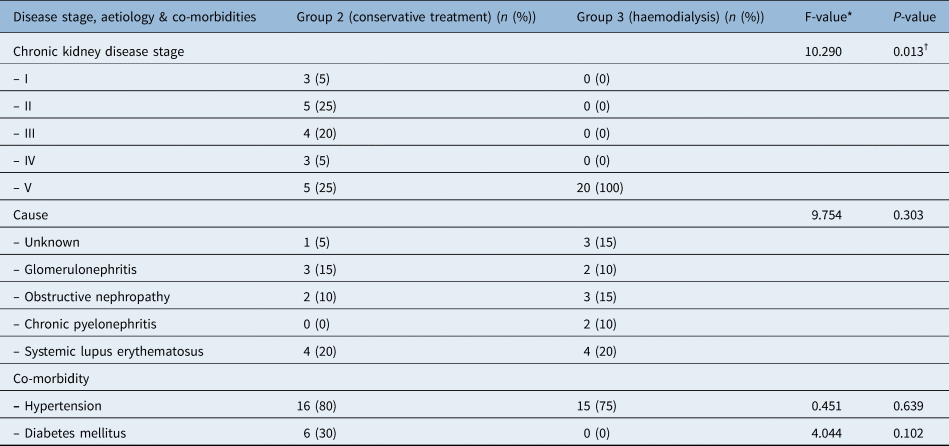
*Fisher's exact test. †Significant at p < 0.05
There were no significant differences in chronic kidney disease duration, haemoglobin level, blood urea, or sodium or potassium levels; however, the creatinine level was statistically significantly higher in group 3 than in group 2 (p < 0.05).
All subjects in this study had normal middle-ear function, as confirmed by otoscopic examination and immittancemetry. Pure tone audiometry showed that all subjects in group 1 had bilateral normal peripheral hearing, while 50 per cent of patients (10 out of 20) in group 2 had bilateral SNHL: one patient had low-frequency SNHL (at 0.25–1 kHz), three patients had flat SNHL (across all tested frequencies) and six patients had high-frequency SNHL (2–8 kHz). With regard to group 3, 60 per cent of patients (12 out of 20) had bilateral SNHL: 10 patients had high-frequency SNHL, 1 patient had low-frequency SNHL and 1 one patient had flat SNHL.
Comparison of pure tone audiometry results revealed no difference in hearing thresholds between the three groups at 0.25 kHz and 0.5 kHz, for both right and left ears. For the other frequencies (1, 2, 4 and 8 kHz), the control group showed significantly lower hearing thresholds compared to the two study groups, but with no difference in hearing thresholds between groups 2 and 3, for the right or left ears (Table 2).
Table 2. Pure tone audiometry results for both ears of all three groups

Data represent mean (± standard deviation) hearing levels (in decibels), unless indicated otherwise. *Welch's analysis of variance (ANOVA); †one-way ANOVA; ‡Kruskal–Wallis test. **Significant at p < 0.05. P 1–2 = comparison between controls and group 2; P 1–3 = comparison between controls and group 3; and P 2–3 = comparison between groups 2 and 3
The results of extended high-frequency pure tone audiometry are summarised in Tables 3 and 4. Hearing thresholds could be detected in 100 per cent of the control group at the three tested frequencies. Groups 2 and 3 showed maximum detectability of hearing thresholds at 10 kHz (80 per cent and 90 per cent respectively). At 12 kHz and 16 kHz, groups 2 and 3 showed significantly reduced detectability in comparison to the control group.
Table 3. Detectability of high-frequency pure tone audiometry hearing thresholds for right ears of all three groups

Detectability data represent numbers (percentages) of right ears with elicited response; threshold data represent mean (± standard deviation) hearing levels (in decibels). *Kruskal–Wallis test; †one-way analysis of variance. ‡Significant at p < 0.05. P 1–2 = comparison between controls and group 2; P 1–3 = comparison between controls and group 3; P 2–3 = comparison between groups 2 and 3
Table 4. Detectability of high-frequency pure tone audiometry hearing thresholds for left ears of all three groups

Detectability data represent numbers (percentages) of left ears with elicited response; threshold data represent mean (± standard deviation) hearing levels (in decibels). *Kruskal–Wallis test; †Welch's analysis of variance. ‡Significant at p < 0.05. P 1–2 = comparison between controls and group 2; P 1–3 = comparison between controls and group 3; P 2–3 = comparison between groups 2 and 3
Clinical high-frequency SNHL was defined as a hearing threshold level of more than 40 dB HL at 1 kHz, and of more than 55 dB HL at 12 kHz and 16 kHz, with a normal pure tone average on standard pure tone audiometry (according to the normative data of high-frequency audiometry in our clinic). In this study, 7 (out of 10) patients in group 2, and 6 (out of the 8) patients in group 3, with normal hearing thresholds on standard pure tone audiometry, had high-frequency SNHL. In both right and left ears, the comparison of hearing thresholds at 10 kHz showed significantly lower hearing thresholds in the control group when compared with the other two groups, with groups 2 and 3 having similar hearing thresholds. At 12 kHz and 16 kHz, there was no significant difference between the three groups in left ears; the comparison in right ears showed significantly lower hearing thresholds in the control group when compared with the two study groups, with no significant difference between groups 2 and 3 (Tables 3 and 4).
