Introduction
Head and neck cancer encompasses a heterogeneous selection of cancers, with prognosis depending on anatomical site, staging and various epidemiological factors.Reference Longo and Chow1 They include cancers of the sinuses and nasal cavity, oral cavity, pharynx, larynx and salivary glands. In 2018, there were over 800 000 new cases of head and neck cancer accounting for 4.9 per cent of global cancer cases, 4.8 per cent of cancer deaths and making it the 7th most common cancer worldwide.Reference Bray, Ferlay, Soerjomataram, Siegel, Torre and Jemal2
Despite being relatively common, public awareness is poor. The public generally underestimates incidence, are unaware that smoking and alcohol are significant risk factors, and do not appreciate the significance of red flag symptoms.Reference Montgomery, Douglas, Begbie, Hitchings and MacKenzie3 The onset of the coronavirus disease 2019 (Covid-19) pandemic and lockdown in March 2020 in the UK caused significant disruption to out-patient services. This was associated with a sudden reduction in urgent cancer referrals that remained below pre-pandemic levels until August 2020.4 It is likely that, in addition to disrupted services, this resulted from patients’ reluctance to seek medical attention. In the USA, an estimated 41 per cent of patients avoided emergency and routine medical care because of concerns regarding Covid-19.Reference Czeisler, Marynak, Clarke, Salah, Shakya and Thierry5
A combination of poor awareness of red flag symptoms, pandemic-related healthcare avoidance and disrupted out-patient services has fuelled concerns that there may be particularly significant delays in the presentation of patients with head and neck cancer.Reference Werner, Carey, Albergotti, Lukens and Brody6,Reference Sud, Jones, Broggio, Loveday, Torr and Garrett7
Head and neck tumours have been found to double in size in around 3 months, and even a single month delay in treatment increases tumour–node–metastasis (TNM) staging and can reduce rate of local control by 10 per cent.Reference Jensen, Nellemann and Overgaard8–Reference Wyatt, Beddoe and Dale10 Higher staging is associated with worse prognosis, and the need for more aggressive therapeutic options.Reference O'Sullivan, Huang, Su, Garden, Sturgis and Dahlstrom11,Reference Adelstein, Gillison, Pfister, Spencer, Adkins and Brizel12
UK national population-based modelling has suggested disruption to health services will cause substantial increases in avoidable cancer deaths,Reference Maringe, Spicer, Morris, Purushotham, Nolte and Sullivan13 although the true impact of the pandemic is unknown. Assessing excess head and neck cancer mortality and morbidity attributable to the pandemic will take many years. However, we can use indicators to predict likely trends, including the initial staging of disease, incidence of emergency hospital admissions and whether initial management had curative or palliative intent. We aim to assess whether the Covid-19 pandemic has been associated with delays to patient presentation by investigating these before and after the pandemic.
Materials and methods
Setting and participants
This retrospective cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology guideline for reporting cohort studies. It involved the extraction of routinely documented clinical information and received local clinical governance approval. Participants were identified from prospectively collected records of patients discussed at two regular regional head and neck cancer tumour board meetings, to which all patients with confirmed head and neck cancer in West Scotland are routinely referred. This centralised service captures all newly diagnosed head and neck cancer patients in West Scotland, an area with a population of 2.5 million people.14 Tumour board records from June to October of 2019 and 2020 were reviewed for the study; these months were chosen to capture the impact of the pandemic, which became prevalent in the UK in March 2020 and account for the lag between referral date and tumour board discussion. From these records, participants were identified for inclusion if they had a new diagnosis of head and neck cancer (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes C00–C14, C30–C32). Those presenting with recurrent disease were excluded, in addition to those with lymphoma, skin cancer, melanoma and thyroid cancer, which are managed through other dedicated referral pathways and tumour boards.
