Introduction
The ossicles in the middle ear function as a pure physical oscillating system when conducting the sound from the external ear to the inner ear. As it does not evoke electrical signals during this process, optical observation is the only way to analyse the movement of the ossicles. To date, laser Doppler Vibrometry has been the ‘gold standard’ research method used to analyse the ossicular movement at high temporal resolution.Reference Goode, Ball, Nishihara and Nakamura1
We have recently found that commercially available high-speed cameras could record sound-induced vibration of the ossicles in the near-intact middle ear of guinea pigs.Reference Ishizu, Yasui, Ohashi, Matsumoto and Komune2 Although our current video analysis had a much lower temporal resolution (up to 4 kHz) than laser Doppler vibrometry, the acquired video was easy to understand and analyse. Upon analysing the video files, we found that the stapes showed an additional oscillation before making a complete stop, which we interpreted as inertia-induced passive movement. The inertial movement of an oscillating system without any external force depends only on the characteristic physical properties of the oscillating system. Therefore, the measurement of this passive oscillation may reveal important physical properties of the middle ear. We herein report the findings of our analysis of the oscillation immediately after the sound had stopped.
Materials and methods
Preparation
The preparation of the specimen and the experimental setup were described in our previous report.Reference Ishizu, Yasui, Ohashi, Matsumoto and Komune2 Briefly, guinea pigs (200–300 g) were euthanised by decapitation after being intraperitoneally administered a sedative (20 mg/kg), and then a lethal dose (200 mg/kg) of pentobarbital, following procedures that were approved by the institutional committees on animal experimentation of the Graduate School of Medical Sciences, Kyushu University. We prepared the middle-ear specimens to provide optical access to the intact middle-ear structures. The specimen was then placed under a high-speed camera (VW-5000, Keyence, Osaka, Japan) equipped with a ×20 zoom lens (VH-20w, Keyence). Stimulating sound was delivered through an earphone (YE-103J, Nihon Kohden, Tokyo, Japan).
Recording and analysis
Tone bursts of various frequencies (125, 250, 500 and 1000 Hz) were generated in an auditory brainstem response system (Neuropack M1: MEB-9204, Nihon Kohden, Tokyo, Japan) and delivered for a duration of 100 milliseconds every 1 second. The intensity of each tone burst was set at 105 dB SPL. The high-speed video was acquired at a recording rate of 1000–4000 frames per second (fps). The recording rate was chosen in each experiment to acquire analysable video at the highest time resolution. The low-contrast image obtained due to the limited light intensity was the major reason for decreasing the recording rate. The recorded video files were analysed using a software program developed for moving target analysis (VW-H1MA, Keyence), as reported previously. A point of interest was placed on the head of the stapes, and the coordinates of the point of interest were sent for further analysis.
We manually calculated the frequency of the oscillations. We recorded the time (t n) in seconds during which the stapes showed the peak oscillation as the ‘nth’ time. The frequency of the oscillation (ω) was calculated using the following equation:
where n and m are integers ≥1, and t 0 is the time when the sound started.