The OAE results showed that transient evoked OAEs (TEOAEs) were absent in 10 cases in group 2 (50 per cent) and absent in 8 cases (40 per cent) in group 3 (despite their bilateral normal hearing sensitivity), with recordings in 100 per cent of cases in group 1. In contrast, distortion product OAEs (DPOAEs) were elicited in all patients of all three groups. The comparison of TEOAE and DPOAE amplitudes between the three groups showed significantly higher amplitudes in group 1 in comparison to groups 2 and 3, with no significant difference between groups 2 and 3 (p < 0.05).
This study assessed vestibular function in patients with chronic kidney disease by recording oculomotor tests of videonystagmography and combined vestibular-evoked myogenic potentials. Results of the horizontal tracking test showed successful recording in 100 per cent of all control and study group patients; however, gain was normal in the control group (ratio of more than 0.7), but reduced in the study groups (ratio of less than 0.7). Statistically, there was significantly reduced gain in both study groups when compared with the control group. There was no statistically significant difference between the two study groups. Qualitatively, the tracking test results in group 2 (patients with conservative treatment) showed low gain (less than 0.7), in both the rightward and leftward directions, at the low- and mid-frequency range in about 40 per cent of the cases, which increased to approximately 55 per cent at the high-frequency range. In group 3 (patients undergoing haemodialysis), tracking in rightward or leftward directions showed low gain (less than 0.7) at the low- and mid-frequency range in approximately 25 per cent of cases, which increased to approximately 38.5–60 per cent at the high-frequency range.
The saccade test results showed normal latency, accuracy and velocity in the control group. With regard to the study groups, there was a significantly delayed latency when compared to the control group, but with similar saccadic accuracy and velocity amongst the three groups. The three groups showed similar results on the gaze test. However, the optokinetic test results showed significantly reduced nystagmus velocity in both study groups when compared to the control group, but with no significant difference between groups 2 and 3 (group 1, 22.7 ± 4.2 degrees per second; group 2, 16.4 ± 3.8 degrees per second; and group 3, 17.9 ± 2.3 degrees per second).
Cervical vestibular-evoked myogenic potentials were present in 100 per cent of subjects in the control group. The mean (± SD) P13 and N23 latencies were 13.1 ± 1.4 ms and 19.7 ± 2.2 ms, respectively (Table 5). The mean (± SD) peak-to-peak amplitude of P13–N23 was 22.7 ± 11.7 μV. In the two study groups, cervical vestibular-evoked myogenic potentials were bilaterally absent in eight patients (40 per cent) in group 2, and in seven patients (38.5 per cent) in group 3. This difference between these cases and the control group was significant (Fisher's exact test value = 10.553, p = 0.003). In the rest of cases, both groups showed similarities in terms of delayed P13 and N23 latencies and reduced P13–N23 amplitudes, when compared with the control group.
Table 5. Combined vestibular-evoked myogenic potentials for all three groups

Data represent mean (± standard deviation) values, unless indicated otherwise. *One-way analysis of variance; †Welch's analysis of variance. ‡Significant at p < 0.05. **’nI’ and ‘pI’ refer to the negative and positive peaks, respectively, in the response trace. P 1–2 = comparison between controls and group 2; P 1–3 = comparison between controls and group 3; P 2–3 = comparison between groups 2 and 3
Ocular vestibular-evoked myogenic potentials were detected in 100 per cent of patients in the control group, but were absent in 11 patients (73.3 per cent) in group 2 and in 9 patients (69.2 per cent) in group 3. The rest of the patients showed no significant differences from the control group in either latency or amplitude (Table 5).