Data collection
For eligible patients, data were extracted from the tumour board database and centrally stored electronic health records. These were collated in Excel® spreadsheet software (version 12.0) and were anonymised, with participants allocated unique identification numbers. Basic demographic information extracted included age, sex and Scottish Index of Multiple Deprivation quintile. Scottish Index of Multiple Deprivation is a measure of relative socioeconomic deprivation in Scotland, linked to patients by postcode. For the purposes of this study, rankings were consolidated into quintiles of relative deprivation. Clinical information extracted included history of smoking or alcohol excess as defined by the referring clinician, Eastern Cooperative Oncology Group performance status, symptom duration, TNM and American Joint Committee on Cancer head and neck cancer staging (8th edition) at presentation, cancer subsite, and intended treatment. Symptom duration was defined as clinician-estimated duration in weeks of red flag symptoms at time of referral. Common red flag symptoms of head and neck cancer include unexplained head and neck lumps, hoarseness, oral ulceration and throat pain.15
Data analysis
Statistical analysis was performed using SPSS® statistical software (version 27.0). Descriptive statistics were used to summarise patient characteristics including basic demographic data, tumour subsites, American Joint Committee on Cancer staging, and symptom duration in both 2019 and 2020 cohorts. The primary outcome of this study was American Joint Committee on Cancer staging at time of first multidisciplinary team (MDT) review. Univariate analysis was conducted on year of presentation as well as potential covariables. These included age, sex, cancer subsite, Scottish Index of Multiple Deprivation score, Eastern Cooperative Oncology Group performance status, smoking and alcohol history, which were chosen because of their association with developing head and neck cancer or having poorer outcomes from it.16,17 Only cancers of the oral cavity, larynx and cancer of unknown primary significantly differed in incidence between 2019 and 2020 cohorts and were included in univariate analysis. Univariate analysis was performed using the Mann–Whitney U test for dichotomous variables, the Kruskal–Wallis test for ordinal variables and the Spearman's rank test for continuous variables. In order to preserve sample size, only covariables with statistically significant (p < 0.05) results in univariate analysis were included in multivariate analysis, which employed ordinal logistic regression assuming a proportional odds model.
Symptom duration was investigated in a similar manner to American Joint Committee on Cancer staging involving screening of potential covariables and multivariate analysis with ordinal logistic regression. Other secondary outcomes included emergency presentation, emergency tracheostomy and decision for palliative management at first presentation, which were compared using chi-squared tests of homogeneity. Further analysis to calculate the association between these outcomes and year of presentation was performed using Spearman's rank test. Participants with missing data for an outcome were excluded from the analysis of that outcome.
Results
Patient demographic data
A total of 528 patients met eligibility criteria and were included in the study, with 250 patients in 2019 and 278 patients in 2020 (Table 1). With a mean age of 65 years and 70 per cent of patients being male, the demographic data of this group were comparable with that of previously described head and neck cancer populations.17 Most patients came from higher areas of social deprivation, with 38 per cent coming from the most deprived areas in Scotland. The majority had a history of smoking (74 per cent) and around a third had a history of drinking alcohol to excess. A total of 75 per cent of patients were performance status 0 or 1 at time of referral. These demographic data were similar between 2019 and 2020 cohorts.
Table 1. Patient demographic data, smoking and alcohol history and performance status*

* Numbers do not add to 100 per cent because of rounding
Cancer demographic information
Oropharyngeal (30 per cent), oral (28 per cent) and laryngeal (20 per cent) were the most common cancer types, followed by hypopharyngeal (8 per cent), salivary gland (3 per cent) and nasopharyngeal cancer (3 per cent) (Figure 1). In 2020, laryngeal cancer presented less commonly (odds ratio, 0.569; 95 per cent confidence interval (CI), 0.379 to 0.855, p = 0.006) and oral cancer presented more commonly (odds ratio, 1.714; 95 per cent CI = 1.162 to 2.527; p = 0.006). Cancer of unknown primary was also more common in 2020 (odds ratio, 3.261; 95 per cent CI = 1.059 to 10.043; p = 0.03). The proportions of other cancer subsites did not differ significantly between cohorts. Patients most commonly (29 per cent) presented with T4a disease, with T1, T2 or T3 disease each accounting for a further 21–22 per cent of the population (Table 2). Overall, 4 per cent of patients presented with T4b tumours. The majority (54 per cent) of patients had nodal spread at time of first investigation, and only a small proportion (4 per cent) had distant metastasis.
