Introduction
Baird's tapir Tapirus bairdii (Gill, 1865), is the largest mammal of the Neotropics known today. This perissodactyl species inhabits woods and wetlands, ranging from 0 to 2000 metres above sea level. However, due to habitat loss and hunting pressure, the species is endangered and its present distribution is fragmented, from southern Oaxaca State in Mexico to northern Colombia (Botello et al., Reference Botello, Sánchez-Hernández, Hernández, Reyes-Chávez and Sánchez-Cordero2014).
Despite its important ecological role in tropical forests and its wide distribution, parasitological studies of this mammal are scarce. In addition, its status as an endangered species has determined that parasitological investigations must be restricted only to the study of faecal samples. To date, only four studies dealing with the helminth fauna of T. bairdii are known. Three of them have been conducted in Chiapas, Mexico and the fourth in Costa Rica; according to these studies, the helminthological parasites of T. bairdii consist of ten nematode taxa: Agriostomum sp., Ancylostomatidae gen. sp., Neomurshidia sp., Strongylus sp., Trichostrongylus sp. (Cruz-Aldán et al., Reference Cruz-Aldán, Lira-Torres, Güiris-Andrade, Osorio-Sarabia and Quintero2006); Bunostomum sp., Cyathostomum sp., Nematodirus sp., Tapironema coronatum (Romero-Castañón et al., Reference Romero-Castañón, Ferguson, Güiris, González, López, Paredes and Weber2008); Probstmayria tapiri (Güiris-Andrade et al., Reference Güiris-Andrade, Rojas-Hernández, Berovides-Álvarez, Cruz-Aldán, Moguel-Acuña, Pérez-Escobar and Palacios-Mendoza2009) and one hirudinean species found in the respiratory tract, Pintobdella chiapasensis (Caballero, Reference Caballero1957).
As result of the necropsy of one tapir found dead at the Reserva de la Biósfera El Triunfo, Chiapas, Mexico, 276 specimens of intestinal nematodes belonging to the Strongylinae were collected. After morphological comparison with the diagnosis of the nine genera included in Strongylinae (Gibbons, Reference Gibbons2010; Beveridge et al., Reference Beveridge, Spratt, Durette-Desset and Schmidt-Rhaesa2014), we propose that this material represents a new genus and species. The main goal of this work is to describe this new taxon, differentiating it from other members of Strongylinae based on morphological evidence and mitochondrial and nuclear DNA sequences.
Materials and methods
Collection and examination of nematodes
In December 2010, one specimen of Baird's tapir, T. bairdii, found dead in Rancho San José Los Pinos, Reserva de la Biósfera El Triunfo, Polígono 5 (15°48′09″N, 93°01′52″W), was necropsied. The whole intestine was dissected and 276 nematodes (23 males and 253 females) were recovered and fixed in 70% ethanol. Representative specimens were cleared using Amman's lactophenol and mounted temporarily on slides for morphological study. To study the structure of the buccal capsule and oesophagus, longitudinal sections of the anterior end of nine worms were performed. Characterization of the ovejector was based on the dissection of four females. Measurements are presented in millimetres, as a range, with means, standard deviation and sample size in parentheses. Figures were drawn with the aid of a drawing tube attached to a microscope. Specimens for scanning electron microscopy (SEM) were dehydrated using graded ethanol series, and critical-point dried with carbon dioxide. Dry specimens were mounted on metal stubs, coated with a gold–palladium mixture and examined with a SEM TOPCON® SM510 (TOPCON, Tokyo, Japan). Paragenophores (see Astrin et al., Reference Astrin, Zhoun and Misof2013) were identified based on morphology. Type specimens were deposited at Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico.
Molecular and phylogenetic analyses
The DNeasy Tissue Kit (QIAGEN Inc., Valencia, California, USA) was used to extract total DNA from three specimens of the new taxon; polymerase chain reactions (PCRs) were performed to amplify a partial sequence of the internal transcribed spacer 1, the complete sequence of the 5.8S and a partial sequence of the internal transcribed spacer 2 (ITS1, 5.8S and ITS2), as well as the mitochondrial cytochrome c oxidase subunit I (COI) gene, using the primers and PCR protocols shown in table 1. Sequencing reactions were performed at the Laboratorio Nacional de Biodiversidad, Instituto de Biología using an ABI PRISM 3730xl DNA analyser (Applied Biosystems, Carlsbad, California, USA).
