Introduction
Otitis media with effusion (OME) is a clinical manifestation characterised by inflammation of middle-ear mucosa, leading to non-purulent fluid accumulation in the middle ear and variable conductive-type hearing loss, without symptoms and findings of acute infection.Reference Aktan, Gundogdu, Ucuncu, Unal, Sütbeyaz and Altas1 Otitis media with effusion is an inflammatory response of the middle ear caused by multiple factors, such as viral or bacterial infection, Eustachian tube dysfunction, or allergy. Although OME can affect people of all ages, it is more common in babies and children. Antibiotics, decongestants, glucocorticoids, antihistamines, antioxidant substances, non-steroid anti-inflammatory drugs and immunoprophylaxis are used in the treatment of OME. Ventilation tube application is used in cases resistant to treatment.
Macrolide antibiotics have been in clinical use since 1952, and have proven effective in the treatment of chronic diseases such as OME, sinobronchial syndrome and diffuse panbronchiolitis.Reference Iino, Sugita, Toriyama and Kudo2 Macrolide antibiotics exhibit their anti-inflammatory effect by reducing pro-inflammatory mediator and cytokine production, and preventing the synthesis of adhesion molecules.Reference Culic, Erakovic and Parnham3 Other anti-inflammatory effects include an increase in the mucociliary transport of macrolides and a reduction in goblet cell secretion.Reference Cervin4 The clinical effects of macrolide antibiotics may be related to their anti-inflammatory and anti-secretory properties as much as to their antimicrobial characteristics.
Researchers have used animal models to study OME, with the intra-tympanic application of various chemicals. Cortisporin, Streptococcus pneumoniae, Escherichia coli endotoxin and histamine have been used as irritant substances in such studies.Reference Aktan, Taysi, Gumustekin, Bakan and Sutbeyaz5, Reference Kozan, Aktan, Sakat, Kurt, Öner and Kara6 Aktan et al. observed that histamine can induce inflammatory changes in the middle ear of laboratory animals.Reference Aktan, Gundogdu, Ucuncu, Unal, Sütbeyaz and Altas1 Although many studies have investigated the aetiopathogenesis of OME, there is no clear consensus regarding an effective therapeutic approach.
The present study investigated the therapeutic effects of erythromycin, clarithromycin, azithromycin and roxithromycin on a histamine-induced animal model of OME. In particular, we assessed histopathological and stereological findings during the course of these agent therapies.
Materials and methods
Thirty-five healthy guinea pigs weighing 600–700 g, obtained from the Ankara Refik Saydam Hygiene Institute, were used in the study. These were bred and maintained in the animal facility at the Atatürk University Faculty of Veterinary Medicine, with the approval of the Institutional Animal Care and Use Committee. Prior to inclusion in the study, all animals underwent otoscopic and tympanometric examinations, in order to exclude middle-ear infection or pathology.
At the beginning of the study, the animals were divided into five groups, each containing seven animals, receiving: erythromycin, clarithromycin, azithromycin, roxithromycin or saline solution. The guinea pigs received erythromycin (40 mg/kg/day), clarithromycin (15 mg/kg/day), azithromycin (10 mg/kg/day) or roxithromycin (10 mg/kg/day) for 3 days by gastric tube. The control group received an equal volume of saline solution by gastric tube for 3 days.
Four hours after the end of the administration, the guinea pigs were anaesthetised (100 mg/kg ketamine hydrochloride and 3 mg/kg diazepam intraperitoneally). Histamine solution was subsequently injected into the right middle ear. Two hours after histamine administration, the experimental animals were sacrificed painlessly with a high-dose (100 mg/kg) sodium pentobarbital injection.