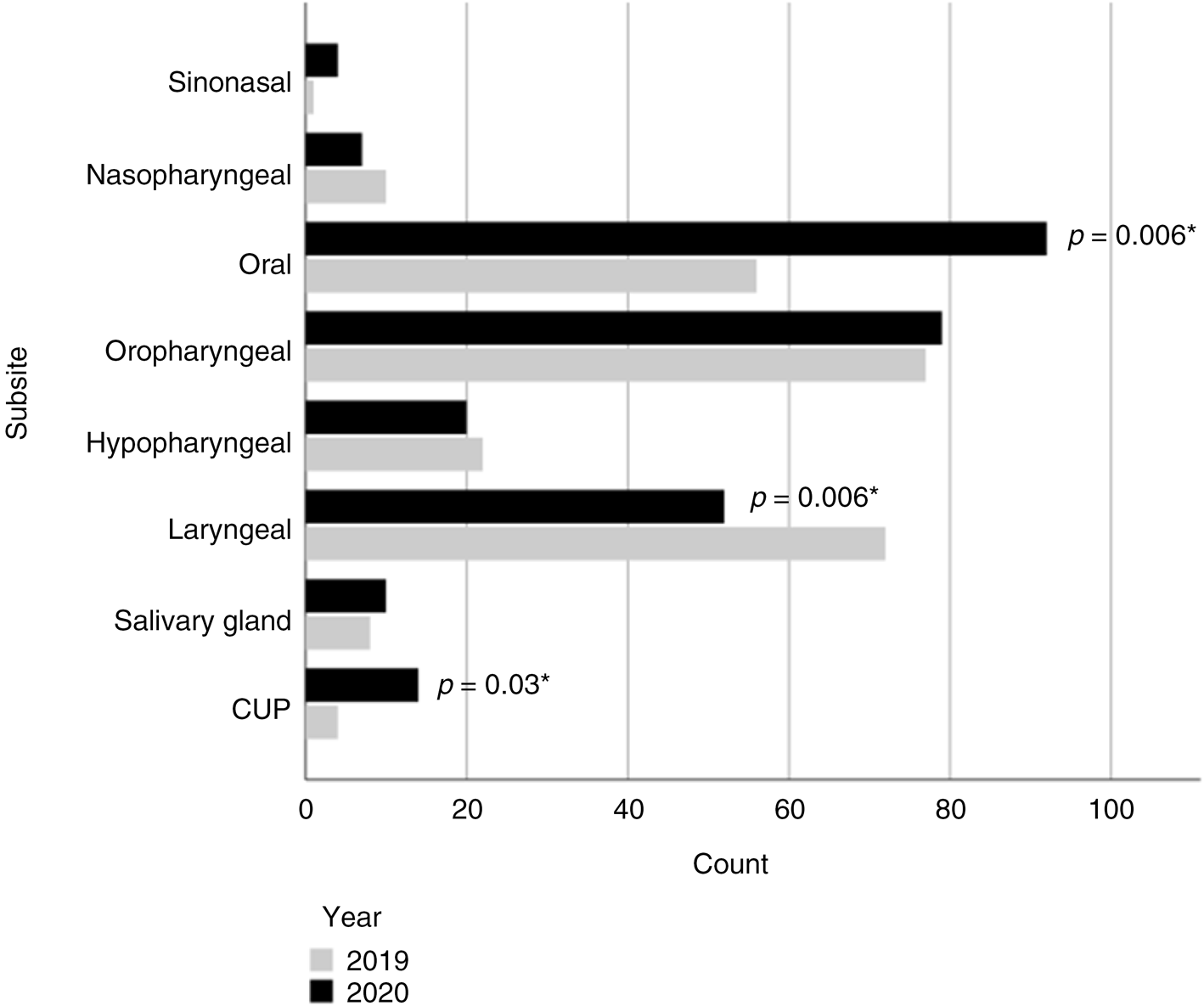
Fig. 1. Counts of cancer by subsite in 2019 and 2020. *Chi-squared test for independence. CUP = cancer of unknown primary
Table 2. Tumour characteristics*
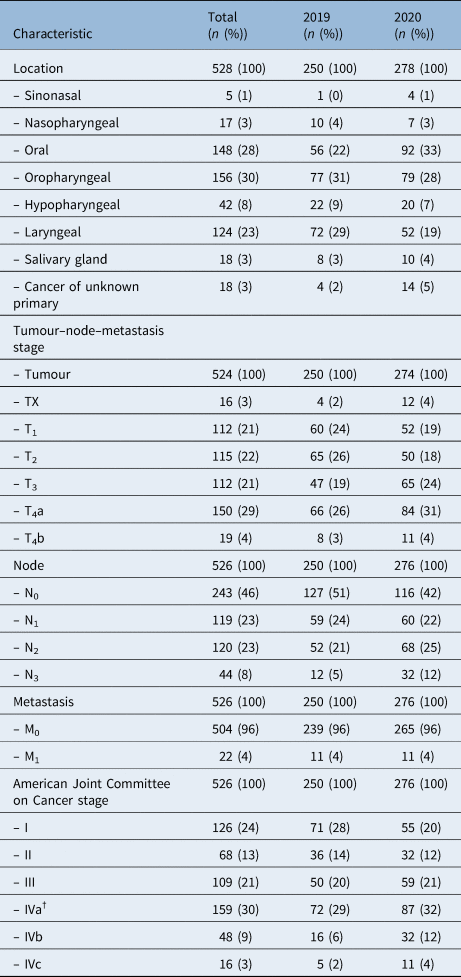
* Numbers do not add to 100 per cent because of rounding; †human papilloma virus positive oropharyngeal cancers stage IV are equated to IVa for summary
Staging at presentation
Overall, most patients (42 per cent) presented with stage IV disease, with 24 per cent having stage I, 13 per cent stage II and 21 per cent stage III disease. Data were missing on staging for two patients who were excluded from this analysis. Univariate analysis found age, performance status, alcohol and smoking history, and cancer of unknown primary to be predictive of American Joint Committee on Cancer staging (p < 0.05), and these were identified for inclusion in the multivariate testing model (Table 3). Male sex, Scottish Index of Multiple Deprivation, laryngeal and oral cancer were not found to be predictive (Table 4).
Table 3. Univariate analysis of explanatory factors for American Joint Committee on Cancer staging
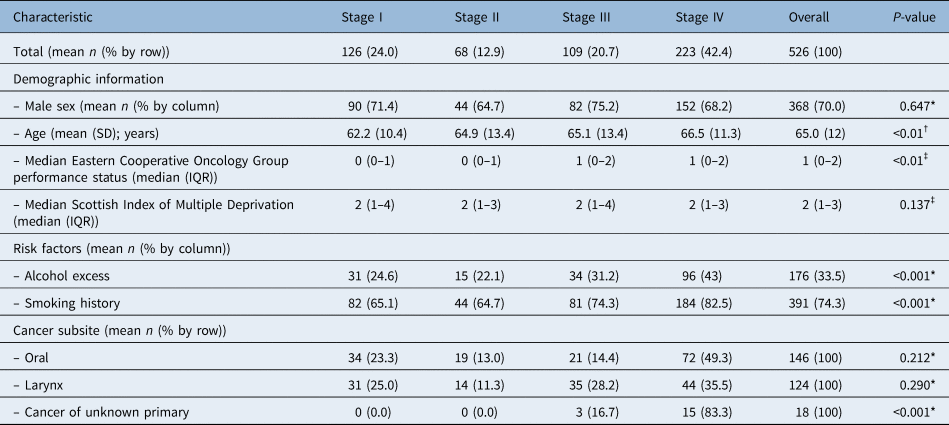
*Mann–Whitney U testing; †Spearman's correlation testing; ‡ Kruskal–Wallis H testing. IQR = interquartile range
Table 4. Univariate and multivariate ordinal regression analysis of explanatory factors for American Joint Committee on Cancer staging*

* Odds ratios provided for a one stage increase in American Joint Committee on Cancer staging. Model 1 is unadjusted. Model 2 is adjusted for age and Eastern Cooperative Oncology Group performance status. Model 3 is adjusted for age, Eastern Cooperative Oncology Group performance status, smoking and alcohol history, and cancer of unknown primary. OR = odds ratio; CI = confidence interval