Table 1. Primers and PCR protocols used in the present study.

DNA sequences generated in the present work were assembled in two matrices: one including the sequence of the nuclear ITS1, 5.8S–ITS2 of 12 species of Strongylinae and three species of Cyathostomum Molin, 1861 used as the outgroup; the second matrix included the mitochondrial COI sequences of seven species of Strongylinae and one sequence of an unidentified Cyathostominae available in GenBank used as the outgroup. Both datasets were aligned using Muscle (Edgar, Reference Edgar2004) through the European Bioinformatics Institute server (http://www.ebi.ac.uk/Tools/msa/muscle/). The final dataset of the nuclear markers included 19 terminals representing 15 species and 909 aligned characters. The final matrix of the COI gene sequences included 33 terminals, representing eight species and 806 aligned characters. Phylogenetic analyses of the nuclear DNA dataset were conducted as follows. Parsimony analysis was made in PAUP* (Swofford, Reference Swofford2002); an heuristic search used 1000 replicates of random taxon addition and tree bisection–reconnection branch swapping. All characters were unweighted and non-additive. Bootstrap values were also obtained with PAUP* 4.0b10 (Swofford, Reference Swofford2002) performing 1000 pseudoreplicates. Maximum Likelihood (ML) analysis was performed in RAxML v. 7.0.4 (Stamatakis, Reference Stamatakis2006) using the GTR + GAMMA + I for each partition (three partitions in total) as implemented in the program. Bootstrap (BS) values were calculated in the same program through 1000 pseudoreplicates. The phylogenetic tree obtained from the analysis was visualized with FigTree v. 1.4.2 (Rambaut, Reference Rambaut2012). The COI dataset was used to calculate the genetic distances using the Kimura 2-parameters algorithm (Kimura, Reference Kimura1980). We followed this approach given the poor and unequal representation of members of Strongylinae in GenBank; for example, the nuclear ITS and 5.8S sequences of 12 species of the subfamily are in GenBank and only five of them also have COI gene sequences. Based on this, we performed phylogenetic analyses using only the nuclear sequences and, in addition, the COI gene sequences were generated following the genetic barcodes initiative, as proposed by Hebert et al. (Reference Hebert, Cywinska, Ball and deWaard2003), in an attempt to facilitate future studies of taxonomic identification based on molecular data.
Results
Strongylidae Baird, 1853; Strongylinae Railliet, 1885; Tziminema Güiris-Andrade, Osorio-Sarabia and García-Prieto n. gen.
Description
Whitish nematodes with cylindrical body in cross-section, tapering at caudal end in females; cuticle finely striated with transverse annulations (fig. 1A, B). Females larger than males. Four cephalic papillae, short and conical (figs 1C, 2A, C); two lateral amphids (figs 1C, 2A). Oral opening oval, directed anteriorly; external leaf crown of more than 240 elements (figs 1C, 2A, C). Internal leaf crown constituted by 40–42 rectangular elements (figs 1B, 2B). External surface of buccal capsule heavily striated (fig. 3A). Buccal capsule subglobular (fig. 1A, B). Dorsal gutter absent. Opening of buccal capsule triangular (fig. 3B). Seven to nine tooth-like, posteriorly directed structures arise from the lateral walls of the buccal capsule (figs 1B, 2B). Internal lining of buccal capsule smooth. Oesophagus globular, cylindrical at base (fig. 1A); in transverse section, formed by three thick muscular bundles with a wide tri-radiated lumen; oesophagus–intestinal valve pentacuspid, with large projections located at terminal end of oesophagus. Acicular deirids situated at posterior third of buccal capsule in males and posterior to this structure in females (fig. 1A).

Fig. 1. The morphology of Tziminema unachi n. sp. to show: (A) anterior end; (B) buccal capsule, longitudinal section; (C) anterior end, apical view; (D) copulatory bursa, ventral view; (E) dorsal ray, ventral view; (F) copulatory bursa, lateral view; (G) ovejector, lateral view; (H) mature eggs.