Otitis media with effusion induction
A histamine solution was prepared by adding histamine dihydrochloride (Sigma, Germany) to a 0.9 per cent saline solution. The pH of the solution was adjusted to 7.4 using potassium hydroxide. The guinea pigs were anaesthetised (100 mg/kg ketamine hydrochloride and 3 mg/kg diazepam intraperitoneally). The histamine solution (0.1 ml) was injected into the middle-ear cavity through the right tympanic membrane using a 27-gauge needle.Reference Taysi, Ucuncu, Elmastas, Aktan and Emin7
Histopathological procedures
The right temporal bones were removed by decapitation immediately after sacrifice. The temporal bones were placed in 10 per cent buffered formalin and kept for 24 hours at 4 °C for fixation purposes. At the end of 24 hours, they were removed from the formalin solution and placed into a 10 per cent ethylenediamine tetra-acetic acid solution for decalcification. The decalcified specimens were embedded in paraffin after approximately 20 days.
Stereological procedures
Thirty-micron sections were taken from the paraffin blocks from all 35 cases using systematic random sampling. One section in 15 was taken during the procedure. These sections were stained with routine haematoxylin and eosin, and prepared in such a way as to avoid closure.
A step interval of 100 µm on the x-axis and 100 µm on the y-axis was used for scanning on all observed projections. All 30-micron sections from all cases were screened from the first section at predetermined step intervals. An unbiased counting frame with a 100-μm x-axis and a 100-μm y-axis was produced for resection materials at each step interval. Area sampling at a rate of 1/400 was thus established at every step using this method. These values were recorded as the ‘area sampling fraction’. The unbiased counting frame area was determined as 25 μmReference Iino, Sugita, Toriyama and Kudo2.
Optical dissector counting rules were used as a statistical analysis technique in the counting procedures. In the optical dissector technique, a 5-micron safety margin was left on the upper and lower surfaces of the sections in order to exclude potential irregularities occurring during the section cutting procedures. The height of the dissector probe was taken as 20 µm with a mean section thickness, and a ‘thickness sampling factor’ was calculated for each case.
In the final stage of the study, particle counting was performed using a stereological dissector. A previously determined unbiased counting frame was placed over each consecutive area, and the lens was moved through the section. The counting frame was thus enabled to move inside the section, and a three-dimensional counting volume was established. Polymorphonuclear leukocyte numbers were counted in all areas within the margins of the unbiased counting frame, within the defined volume throughout the depth of the dissector probe. No change was made in the determined value at any stage during counting, and each case was listed automatically by the system under a file name consisting of the protocol number.
Total dissector volume (‘ΣVdis’) and the total dissector particle (‘EQ−’) number counted within this volume were obtained, and the neutrophil number density (‘Nv’) was calculated using the formula: Nv = ΣQ−/ΣVdis.
Statistical analysis
The neutrophil number density values obtained were subjected to statistical analysis using SPSS® version 11.0 software on Microsoft® Windows XP. The non-parametric Wilcoxon signed-rank test was used in the comparison of all parameters examined in the study and control groups. P-values of less than 0.05 were regarded as statistically significant.
Results
No sign of systemic disease, impairment of general condition or mortality was observed in any animal throughout this experimental study.
Neutrophil density in middle-ear mucosa, selected as a major marker of inflammation, was measured in the study and control groups. Intense neutrophil infiltration in the mucosa of the middle ear was determined in the control group during histopathological examination (Figure 1). Neutrophil infiltration was lower in the study groups compared to the control group (Figures 2–5). The neutrophil number density values obtained using stereological methods are shown in Table I and Figure 6.