Discussion
Chronic kidney disease is a major health problem, with serious adverse outcomes. Clinically, there are five stages of disease severity, according to the presence or absence of kidney damage and the level of kidney function.Reference Cabrera13 This study included two groups of patients with chronic kidney disease (groups 2 and 3) in addition to a control group (group 1). Group 2 included 20 patients with different stages of chronic kidney disease who were receiving conservative treatment (no haemodialysis). In contrast, all patients in group 3 had stage V chronic kidney disease (renal failure), and were undergoing regular haemodialysis. The three groups were matched in terms of age and sex, and had normal middle-ear function.
• The auditory and vestibular pathway can be affected in patients with different stages of chronic kidney disease
• This study showed that hearing thresholds were affected mainly at high frequencies
• Transient evoked otoacoustic emissions (OAEs) were absent in 40–50 per cent of chronic kidney disease cases
• Distortion product OAEs were recorded in all cases, which could be related to hearing thresholds
• However, both OAE types showed significantly reduced amplitudes in patients compared to controls
• Oculomotor and combined vestibular-evoked myogenic potential testing showed abnormal results in chronic kidney disease cases
Different co-morbidities, especially hypertension, were present in groups 2 and 3, with no significant difference between the groups. The laboratory test results showed significantly higher serum creatinine levels in group 3 compared to group 2. This is consistent with National Kidney Foundation guidelines (2002),2 in which glomerular filtration rate is still considered the best overall index of kidney function, even in stable, non-hospitalised patients. The glomerular filtration rate is primarily determined by serum creatinine level.
In this study, hearing thresholds were evaluated with standard pure tone audiometry, in addition to high-frequency audiometry. The standard pure tone audiometry results showed no significant difference between the three groups at 0.25 kHz and 0.5 kHz, in either the right or left ears. Other frequencies (1–8 kHz) showed significantly elevated hearing thresholds in both groups with chronic kidney disease when compared with the control group. However, there was no significant difference in hearing thresholds within the same range of frequencies for groups 2 and 3.
High-frequency audiometry showed that frequencies higher than 8 kHz were affected in patients with chronic kidney disease. This presented as a reduction in hearing threshold detection with increased test frequencies in both study groups when compared to the control group. The number of cases with non-measurable hearing thresholds increased significantly as the test frequency increased from 10 kHz to 16 kHz. In addition, the measured hearing thresholds in the rest of the patients were similar in both chronic kidney disease groups, and were significantly elevated when compared with the control group, in both the right and left ears. Similar elevated hearing thresholds, predominantly for the high frequencies, were reported by Gatland et al.,Reference Gatland, Tucker, Chalstrey, Keene and Baker4 and Alder and Ritz.Reference Adler and Ritz7
Regarding the aetiology of SNHL in chronic kidney disease, several possible mechanisms have been reported. First, there is the effect of the disease itself, due to disturbance of the homeostasis of water and electrolytes, with a subsequent effect on endolymphatic fluid. Second, there is the possibility of endolymphatic hydrops.Reference Adler and Ritz7 Third, one might consider the antigenic similarities between the glomerular basement membrane and cochlear stria vascularis. Fourth, some drugs (e.g. loop diuretics and aminoglycosides) used in the treatment of renal disease are known for their otoxicity.Reference Mitschke, Schmidt, Zazgorlik, Kopsa and Pils14 Finally, haemodialysis and renal transplants may induce electrolytic disturbances, and osmotic and immunological alterations at the inner-ear level, resulting in tinnitus, vertigo and hearing loss. Prolonged haemodialysis also may result in the accumulation of amyloid materials in many tissues, including the inner ear.
Aluminium toxicity resulting from antacid use in hyperphosphataemia treatment or dialysate water contamination may also have a role in hearing loss.Reference Nikolopoulos, Kandiloros, Segas, Nomicos, Ferekidis and Michelis15 However, in our study, both groups with chronic kidney disease were not significantly different from each other. This finding is not consistent with that of Lopez et al.,Reference Lopez, da Silva, Martin and Montovania16 who reported worse hearing thresholds for patients receiving conservative treatment than patients undergoing haemodialysis, due to the accumulation of toxic metabolites in the bloodstream with consequent impaired auditory functions.