Fig. 2. SEM micrographs of Tziminema unachi n. sp. to show: (A) anterior end, apical view; (B) buccal capsule, longitudinal section; (C) oral opening, apical view; (D) caudal region (male), showing genital cone and one spicule; (E) ovejector. Va. vagina; Vs, vestibule; S, sphincter; In, infundibulum; Ut, uterus.

Fig. 3. Light micrographs of Tziminema unachi n. sp. to show: (A) buccal capsule, lateral view, showing external surface heavily striated; (B) oral opening, apical view.
Etymology
The generic name of this nematode relates to the Mayan–Lacandon word ‘Tzimin’, meaning tapir.
Tziminema unachi Güiris-Andrade, Osorio-Sarabia and García-Prieto n. sp.
Description
Male
Based on 11 specimens. Body length 11.80 ± 1.7 (10.29–15.47); maximum width at oesophagus 0.6 ± 0.1 (0.5–0.8), at mid-length 0.8 ± 0.1 (0.6–0.11), at bursa 0.7 ± 0.1 (0.6–0.8). Oesophagus 0.6 ± 0.3 (0.6–0.7) long; width 0.4 ± 0.3 (0.4–0.5). Buccal capsule 0.2 ± 0.2 (0.2–0.3) deep; width 0.3 ± 0.3 (0.2–0.3) at anterior end and 0.3 ± 0.3 (0.3–0.4) at posterior end. Anterior end to deirids 0.3 ± 0.1 (0.2–0.5), to excretory pore 0.9 ± 0.1 (0.8–0.10) and to nerve ring 0.3 ± 0.2 (0.3–0.4). Spicules alate and filiform, terminating in straight tip (figs 1F, 2D), 3.7 ± 3 (3.1–4) length. Gubernaculum tubular, with longitudinal ventral groove, 0.199 ± 0.008 (0.191–0.22) length by 0.048 ± 0.002 (0.046–0.050) width (fig. 1F). Papilla 0 at distal end of genital cone, which is wide at base (0.2 ± 0.13 (0.21–0.24) length, 0.147 ± 0.002 (0.145–0.150) width) (fig. 2D); one pair of pre-bursal lateral papillae present. Copulatory bursa closed and symmetrical (figs 1D, F, 2D); dorsal ray typical of the Strongylinae (three branches on each side of the median fissure, two long and one short) (fig. 1E); lateral lobes ventrally connected by a cuticular fold. Dorsal lobe not differentiated, slightly larger than lateral lobes (fig. 1D). Bursal rays do not reach the edge of the bursa; each ray with button-like papillae at distal end (fig. 1E, F); externo-lateral and externo-dorsal ray papillae located on external surface of bursa (fig. 2D). Length to base of externo-dorsal ray: 0.768 ± 0.010 (0.755–0.782). Externo-dorsal rays originate from the base of dorsal ray; rays straight with two short branches at mid length. Posterolateral, mediolateral and externo-lateral rays wide, parallel throughout their length; posterolateral with mediolateral rays diverging distally. Ventro-ventral and ventro-lateral rays parallel, shorter and slender than lateral rays (fig. 1D, E).
