Introduction
The inner ear is composed of two main parts: the cochlea and the vestibule. Vestibular function is controlled by the semicircular canals and the otoliths (in the utricle and the saccule). Auditory brainstem response (ABR) testing assesses the auditory pathway comprising the cochlea, cochlear nerve and brainstem. The caloric test and the rotatory chair test assess the activity of the semicircular canals and the superior vestibular nerve.Reference Sazgar, Akrami, Akrami and Karimi Yazdi1
Kato et al. Reference Kato, Shiraishi, Eura, Shibata, Sakata and Morizono2 reported that a large, negative deflection with a latency of 3 milliseconds could be recorded in patients with profound hearing loss. A peculiar, V-shaped, acoustically evoked, short latency negative response was also recorded at approximately 3 to 4 milliseconds during ABR testing.Reference Nong, Ura and Owa3 This acoustically evoked, short latency negative response was present only in ears with profound hearing loss, under intense stimulation. Its neural response characteristics included the occurrence of a shortened latency and an increased amplitude when the stimulus intensity was increased.
Acoustically evoked, short latency negative responses were not interpreted as potentials generated from the conventional auditory pathway, because their peculiar, V-shaped waveforms obviously differed from those associated with ABR. However, acoustically evoked, short latency negative responses morphologically resemble vestibular evoked myogenic potentials.Reference Hausler, Kasper and Demierre4
Ears with acoustically evoked, short latency negative responses have been found to have good vestibular function, in sharp contrast to their profoundly impaired hearing. This suggests a possible relation between acoustically evoked, short latency negative responses and the vestibular system. Both acoustically evoked, short latency negative responses and vestibular evoked myogenic potentials are acoustically evoked, non-auditory responses; therefore, they may reflect different neuronal activities along the same neural pathway.
The acoustically evoked, short latency negative response may be of vestibular, particularly saccular, origin, as are vestibular evoked myogenic potentials.Reference Ochi, Ohashi and Nishino5 Considering their short latency (3 to 4 milliseconds), Nong et al. Reference Nong, Ura, Kyuna, Owa and Noda6 speculated that acoustically evoked, short latency negative responses represented one type of vestibular evoked potential emanating from the second-order neurons at the lower part of the brainstem. The saccule and vestibular nuclei are hypothesised to be the sense organ and the generator of the acoustically evoked, short latency negative response. This hypothesis explains why acoustically evoked, short latency negative responses appear exclusively in ears with normal vestibular function and profoundly impaired hearing, which are free from the superimposition of ABR waves I to V.
Routine examination of infants' inner ears does not include vestibular tests, as these are particularly difficult to perform in this age group and their results may be less than reliable. Only some of the vestibular tests performed in adults can be used in young children.Reference Zagolski7
Vestibular evoked myogenic potentials reflect the integrity of the sacculo-collic reflex pathways, and are recorded on the surface of the neck muscles after intense acoustic stimulation of the ear.Reference Zagolski and Jurkiewicz8 They are polysynaptic responses of otolith and vestibular nerve origin, and are now accepted as reliable tests with which to assess the function of the saccule. The afferent pathway is the inferior vestibular nerve and the efferent pathway is the vestibulospinal tract.Reference Brantberg and Fransson9
Caloric stimulation involves introduction of cold water into the external ear canal together with direct observation of nystagmus, and is the only reliable diagnostic tool used to assess the function of the lateral semicircular canal in young children.Reference Vatovec, Velikovic, Smid, Brenk and Zargi10 Vestibular evoked myogenic potential and caloric testing is difficult to perform in infants, due to lack of cooperation between the subject and the examiner.
Aims
The purpose of the current study was to investigate the presence of the acoustically evoked, short latency negative response, and its use as an objective tool with which to assess saccular function, in children with severe and profound hearing loss. The study also aimed to investigate the relationship between the acoustically evoked, short latency negative response and the vestibular evoked myogenic potential in such children.