Multivariate modelling was performed using cumulative odds ordinal logistic regression with proportional odds. Each model predicted American Joint Committee on Cancer stage over and above the intercept model with statistical significance (p < 0.01) and met the assumption of proportional odds after a full likelihood ratio test comparing the proportional odds model fit to a model with varying location parameters. Model 1 represents unadjusted testing of year of presentation against American Joint Committee on Cancer staging. In Model 2, the multivariate model was adjusted for demographic data including age and Eastern Cooperative Oncology Group performance status. In Model 3, all identified covariables including age, performance status, alcohol and smoking history, and cancer of unknown primary were adjusted for. Odds ratios were calculated for a one stage greater cancer. The final model found the odds of presenting with a greater stage cancer was significantly higher in 2020 (p = 0.002) with an odds ratio of 1.67 (95 per cent CI = 1.20–2.31).
Symptom duration at presentation
Overall, patients most commonly (38 per cent) presented with more than 12 weeks symptom duration which is unsurprising as a wide ranging, open-ended category. A quarter presented with an 8–11 week history of symptoms, and 23 per cent presented with a 4–7 week history of symptoms. A total of 13 per cent of patients presented with three weeks of symptoms or fewer. Data were missing on symptom duration for 72 patients who were excluded from this analysis. Univariate analysis found only laryngeal cancer to be predictive of symptom duration (p = 0.007) (Table 5). Multivariate modelling was performed using cumulative odds ordinal logistic regression with proportional odds (Table 6). Each model predicted American Joint Committee on Cancer stage over and above the intercept model with