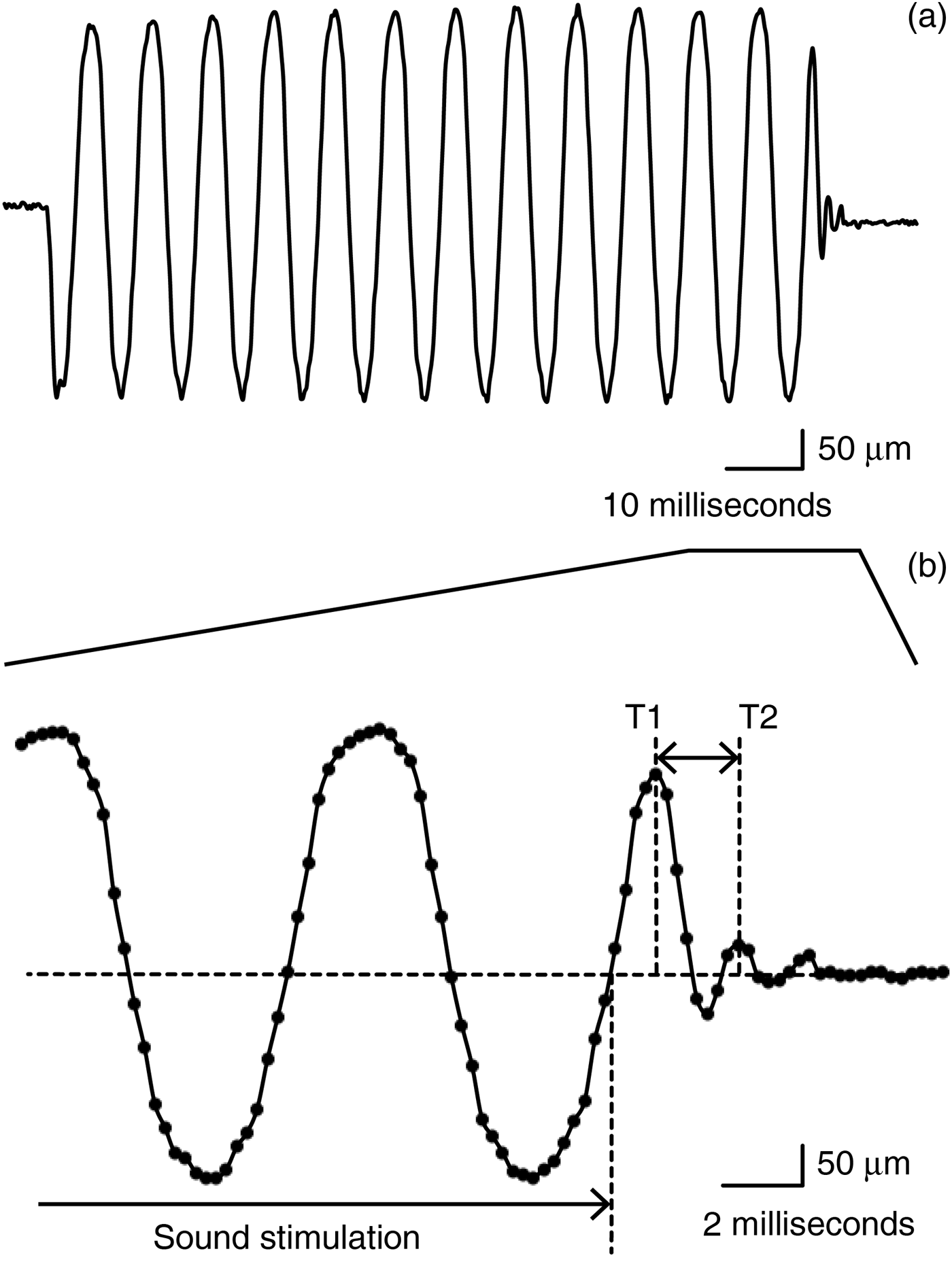
The oscillating frequency (ω) during sound stimulation (t n+m − t 0 ≤ 100 milliseconds) was calculated to confirm that the ω was equal to the input sound frequency. The oscillating frequency (ω) after the sound stimulation (t n – t 0 ≥ 100 milliseconds) was calculated whenever possible (Figure 1b).

Fig. 1 Stapedial motion. (a) The analysed stapedial motion in different time scales. (b) The stapedial motion in the last part of the sound stimulation and its damped oscillation shortly after the sound stimulation had ended. Note that the frequency of the damping oscillation (the difference between T1 and T2) is clearly different from the oscillation under the sound stimulation.