Materials and methods
Twenty-three children suffering bilateral, symmetrical, severe to profound, sensorineural hearing loss (SNHL) were included in this study. Work was performed at the audiology unit of the ENT department of Tanta University Hospital, Tanta, Egypt. Written consent was obtained from the parents of the participating children. All patients underwent: (1) full audiological history-taking and otological examination; (2) basic audiological evaluation (including pure tone audiometry, speech audiometry and immittance testing); (3) ABR testing; (4) vestibular evoked myogenic potential testing; and (5) caloric testing.
Auditory brainstem response
Auditory brainstem response testing was elicited by clicks through insert headphones, using the Smart-EP TM, Intelligent Hearing system (Smart-EP TM, Intelligent Hearing system, Miami, USA). Each ear was stimulated separately. Testing used rarefaction clicks with a duration of 100 milliseconds; 2000 sweeps were used, with a repetition rate of 21.1 pulses/second at the intensities 100 and 90 dBnHL. The time window was 10 milliseconds. The active electrode was placed in the centre of the forehead, the reference electrode was located on the ipsilateral mastoid and the ground electrode was placed on the contralateral mastoid.
Detection of acoustically evoked, short latency negative responses was based on the method of Murofushi et al. Reference Murofushi, Iwasaki, Takai and Takegoshi11 An acoustically evoked, short latency negative response was defined as a negative peak occurring under the following conditions. Firstly, the peak at the vertex to the ipsilateral mastoid should be reproducible. Secondly, the peak should appear 3–5 milliseconds after the onset of stimulation. Thirdly, the onset to peak amplitude (with onset defined as the starting point of the deflection toward the negative peak) should be more than 0.05 mV. If there were two or more acoustically evoked, short latency negative responses, we regarded the largest peak as the definitive response. Fourthly, acoustically evoked, short latency negative responses became absent after external auditory canal occlusion, which blocked air conduction without influencing scalp potentials.Reference Ochi and Ohashi12
Vestibular evoked myogenic potential
Vestibular evoked myogenic potential testing was performed using the Smart-EPTM, Intelligent Hearing system, Miami, USA. Monaurally delivered rarefaction clicks were generated according to the method of Cheng et al. Reference Cheng, Huang and Young13 The response was recorded using surface electrodes placed over the isometrically contracting sternocleidomastoid muscle. Contraction of the muscles was obtained by bending the child's head slightly backwards and holding it in this position, which provoked a relatively strong reflex muscle contraction, making it possible to record the responses. The middle part of the sternocleidomastoid muscle was chosen as the position of the active electrode, in order to obtain consistent recordings. The negative electrode was placed on the middle part of the clavicle, and the reference electrode on the forehead. Stimuli were delivered via a headphone at 95 dBnHL, with a repetition rate of one per second. The analysis time was 50 milliseconds. The signals were amplified and band pass filtered between 30 and 3000 Hz. Responses to 128 stimuli were averaged, and each response was obtained three times to ensure reproducibility. This biphasic VEMP wave P13 and N23 represented the largest response wave within an analysis time of 50 milliseconds. Electromyographic activity was monitored during recording to ensure that muscle activity was at a constant level. In the study clinic, the normative ranges for the P13 and N23 waves were 11.7–13.4 and 21.9–24.1 milliseconds, respectively.
Caloric test
Eye movements were recorded using infrared videonystagmography (GN Otometrics ICS VNG/ENG system; ICS mode-NCI-480 with NCA200 Caloric Stimulator, Taastrup, Denmark). Cool water irrigations with a temperature 20°C were applied to each ear and the maximum velocity of the slow component of nystagmus is determined. Bilateral caloric hypofunction was considered if the SPVs were less than 22°/second.
Statistical analysis
Patients were classified according to the presence of acoustically evoked, short latency negative responses, into response and non-response groups. Student's t-test was used to compare the two groups. Pearson correlation was used to assess the relationship between acoustically evoked, short latency negative responses and vestibular evoked myogenic potentials. Fisher's exact test was used to assess the relationship between the presence or absence of acoustically evoked, short latency negative responses and vestibular evoked myogenic potentials. The Statistical Package for the Social Sciences version 12 software program was used for statistical analysis.