Female
Based on 11 specimens. Body length 18.67 ± 0.68 (17.86–20.52). Maximum width at oesophagus 0.9 ± 0.1 (0.8–1.1), at uterus 1.3 ± 0.1 (1.1–1.5), at vulva 0.8 ± 0.1 (0.7–0.9), at anus 0.4 ± 0.02 (0.4). Oesophagus 0.8 ± 0.1 (0.7–1) long; width 0.5 ± 0.04 (0.4–0.5). Buccal capsule 0.3 ± 0.01 (0.2–0.3) deep, width 0.3 ± 0.02 (0.3–0.32) at anterior end and 0.4 ± 0.02 (0.3–0.4) at posterior end. Anterior end to deirids 0.4 ± 0.03 (0.4–0.5), to excretory pore 1 ± 0.1 (0.9–1.1) (fig. 1A) and to nerve ring 0.4 ± 0.02 (0.3–0.4). Ovaries paired, prodelphic; ovejector type I (figs 1G, 2E). Wide vulvar opening located at the posterior third of body in a transverse depression, flanked by thick cuticular lips, situated at 2.5 ± 0.2 (2.3–3) from the tail tip (fig. 1G). Vagina wide and short. Ovejector Y-shaped; vestibule twisted, 12.6 ± 1.3 (12.48–12.71) length and 1.07 ± 1.1 (0.9–1.1) width. Sphincter long and thick walled, 8.4 ± 1.4 (8.2–8.5) (n = 4) length and 1.2 ± 0.8 (1.1–1.2) width. Thin-walled infundibulum, 3.4 ± 1.8 (3.3–3.4) (n = 4) length, 1.1 ± 0.9 (1.1–1.3) width. Sphincters and infundibula clearly distinguishable, connected to the uterus; uterine branches parallel (figs 1G, 2E). Anal opening crescent-shaped, directed posteriorly. Distance from anus to tail 0.9 ± 0.1 (0.8–1). Eggs large, oval and brown (fig. 1H), shell smooth and thin, 0.195 ± 0.099 (0.180–0.215) × 0.089 ± 0.006 (0.080–0.110). Tail robust, conical and straight, tapering at caudal end. Tail robust, conical and straight, tapering at caudal end and ending in a mucron (fig. 1G).
Taxonomic summary
Type host. Baird's tapir, Tapirus bairdii (Gill, 1865) (Perissodactyla: Tapiridae).
Site of infection. Caecum and colon.
Type locality. Rancho San José Los Pinos, Reserva de la Biósfera El Triunfo, Polígono 5 (15°48′09.1″N, 93°01′51.8″W), 1167 metres above sea level (collected in December 2010).
Number of worms collected. Two hundred and seventy-six in one host examined (23 males and 253 females).
Deposition of type specimens. Colección Nacional de Helmintos, Instituto de Biología, UNAM, Mexico City, Mexico: holotype (CNHE 10319), allotype (CNHE 10320) and ten paratypes (CNHE 10321).
Etymology. This species is named after the institution where the preliminary identification of specimens was undertaken: Universidad Autónoma de Chiapas (UNACH).
Remarks
The presence of subglobular buccal capsule, heavily sclerotized, with external and internal leaf crowns, without teeth or cutting plates, allows us to include Tziminema n. gen. into the superfamily Strongyloidea, according to Beveridge et al. (Reference Beveridge, Spratt, Durette-Desset and Schmidt-Rhaesa2014). The new genus is included in Strongylidae by having a paired ovejector Type I, Y-shaped with two anterior parallel branches, and a dorsal ray with three branches on each side (Lichtenfels, Reference Lichtenfels, Anderson, Chabaud and Willmott1980; Beveridge et al., Reference Beveridge, Spratt, Durette-Desset and Schmidt-Rhaesa2014). The new genus described herein cannot be included in the subfamily Cyathostominae as it has a subglobular buccal capsule instead of a cylindrical one (Lichtenfels, Reference Lichtenfels, Anderson, Chabaud and Willmott1980; Beveridge et al., Reference Beveridge, Spratt, Durette-Desset and Schmidt-Rhaesa2014). Based on having a subglobular buccal capsule, the new genus is included in Strongylinae, a subfamily that currently comprises nine genera, according to Beveridge et al. (Reference Beveridge, Spratt, Durette-Desset and Schmidt-Rhaesa2014): Choniangium Railliet, Henry & Bauche, 1914, Decrusia Lane, 1914 and Equinurbia Lane, 1914, with species parasitizing Indian elephants; and five more genera described based on organisms parasitizing equids – Bidentostomum Tshoijo, 1957, Craterostomum Boulenger, 1920, Oesophagodontus Railliet & Henry, 1902, Strongylus Meller, 1780 and Triodontophorus Looss, 1902. Finally, Codiostomum Railliet & Henry, 1911 includes species associated with African ostriches.