Fig. 1 Intense neutrophil infiltration in the middle-ear mucosa of the control (saline) group. (H&E; ×100)

Fig. 2 Neutrophil infiltration in the middle-ear mucosa of the erythromycin group. (H&E; ×100)

Fig. 3 Neutrophil infiltration in the middle-ear mucosa of the clarithromycin group. (H&E; ×100)

Fig. 4 Neutrophil infiltration in the middle-ear mucosa of the azithromycin group. (H&E; ×100)

Fig. 5 Neutrophil infiltration in the middle-ear mucosa of the roxithromycin group. (H&E; ×100)
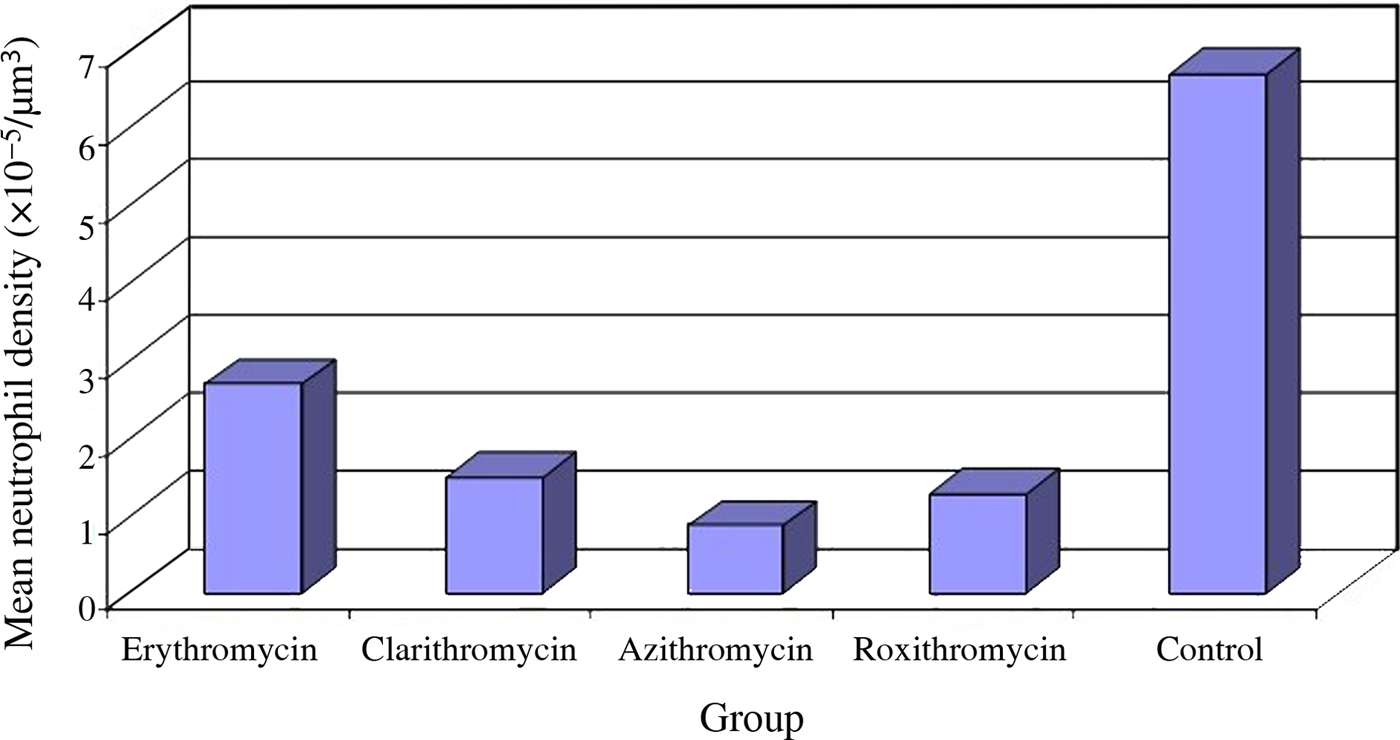
Fig. 6 Mean neutrophil density values, determined using stereological techniques, in the study and control groups.
Table I. Neutrophil density values in study and control groups*

* Neutrophil density values (×10−5/μm3) determined using stereological techniques. SD = standard deviation
The lowest neutrophil density value obtained using stereological techniques in our study was in the azithromycin group (0.86 ± 0.25 × 10−5/μm3), while the highest value was observed in the control group (6.68 ± 3.12 × 10−5/μm3). The difference was statistically significant (p < 0.05). Neutrophil density was statistically significantly lower in the erythromycin group (2.67 ± 0.64 × 10−5/μm3) than in the control group (p < 0.05). Variations between neutrophil density values in the clarithromycin (1.47 ± 0.61 × 10−5/μm3) and azithromycin (0.86 ± 0.25 × 10−5/μm3) groups and the roxithromycin group (1.27 ± 0.90 × 10−5/μm3) were not statistically significant (p > 0.05). Neutrophil density values in these groups were significantly lower than the neutrophil infiltration values in the control (6.68 ± 3.12 × 10−5/μm3) and erythromycin (2.67 ± 0.64 × 10−5/μm3) groups (p < 0.05).