Results
This study included 23 children with severe to profound SNHL (46 ears). Their ages ranged from 4.25 to 14.0 years, with a mean age ± standard deviation (SD) of 7.19 ± 3.86 years. The mean ± SD of the average pure tone threshold was 89.05 ± 6.7 dBHL. The children's ears were classified into either group I (acoustically evoked, short latency negative responses present) or group II (responses absent). Group I included 14/46 ears (30.43 per cent); the mean ± SD for the acoustically evoked, short latency negative response wave latency was 3.54 ± 0.41 milliseconds, and the mean ± SD amplitude was 0.095 ± 0.05 µV. Group II included 32/46 ears (69.57 per cent). Patients' results are shown in Table I and Figure 1.
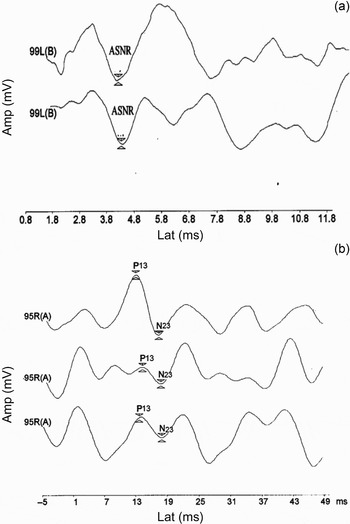
Fig. 1 (a) Acoustically evoked, short latency negative response (ASNR) and (b) vestibular evoked myogenic potential.
Table I Patients' age, PTA, and ASNR and VEMP latencies and amplitudes

PTA = pure tone audiometry; ASNR = acoustically evoked, short latency negative response, VEMP = vestibular evoked myogenic potential; yrs = years; lat = latency; amp = amplitude; R = right; L = left
Vestibular evoked myogenic potentials were recorded in 31/46 ears (67.39 per cent). They were elicited from all ears in group I. In this group, the mean ± SD for wave P13 latency was 12.23 ± 2.6 milliseconds, with a mean ± SD amplitude of 1.43 ± 0.9 µV. The mean ± SD wave N23 latency was 20.94 ± 2.3 milliseconds, with a mean ± SD amplitude of 1.71 ± 1.2 µV. In group II, vestibular evoked myogenic potentials were present in only 17/32 ears (53.13 per cent). The mean ± SD for wave P13 latency was 12.86 ± 1.4 milliseconds, with a mean amplitude of 1.15 ± 0.6 µV. The mean ± SD wave N23 latency was 22.03 ± 2.2 milliseconds, with a mean ± SD amplitude of 1.03 ± 0.4 µV. No significant difference was found between the two groups as regards P13 wave latency and amplitude and N23 wave latency. The mean N23 wave amplitude was significantly larger in group II than in group I (Table II). Decreased N23 amplitude in GII (Non-ASNR) group may be attributed to saccular and/or inferior nerve abnormality. Acoustically evoked, short latency negative responses and vestibular evoked myogenic potentials were both absent in 14 ears, and vestibular evoked myogenic potentials were present in only 17 ears. There was a statistically significant relationship between the presence of acoustically evoked, short latency negative responses and vestibular evoked myogenic potentials (p < 0.001) (Table III). There was also a positive correlation between acoustically evoked, short latency negative response latency and vestibular evoked myogenic potential wave P13 and N23 latencies (p < 0.05) (Table IV).