statistical significance (p < 0.01) and met the assumption of proportional odds after a full likelihood ratio test comparing the proportional odds model fit to a model with varying location parameters. Model 1 represents unadjusted testing of year of presentation against symptom duration. Model 2 adjusted for basic demographic data including age and sex. Model 3 adjusted for these demographic data and the presence of laryngeal cancer. Odds ratios were calculated for a one month increase in symptom duration. The final model found the odds of presenting with a longer symptom duration was significantly higher in 2020 (p < 0.001) with an odds ratio of 2.03 (95 per cent CI = 1.44–2.87).
Table 5. Univariate analysis of explanatory factors for symptom duration
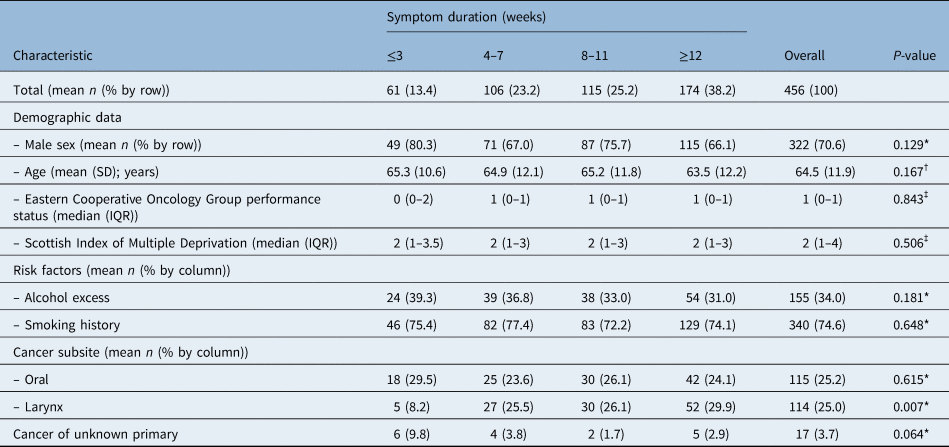
*Mann–Whitney U testing; †Spearman's correlation testing; ‡Kruskal–Wallis H testing. SD = standard deviation; IQR = interquartile range
Table 6. Univariate and multivariate ordinal regression analysis of explanatory factors for symptom duration*

OR = odds ratio; CI = confidence interval
Emergency admissions, tracheostomies and palliation
Overall, a minority of patients (6.3 per cent) were referred following a related emergency hospital admission attributed to head and neck cancer complications or sequela (Table 7). Emergency presentations were significantly more common in 2020 (p = 0.017) with an odds ratio of 2.53 (95 per cent CI = 1.15–5.55). However, there was no significant change in the proportion of emergency tracheostomy procedures performed each year, occurring in 2.5 per cent of patients overall. At the initial MDT, treatment strategies are defined as curative or palliative. In 35 per cent of cases, initial treatment strategy was palliative in nature, and this did not differ statistically significantly between cohorts.