Results
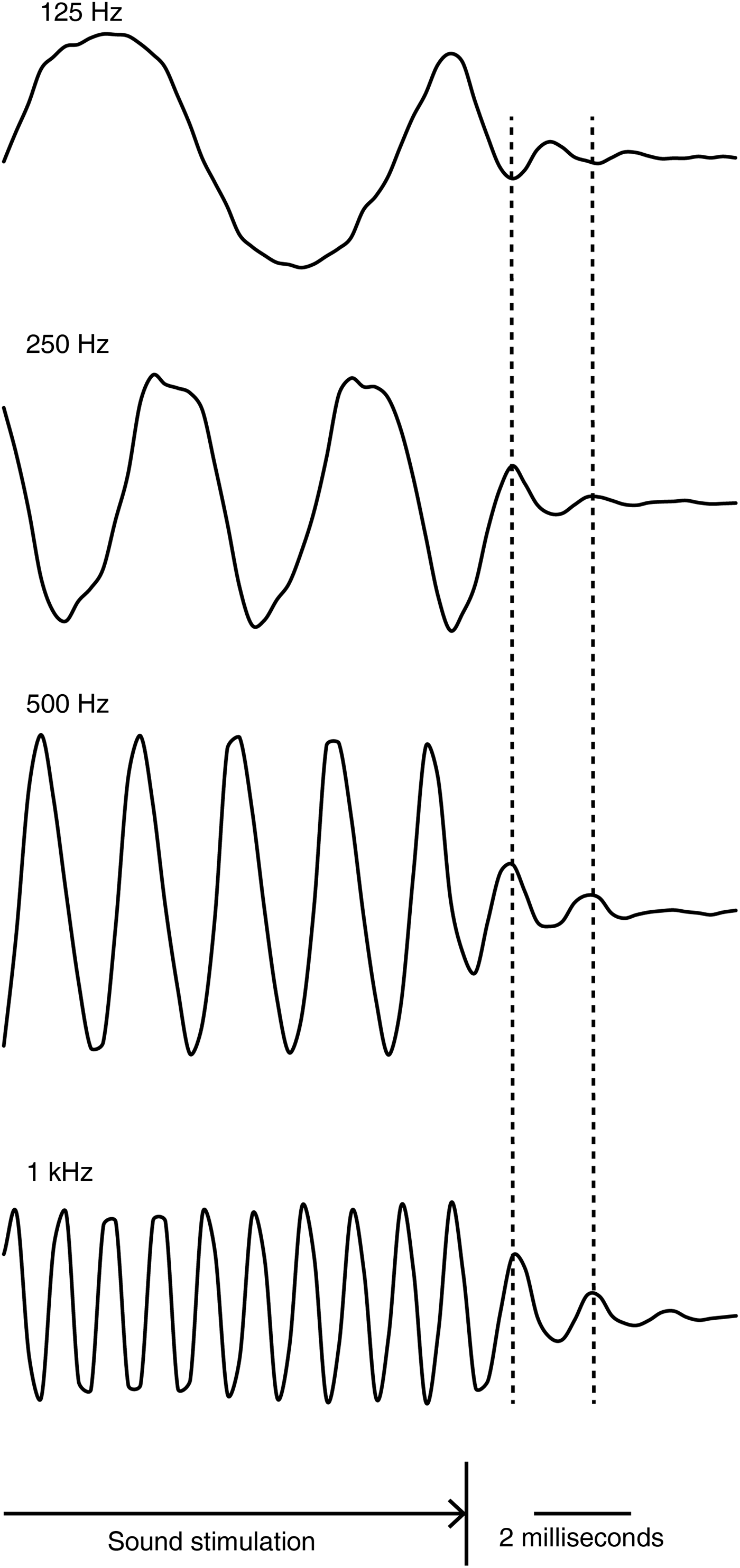
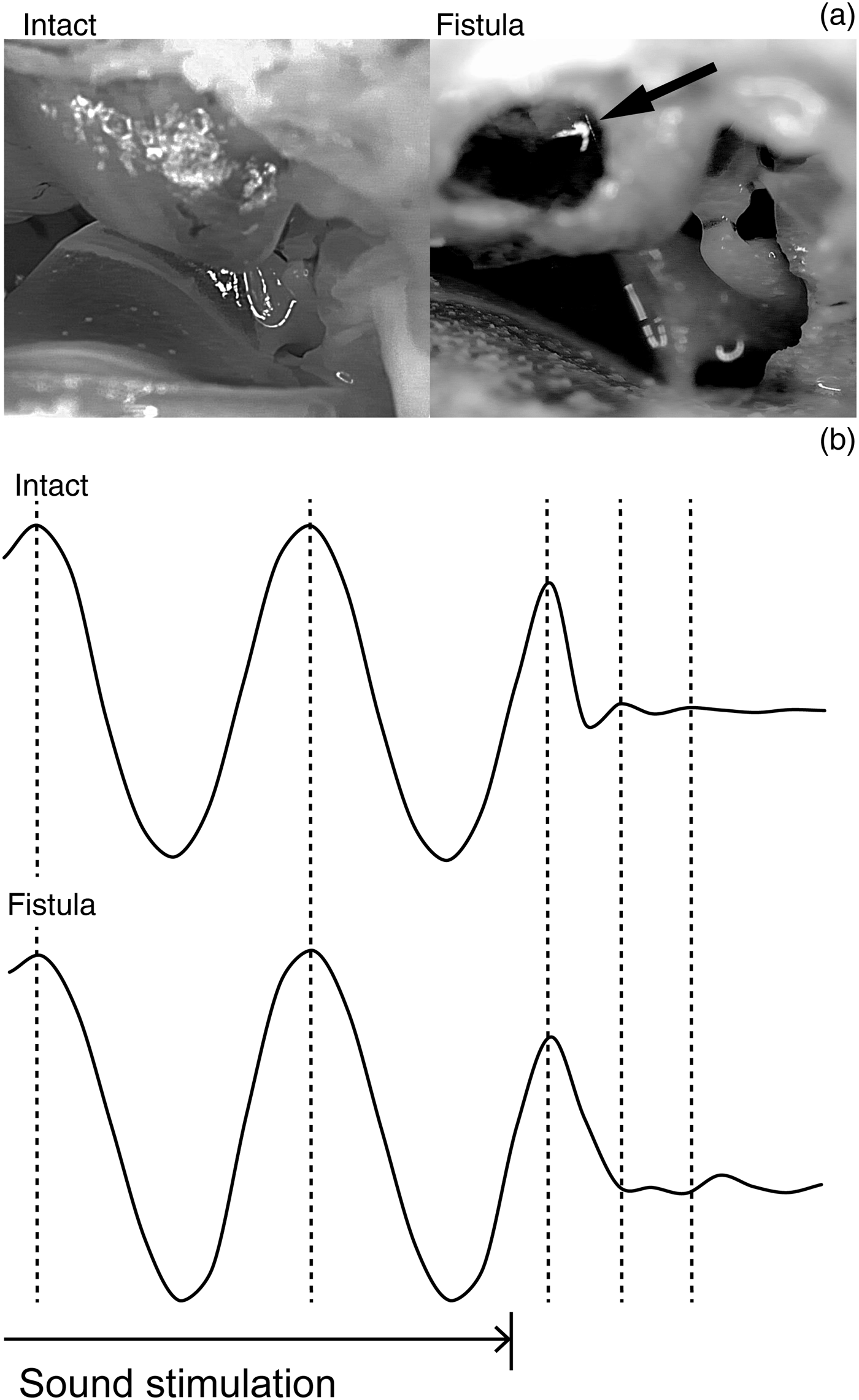
The stapes showed a weakening oscillation before stopping after the sound stimulation had stopped (Figure 1). The waveform of this oscillation resembled a damped sine wave. The frequency of this damped oscillation, which was 500–664 Hz, was specific to the specimen, and was independent of the input sound frequency (Figure 2, Table I). When a fistula was created at the vestibule and the labyrinthine fluid was removed, the same stapes no longer showed this damped oscillation (Figure 3).