Table II Results for VEMP and caloric testing: group II vs I

* statistically significant difference P ≤ 0.05;. VEMP = vestibular evoked myogenic potential; grp II = ears with acoustically evoked, short latency negative response absent; grp I = ears with acoustically evoked, short latency negative response present; SD = standard deviation; lat = latency; amp = amplitude; R = right; L = left
Table III Presence and absence of VEMP: group II vs group I

* n=32; †n = 14. ‡Fisher's exact test. **statistically significant difference P ≤ 0.05. VEMP = vestibular evoked myogenic potential; grp II = ears with acoustically evoked, short latency negative response absent; grp I = ears with acoustically evoked, short latency negative response present
Table IV Correlation between ASNR and VEMP latencies and amplitudes

* Correlation significant at 0.05 level (two-tailed); †correlation significant at 0.001 level. ASNR = acoustically evoked, short latency negative response; VEMP = vestibular evoked myogenic potential; lat = latency; amp = amplitude
Caloric testing was normal in 38/46 ears (82.6 per cent). Bilateral hypofunction was present in eight of the 46 ears (17.4 per cent). Of these eight ears, one showed both acoustically evoked, short latency negative responses and vestibular evoked myogenic potentials, while another showed only vestibular evoked myogenic potentials; the remaining six (all from group II) showed neither acoustically evoked, short latency negative responses nor vestibular evoked myogenic potentials.
Discussion
In this study of children with severe to profound sensorineural hearing loss, acoustically evoked, short latency negative responses were present in 30.43 per cent of patients. These findings agree with those of Zagolski,Reference Zagolski7 who elicited acoustically evoked, short latency negative responses in 10 out of 34 ears. The current study observed acoustically evoked, short latency negative responses with a mean ± SD latency of 3.54 ± 0.41 milliseconds; this agrees with the findings of Dong et al. Reference Dong, Nong, Masaharu, Asanori, Tatsuhito and Yutaka14 The acoustically evoked, short latency negative response is not interpreted as a potential generated by the conventional auditory pathway.Reference Ochi and Ohashi12
Although the origin of the acoustically evoked, short latency negative response is still unclear, its peculiar waveform lends support to the theory of a non-cochlear origin;Reference Nong, Ura and Owa3 however, the ABR results and the poor hearing in these ears suggests otherwise. In the current study, patients with acoustically evoked, short latency negative responses had good vestibular function, in contrast to their poor hearing. This suggests a relationship between this response and the vestibular system. Nong et al. Reference Nong, Ura, Kyuna, Owa and Noda6 have theorised that the saccule and vestibular nucleus are the sense organ and origin of the acoustically evoked, short latency negative response. It has been shown that, of the vestibular organs, only the otolith organs (especially the saccule) respond to sound stimulation, whereas the semicircular canals do not. On the basis of this observation, the acoustically evoked, short latency negative response is thought to be of saccular origin.Reference Nong, Ura and Owa3
The acoustically evoked, short latency negative response is a negative peak while ABR waves (i.e. waves I–V) are positive peaks; the reason for this opposite polarity is not clear. One possible explanation is as follows. The auditory pathway in the brainstem runs in a mainly ascending fashion to the auditory cortex. On the other hand, the sound-evoked vestibular (saccular) pathway may be mainly descending, because inputs from the saccule mainly project to the vestibulospinal tract.Reference Isu, Graf, Sato, Kushiro, Zakir and Imagawa15, Reference Sato, Imagawa, Isu and Uchino16
In the current study, 31/46 ears (67.39 per cent) showed vestibular evoked myogenic potentials evoked by sound stimulation, implying normal saccular function. These results agreed with those of Ochi et al. Reference Ochi, Ohashi and Nishino5 Vestibular evoked myogenic potentials are dependent on normal vestibular function, as they are abolished after vestibular nerve section. However, the vestibular evoked myogenic potential is independent of cochlear function, as it is preserved in patients with severe to profound SNHL.Reference Colebatch and Halmagyi17 This is supported by Sazgar et al.,Reference Sazgar, Akrami, Akrami and Karimi Yazdi1 who reported that patients with SNHL of more than 40 dB HL showed significantly more saccular deterioration, as indicated by absent vestibular evoked myogenic potential responses. This suggests subclinical disturbances of the vestibular system, and especially of the saccule, in these patients. The underlying mechanism may be simultaneous damage to both the cochlea and the saccule, due to the same factors. However, in contrast to the above results, Zhou et al. Reference Zhou, Kenna, Stevens and Licameli18 reported abnormal vestibular evoked myogenic potentials in 21 of 23 children (91 per cent) with SNHL.