Discussion
Otitis media with effusion (OME) is an inflammatory response of the middle ear caused by multiple factors, such as viral or bacterial infection, Eustachian tube dysfunction, or allergy. This inflammation stimulates the release of histamine from free mast cells in the middle-ear mucosa during OME. Inflammation via mast cell degranulation has been reported in these patients.Reference Kozan, Aktan, Sakat, Kurt, Öner and Kara6 The mucosal injury occurring in OME has been associated with the intensity of this inflammation, rather than bacterial invasion.Reference Aktan, Gundogdu, Ucuncu, Unal, Sütbeyaz and Altas1
Macrolide antibiotics have, in recent years, been shown to exhibit anti-inflammatory properties.Reference Nonaka, Pawankar, Tomiyama and Yagi8 However, the mechanism by which macrolides suppress inflammation is as yet unclear. The anti-inflammatory effects of these drugs are reported to be independent of their antibiotic effects. Macrolides are reported to exhibit an anti-inflammatory effect by: inhibiting the secretion of adhesion molecules, leading to neutrophil extravasation; and preventing the synthesis of pro-inflammatory cytokines, such as tumour necrosis factor alpha (TNFα)-1, interleukin (IL)-1 and IL-8.Reference Tamaoki, Kadota and Takizawa9 The expression of many genes involved in the immune and inflammatory response is known to be regulated at the transcriptional level by inducible nitric oxide synthase, cyclo-oxygenase-2, IL-1, IL-6, TNFα, intercellular adhesion molecule 1 and nuclear factor kappa B (NFκB).Reference Cervin4, Reference Yamamoto, Arakawa, Ueda and Yamamoto10 Although the mechanism by which macrolides suppress inflammation is not yet fully understood, studies have focused on NFκB inhibition as the essential factor.Reference Ianaro, Ialenti and Maffia11
The anti-inflammatory effect of macrolides is more marked in the early phase of inflammation.Reference Li, Azuma and Takahashi12 Our study aimed to better identify anti-inflammatory efficacy by pre-treatment with macrolides.
Stereological procedures are fast and efficient methods for performing quantitative analysis, such as determining the number of entities in a biological tissue. We also used stereological methods in this study to ascertain the density of neutrophils in inflamed middle-ear mucosa. Neutrophil densities were calculated in the study and control groups based on neutrophil numbers per unit volume in the middle-ear mucosa. Erythromycin, clarithromycin, azithromycin and roxithromycin were observed to reduce neutrophil infiltration in middle-ear mucosa; this effect was nearly identical in clarithromycin, azithromycin and roxithromycin, but weaker in erythromycin. These findings show that the macrolides used in the study have an anti-inflammatory effect in the middle ear, and this effect was greater following the administration of clarithromycin, azithromycin and roxithromycin. This inhibition of neutrophil infiltration may be ascribed to the inhibitory effect of macrolides on the production of adhesion molecules, cytokines and pro-inflammatory mediators.Reference Tamaoki, Kadota and Takizawa9, Reference Suzuki, Asada, Ikeda, Oshima and Takasaka13, Reference Miyanohara, Ushikai and Matsune14
Ianaro et al. compared the anti-inflammatory effectiveness of roxithromycin, clarithromycin, erythromycin and azithromycin in vivo and in vitro.Reference Ianaro, Ialenti and Maffia11 Their findings showed that in carrageenan pleurisy induced in rats, all macrolides reduced prostaglandin E2, IL-1β and TNFα levels. In addition, with the exception of azithromycin, the macrolides were effective in lowering exudate volume, and prevented an increase in leukocyte levels. Azithromycin had very little effect on inflammatory reaction, but exhibited greater anti-inflammatory activity than roxithromycin, erythromycin and clarithromycin. The authors also suggested that the increase in nitrogen dioxide and 6-keto prostaglandin F1α levels was associated with cyclo-oxygenase-2 and inducible nitric oxide synthase protein synthesis inhibition; however, erythromycin and roxithromycin exhibit anti-inflammatory effects by preventing NFκB activation through an antioxidant effect, rather than through direct inhibition.Reference Ianaro, Ialenti and Maffia11