Fig. 2 Damped oscillation of the stapes immediately after it was stimulated by various input frequencies. All four waveforms were from the same specimen. The stapes during the sound stimulation oscillated at the same frequency as the input stimulation, and then oscillated at the specimen-specific frequency after the stimulus had stopped.

Fig. 3 Stapedial motion before and after injury of the bony labyrinth. (a) A photograph of a specimen before (Intact) and after (Fistula) the bony labyrinth was injured. The arrow indicates the fistula. (b) The sound-induced stapedial motion and the damped oscillation after the sound stimulation stopped. The waveforms were from the same specimen.
Table I The frequency of the oscillation during damping

All numbers are in Hz.
Discussion
The stapes shows a force-specific movement when oscillated actively by an external energy, such as sound. When the energy input stops, the stapes will continue to move passively according to the inertia of the last move under the force. Then the stapes will show a system-specific damped oscillation when there is no external force. This characteristic oscillation depends primarily on the mass, the spring constant, the damping constant and the friction of the oscillating system. Therefore, the characteristic physical properties of the middle ear can be revealed if this passive damped oscillation is precisely analysed. We herein focused on the frequency of this characteristic damped oscillation.
The main question may be whether this oscillation is a passive movement after the sound stimulation or an artifact caused by a remaining force in the external ear. A weakening reverberation and/or a standing wave in the external ear can vibrate the stapes in a way that mimics a passive damped oscillation. However, our results showed that a remaining force was unlikely to be the cause of the oscillation after the sound stimulation. Firstly, the oscillating frequency during the damping motion was constant within each specimen, and was independent of the input sound frequency. Thus, a reverberation in the external ear was ruled out. Secondly, the damping frequency differed among the individual specimens, ranging from 500 to 664 Hz. Thus, a reverberation in the earphone was ruled out, because the frequency was not specific to the experimental setup. Finally, the damped oscillation was no longer observed when the bony labyrinth was injured. Removal of the inner ear fluid through a labyrinthine fistula decreased the mass of the oscillating system. Under a remaining external force, a lighter oscillating system should be more prone to oscillate. On the other hand, if the oscillation is passive, a lighter oscillating system should show a different oscillating frequency, as was seen in our experimental setup (Figure 3). Thus, we concluded that the stapedial motion after the sound stimulation was indeed a passive, damped oscillation.
The resonant frequency of an oscillating system usually has the same value as the characteristic frequency during a passive oscillation. In our study, the frequency of the damped oscillation was 500–664 Hz, which we consider to represent a characteristic frequency. These values are lower than the sound frequency that evoked the maximum movement of the stapes in cadaveric human specimens (about 1 kHz) measured by laser Doppler vibrometry.Reference Chien, Rosowski, Ravicz, Rauch, Smullen and Merchant3 The values are also lower than the resonant frequency measured using multiple frequency tympanometry on the guinea pig middle ear, which was 1211 ± 195 Hz in a condition similar to that used in our experimental protocol.Reference Darrouzet, Dulon and Franco-Vidal4 We do not have a full explanation for these differences at the moment. However, the middle ear is a complex oscillating system consisting of multiple springs (the tympanic membrane, joints, ligaments and round window membrane) and masses (tympanic membrane, ossicles, labyrinthine fluid and round window membrane), and it is possible that we have observed only one of the multiple characteristic frequencies that the oscillating system has.
Conclusion
We have shown that a high-speed video analysis of a near-intact middle ear is feasible, and may reveal physical properties of the oscillating system in a simple and straightforward experiment. Refinement of this system with a higher acquisition speed and a better signal-to-noise ratio may enhance basic and clinical research on the middle ear in a more intuitive way.
Financial support
This study was supported by JSPS KAKENHI (Grant No. 24500549) to NM.