Sheykholeslami et al. Reference Sheykholesami, Kaga, Megerian and Arnold19 successfully obtained vestibular evoked myogenic potential responses in normally developing neonates. The waveform morphology of these responses was found to be similar to that of adults, although latencies appeared shorter and amplitudes more variable compared with adults. Other authorsReference Kelsch, Schaefer and Esquivel20, Reference Valente21 have recorded vestibular evoked myogenic potentials in preschool and preadolescent children, and have found shorter latencies than in adults. These researchers concluded that recording such potentials constituted a well tolerated screening test for vestibular function in children. The characteristics of vestibular responses may vary among infants, due to the impossibility of cooperation with the procedure, which is required in order to optimise results.Reference Akin and Murnane22
• The acoustically evoked, short latency negative response is a large, negative deflection with a latency of 3 milliseconds which has been reported in patients with profound hearing loss
• This study aimed to assess the presence of acoustically evoked, short latency negative responses in children with severe to profound sensorineural hearing loss (SNHL)
• Impairment of saccular function, indicated by abnormal vestibular evoked myogenic potential results, is often associated with SNHL in children
• Although saccular dysfunction may create a vestibular deficit, its manifestations can vary and be easily overlooked in children
Vestibular evoked myogenic potentials were recorded in all ears in group I (acoustically evoked, short latency negative responses present). This result agrees with that of Dong et al.,Reference Dong, Nong, Masaharu, Asanori, Tatsuhito and Yutaka14 who found that vestibular evoked myogenic potentials were evoked in all ears with acoustically evoked, short latency negative responses present; this implies that ears with the latter response have normal saccular function. Vestibular evoked myogenic potentials were recorded in only 17/32 (53.12 per cent) of the ears in group II (acoustically evoked, short latency negative responses absent). These findings are comparable with those of Nong et al.,Reference Nong, Ura, Kyuna, Owa and Noda6 who recorded vestibular evoked myogenic potentials in two-thirds of ears which had an absent acoustically evoked, short latency negative response. There was a relationship between the presence of acoustically evoked, short latency negative responses and vestibular evoked myogenic potentials.Reference Zagolski7 The fact that vestibular evoked myogenic potentials were recorded in all the ears with a recorded acoustically evoked, short latency negative response implies normal saccular function in the ears with the latter finding. This was consistent with the findings of Zagolski.Reference Zagolski7
No spontaneous symptoms of vestibular dysfunction, such as nystagmus, were found in our patients. In addition, a normal reaction to caloric stimulation was obtained bilaterally in 38/46 ears (82.6 per cent). These results disagree with those of Angeli,Reference Angeli23 who reported an estimated incidence of semicircular canal disorders in infants of approximately 20–70 per cent, this figure being higher in children with profound SNHL.
The acoustically evoked, short latency negative response could be of vestibular, especially saccular, origin. If the acoustically evoked, short latency negative response was of vestibular origin, new information would be gained by measuring this response in addition to the vestibular evoked myogenic potential? The acoustically evoked, short latency negative response may be recorded in patients who cannot contract their neck muscles due to their age, mental state or level of consciousness. When used in combination with vestibular evoked myogenic potentials, acoustically evoked, short latency negative responses may be useful in the detection of lesion sites, because the central nervous system pathway of the acoustically evoked, short latency negative response may be somewhat different from that of the vestibular evoked myogenic potential.
Conclusion
Impairment of saccular function, indicated by abnormal vestibular evoked myogenic potential findings, is often associated with SNHL in the paediatric population. Although saccular dysfunction may create a vestibular deficit, its manifestations can vary and be easily overlooked in children. The results of this study suggest the necessity of thorough, routine examination of not only hearing but also vestibular function in children with severe and profound SNHL.
Thus far, acoustically evoked, short latency negative responses have been recorded only in patients with peripheral, profound hearing loss. If acoustically evoked, short latency negative responses can be recorded in subjects with preserved hearing, such testing might constitute a new clinical test of the vestibular system.