Labro et al. showed that roxithromycin powerfully impaired myeloperoxidase-mediated protein iodination, superoxide anion production and polymorphonuclear leukocyte oxidative burst.Reference Labro, el Benna and Babin-Chevaye15 These effects of the drug were observed only at high concentrations (100 and 50 mg intraperitoneally). Chemotaxis was also impaired with roxithromycin (100 mg intraperitoneally), but Chlamydia pneumoniae phagocytosis remained unchanged, even at high concentrations. This effect was thought to be due to roxithromycin having a higher intracellular uptake compared to other macrolides. This effect of roxithromycin may be useful in controlling inflammation in severe infectious diseases.Reference Labro, el Benna and Babin-Chevaye15
Previous studies have investigated the anti-inflammatory effects of macrolide antibiotics in OME. In a study of OME induced in guinea pigs with histamine, Aktan et al. reported that erythromycin significantly reduced middle-ear mucosa neutrophil infiltration.Reference Aktan, Gundogdu, Ucuncu, Unal, Sütbeyaz and Altas1 Similarly, Kozan et al. investigated the anti-inflammatory effects of clarithromycin and prednisolone in OME, and observed that both clarithromycin and corticosteroids reduced neutrophil infiltration; however, they found that the combined use of the two produced a greater anti-inflammatory effect.Reference Kozan, Aktan, Sakat, Kurt, Öner and Kara6
Inhibition in the initial stage of OME with drugs exhibiting anti-inflammatory effects, such as erythromycin, clarithromycin, azithromycin and roxithromycin, may have a positive effect on the disease course. Macrolides suppressing neutrophil inflammation to the inflamed middle-ear mucosa in the early stage suggests that inhibition needs to be applied from the onset of the disease. Identifying patients at such an early stage may appear to be a difficult procedure in practice. Moreover, it is impossible to determine at such an early stage which patients will develop OME and in which individuals the disease will become chronic.
• Erythromycin, clarithromycin, azithromycin and roxithromycin effects were investigated in a histamine-induced otitis media with effusion (OME) animal model
• Stereological methods were used to determine neutrophil density in inflamed middle-ear mucosa
• The lowest neutrophil density values were found in the azithromycin group; the highest were in the control group
• Anti-inflammatory properties of clarithromycin, azithromycin and roxithromycin were similar, but better than erythromycin
• Macrolide antibiotic use is recommended, as it shows antibacterial and anti-inflammatory efficacy in OME
Further research is required to determine the effectiveness of macrolides and the benefits arising from their anti-inflammatory effects in chronic OME. In addition, as the majority of OME cases begin with acute otitis media, we think that starting treatment using antibiotics with anti-inflammatory effects, such as erythromycin, clarithromycin, azithromycin and roxithromycin, through early diagnosis of acute otitis media, will provide significant benefits. Benefitting from powerful antibacterial effects through using macrolide antibiotics with anti-inflammatory effectiveness, while the infection is still at the acute stage, represents a rational solution. This will also contribute to the avoidance of several side-effects and complications that may develop secondary to glucocorticosteroid use, and will benefit the patient and national budget in terms of treatment costs. No previous studies have compared the anti-inflammatory efficacy of macrolide antibiotics used in the treatment of OME.
This research demonstrates the anti-inflammatory efficacy of erythromycin, clarithromycin, azithromycin and roxithromycin. The anti-inflammatory properties of these three drugs were similar to one another, but better than that of erythromycin. We recommend the use of macrolide antibiotics, which have antibacterial and anti-inflammatory efficacy in OME. However, further studies are needed to determine the appropriate treatment duration and dosage.
Competing interests
None declared.