Table 7. Emergency admissions, tracheostomy procedures and palliation in 2019 and 2020

*n = 528; †n = 250; ‡n = 278; **chi-squared test of homogeneity. CI = confidence interval
Discussion
The public health emergency caused by the Covid-19 pandemic resulted in major societal disruption. Traditional public health messaging was overridden, and the public was asked to stay at home to both prevent transmission of Covid-19 and reduce pressure on health systems. Although societal restrictions have been eased in the UK, and life in many sectors has returned to normal, healthcare continues to face a crisis. This is not only from the ongoing coronavirus burden but a hangover of a myriad of other health issues neglected during the pandemic, notably cancer. Delays in the treatment of head and neck cancers are particularly worrisome. A single month delay in treatment for stage I, II and III head and neck cancer increases 5-year mortality (hazard ratio, 1.061–1.161, varying by stage of disease).Reference Hartman, Sun, Devasia, Chase, Jairath and Dess18
In the absence of routine screening services for most head and neck cancers, early diagnosis and treatment relies on patient or primary care doctor recognition and referral of red flag symptoms. Post-pandemic, patients in our region have a two times greater odds of an extra month of symptom duration by time of referral. A study in Turkey also reported increases in time that head and neck cancer patients spent with symptoms, although this was measured from date of admission, not referral, and could also be explained by delays to secondary service provision.Reference Tevetoğlu, Kara, Aliyeva, Yıldırım and Yener19
Consistent with delay in presentation and the relatively rapid growth of head and neck cancer, we have found the odds of a higher American Joint Committee on Cancer stage to be 1.67 times higher following the pandemic. Smaller unadjusted analyses have shown similar concerning trends in the USAReference Kiong, Diaz, Gross, Diaz and Hanna20 and Europe.Reference Tevetoğlu, Kara, Aliyeva, Yıldırım and Yener19,Reference Wilkie, Gaskell, Hall, Jones and Kinshuck21,Reference Iqbal, Uzzaman, Fox, Munro and Kelly22 Although the true impact of the pandemic on head and neck cancer will be unknown for some years when information on mortality is available, morbidity is already demonstrably on the rise. We found the odds of emergency admission arising from complications of head and neck cancer to be 2.53 times greater following the pandemic. One other UK institution reported a similar rise in emergency admissions with significantly higher rates of emergency tracheostomy procedures.Reference Wilkie, Gaskell, Hall, Jones and Kinshuck21 We did not find a difference in the odds of emergency tracheostomy procedures, although this was perhaps obscured by a lower proportion of laryngeal cancer seen in 2020, which is typically associated with airway emergencies, and a higher proportion of oral cancer which is not.