The OAE results showed absent transient evoked OAEs (TEOAEs) in 50 per cent of patients in group 2 and in 40 per cent of those in group 3, despite normal hearing sensitivity in both ears. Comparison of TEOAEs between the three groups showed significantly higher amplitudes in the control group when compared with the two groups with chronic kidney disease. Distortion product OAEs (DPOAEs) were elicited in all patients of the three groups. However, their amplitude was reduced in patients with chronic kidney disease when compared to controls. Once again, both groups with chronic kidney disease had similar DPOAE amplitudes. These findings support the previous finding of OAE sensitivity to subtle cochlear pathology before the observed elevated hearing thresholds. Similar results were reported by Samir et al.,Reference Samir, Riad, Mahgoub, Awad and Kamal17 Pandy et al.,Reference Pandey, Gore, Valame and Mehta18 and Ozturan and Lam.Reference Ozturan and Lam19
In this study, oculomotor tests were used to examine the vestibular-ocular reflex in patients with chronic kidney disease. The results showed low gain tracking in both chronic kidney disease groups, regardless of whether receiving conservative treatment or undergoing haemodialysis. The impairment worsened with increased target velocity. Moreover, saccade latencies were prolonged in both study groups. In addition, the optokinetic test showed significantly similar reduced nystagmus velocity in both study groups when compared to the control group. These findings might be related to several factors, including the following. First, there is a higher risk of age-related ocular diseases in patients with chronic kidney disease. These age-related ocular diseases include cataracts, retinopathy, glaucoma and age-related macular degeneration, and are the leading causes of blindness in middle-aged and elderly adults.Reference Grunwald, Alexander and Maguire20 Second, patients may have impaired cognitive function, including deficiencies in memory, perceptual motor abilities, executive functioning, attention and processing speed, all of which can affect the ability to concentrate and follow visual targets. Third, the patients are at risk of developing uremic encephalopathy (D Cosgrove, unpublished data).
Combined vestibular-evoked myogenic potentials were used to assess otolithic function in this study. The results showed absent cervical vestibular-evoked myogenic potentials in both ears in about 40 per cent of cases in each group with chronic kidney disease, regardless of whether receiving conservative treatment or undergoing haemodialysis. The rest of the study group cases had similar significantly delayed latencies for cervical vestibular-evoked myogenic potentials P13 and N23, with no significant differences between the two groups. The P13–N23 amplitude was significantly reduced in both study groups when compared with the control group. These vestibular-evoked myogenic potential findings suggest vestibulospinal tract pathology.Reference Murofushi, Shimizu, Takegoshi and Cheng21 Chronic kidney disease may be associated with a disturbance in the blood level of sodium and potassium. This would, in turn, affect their concentration in perilymph and endolymph, leading to poor coupling of energy from the saccular macula to the hair cells. Ocular vestibular-evoked myogenic potentials were absent in almost 70 per cent of study group cases. The cervical vestibular-evoked myogenic potential results suggest impaired otolithic function (saccule and utricle) and/or their innervations (inferior or superior vestibular nerves), in either the ipsilateral (cervical vestibular-evoked myogenic potentials) or contralateral (ocular vestibular-evoked myogenic potentials) side of stimulation. Similar vestibular-evoked myogenic potential results were reported by Sazgar et al.Reference Sazgar, Ahmadi, Akrami and Akrami22
Chronic kidney disease is considered a major health problem, which is prevalent in both adults and children due to the high incidence of hypertension and diabetes. Cochlear function is dependent on normal kidney function. Normal cochlear homeostasis is achieved through the sodium–potassium pump, which ensures the regulation of electrolytes in cochlear fluids: endolymph has a high potassium concentration and perilymph has a high sodium concentration. Both electrolytes are essential for the transmission of electrical signals within the auditory system, with normal electromotility of the outer hair cells.Reference Couloigner, Sterkers and Ferrary23 Interruption of the electrolytic balance that occurs in chronic kidney disease patients is likely to result in a disturbance to the cochlear fluids, with specific changes in the stria vascularis leading to auditory dysfunction.Reference Thodi, Thodis, Danielides, Pasadakis and Vargemezis8 Moreover, changes in sympathetic control of blood pressure can occur in chronic kidney disease patients, and could affect the blood supply of the inner ear, resulting in cochleovestibular dysfunction.Reference Samir, Riad, Mahgoub, Awad and Kamal17 Additionally, these patients typically receive loop diuretics and aminoglycoside antibiotics; these alter excretion from the body because of renal failure, in addition to their ototoxicity and vestibulotoxicity (already increased because of renal impairment).Reference Sazgar, Ahmadi, Akrami and Akrami22
In conclusion, this work provides further support for the finding of high-frequency hearing loss in patients with chronic kidney disease, which can be missed on standard audiometry. It also revealed outer hair cell dysfunction, as shown by OAEs testing. Additional vestibular effects were also apparent, as revealed by abnormal oculomotor tests results, and impaired otolithic function (saccule and utricle) and/or their innervations, in either the ipsilateral or contralateral side of stimulation. We recommend the implementation of high-frequency audiometry evaluation and OAEs testing routinely in the follow up of patients with chronic kidney disease, regardless of their disease staging.
Competing interests
None declared