Oral cancer is often diagnosed by dental practitioners as chronic ulceration. The higher rates of diagnosis of oral cancer in 2020 that were found in our study may reflect a backlog of diagnosis because of the closure of dental practices from March and reopening in June of 202023 when our data collection began. An explanation for higher rates of cancer of unknown primary found in 2020 is less clear. Although general practitioner practices remained open during the pandemic, between April and July around 83–89 per cent of consultations were by telephone, compared with 30–31 per cent in the same period in 2019.Reference Murphy, Scott, Salisbury, Turner, Scott and Denholm24 Cancer of unknown primary in the head and neck often presents as a persistent neck lump, and it is possible a move to telephone consultations may have delayed examination findings that would prompt head and neck cancer referral. This backlog may then have been picked up following increasing face-to-face and e-video consultations later in 2020.Reference Turner, Scott, Horwood, Salisbury, Denholm and Scott25
• The public health emergency caused by the coronavirus disease 2019 pandemic created major societal disruption
• Small datasets found that emergency presentations of head and neck cancer are more common after the pandemic
• It is unknown whether patients have longer symptomatic periods before presenting following the pandemic
• There is a lack of evidence of how presentations have changed following the pandemic
• This retrospective study compared over 500 pre- and intra-pandemic head and neck cancer presentations
• Following the pandemic, patients have been presenting with more advanced head and neck cancer
Although a backlog of delayed cancer presentations is perhaps unsurprising following lockdown, it is unknown whether these delays are likely to be temporary or long lasting. Even prior to the pandemic, head and neck cancer care suffered from poor public awareness of potential red flag symptoms.Reference Montgomery, Douglas, Begbie, Hitchings and MacKenzie3 Following over a year of overshadowing or absence of cancer public health warnings during the pandemic, it is likely this awareness is worse now. Combined with ongoing coronavirus transmission and potential associated public reluctance or difficulty in accessing primary care, it is prudent to assume ongoing delay in presentation of patients with head and neck cancer. Public health campaigns addressing red flag symptoms of gastrointestinal, urological and respiratory carcinoma have restarted.26 Similar efforts need to be made for head and neck cancer to accelerate the presentation of patients and reduce excess morbidity, mortality and the burden of emergency admissions.
Limitations
Head and neck cancer represents a heterogeneous group of cancers in which the natural and clinical history may vary significantly. Further subgroup analysis of cancer of specific anatomical locations could explain, reveal, or demonstrate differing trends in the data. However, adjustments for cancer type were made in multivariate analyses of American Joint Committee on Cancer stage and symptom duration.
This was a retrospective, observational study, and as such a causative effect of the pandemic and lockdown on changes in the data between 2019 and 2020 can only be assumed and not proven. There may be alternative explanations currently unknown. Measurement of symptom duration may be particularly at risk of recall bias when reported by patients or documented by clinicians on referrals; however, there is no obvious suggestion that this bias might vary in extent pre- and post-pandemic. This study also compared only 2019 and 2020 and would not have taken into account potential pre-pandemic trends in head and neck cancer epidemiology.
Generalisability
This study represents the population of 2.5 million people of West Scotland; however, frequencies and types of individual head and neck cancers vary worldwide. This study does have findings consistent with similar studies in the literature from Europe and the USA and offers a significantly larger sample size compared with similar studies of its type to date.Reference Tevetoğlu, Kara, Aliyeva, Yıldırım and Yener19–Reference Wilkie, Gaskell, Hall, Jones and Kinshuck21 Societal impacts of the pandemic and public health measures will also vary both in extent and chronologically between nations. These studies may also offer some foresight to other countries such as New Zealand that are yet to experience the full weight of a similar pandemic.
Conclusion
Patients are presenting later and with more advanced head and neck cancer during the Covid-19 pandemic, which may reflect pandemic-related reluctance or difficulty for patients in engaging with primary healthcare. Although survival outcomes will not be known for some time, modelling already predicts delays in treatment will worsen head and neck cancer related mortality. Concerted public health measures are warranted to encourage earlier patient presentation with red flag symptoms to reduce avoidable morbidity and mortality associated with late presentation of head and neck cancer.
Competing interests
None declared