Tziminema n. gen. differs from all other genera included in Strongylinae by having 7–9 posteriorly directed, tooth-like structures arising from the anterior end of the buccal capsule, which are absent in the other members of the subfamily. The new genus shares the presence of two leaf crowns in the cephalic region with Equinurbia, Strongylus, Oesophagodontus, Bidentostomum, Codiostomum, Craterostomum and Triodontophorus (Carreno & Kinsella, Reference Carreno and Kinsella2008; Ederli et al., Reference Ederli, Rodrigues de Oliveira and de Azevedo2008; Lichtenfels et al., Reference Lichtenfels, Kharchenko and Dvojnos2008). However, Equinurbia lacks a gubernaculum (Khalil, Reference Khalil1922), which is present in Tziminema; Strongylus has a dorsal lobe shorter than the laterals (see Lichtenfels et al., Reference Lichtenfels, Kharchenko and Dvojnos2008) in contrast to a dorsal lobe slightly longer than the laterals in the new genus; Oesophagodontus and Bidentostomum have one and three long oesophageal teeth, respectively (Lichtenfels, Reference Lichtenfels, Anderson, Chabaud and Willmott1980), which are absent in Tziminema; the dorsal ray in Codiostomum has eight branches and lacks a median fissure, whereas in Tziminema this ray has six branches and a median fissure (see Ederli et al., Reference Ederli, Rodrigues de Oliveira and de Azevedo2008); and, finally, according to Lichtenfels et al. (Reference Lichtenfels, Kharchenko and Dvojnos2008), in Craterostomum and Triodontophorus the dorsal gutter extends to the anterior end of the buccal capsule, discriminating them from Tziminema, which lacks this structure.
The presence of a double leaf crown allows the new taxon to be differentiated from the remaining two genera included in Strongylinae: Choniangium and Decrusia, which have only one external leaf crown. In addition, the buccal capsule in Choniangium is deeper than wide and the oral opening is directed dorsally (Piana & Stazzi, Reference Piana and Stazzi1900; Railliet et al., Reference Railliet, Henry and Bauche1914) in contrast with the subglobular buccal capsule and the oral opening directed anteriorly of Tziminema. Decrusia can also be distinguished from the new genus by having two subventral teeth at the base of the buccal capsule (absent in Tziminema) and by the lack of a gubernaculum, which is present in the genus described herein (Khalil, Reference Khalil1922; Baylis & Daubney, Reference Baylis and Daubney1926).
Species belonging to two genera of Cyathostominae have been recorded parasitizing Neotropical tapirs (Muniz-Pereira et al., Reference Muniz-Pereira, Vieira and Luque2009) and also have two leaf crowns, like Tziminema: Kiluluma Skrjabin, 1916 and Neomurshidia Chabaud, 1957. However, both these genera have a cylindrical buccal capsule (Beveridge et al., Reference Beveridge, Spratt, Durette-Desset and Schmidt-Rhaesa2014) while in Tziminema the buccal capsule is subglobular. Furthermore, in Kiluluma leaf crowns have few elements (which are numerous in Tziminema) and the externo-dorsal rays originate separately from the dorsal ray (Lichtenfels, Reference Lichtenfels, Anderson, Chabaud and Willmott1980), while in the new genus the externo-dorsal rays arise from the base of the dorsal ray (fig. 2A, C). Finally, the second leaf crown in Neomurshidia is deep in the buccal cavity (while, in the new genus, the second leaf crown is located in the apex of the buccal capsule) and the tips of the spicules are bifid in Neomurshidia (Lichtenfels, Reference Lichtenfels, Anderson, Chabaud and Willmott1980) and entire in Tziminema.
Molecular analysis
Parsimony analysis of the nuclear dataset resulted in a single tree of 688 steps, with a Retention Index of 0.75 and Consistency Index of 0.75 (fig. 4). In this tree, the samples of Tzimanema unachi n. sp. appeared in a monophyletic group with a BS = 100. Some terminal nodes show high bootstrap values; however, most of the values of the internal nodes are below 50%. The Maximum Likelihood tree (fig. 5) also recovered the three samples of the new species, forming a single group with high bootstrap support. However, bootstrap values in the internal nodes were low. Differences between both hypotheses include nodes with low support and the recovery of different sister groups for the new taxon; parsimony analyses recovered Strongylus vulgaris (Looss, 1900) and ML recovered Craterostomum acuticaudatum (Kotlan, 1919), both hypotheses having negligible node support. These two analyses recovered two genera with more than one species as monophyletic, Parapoteriostomum Hartwich, 1986 and Triodontophorus; however, their position varies depending on the analysis. In addition, the methods employed failed to recover Strongylus as monophyletic.
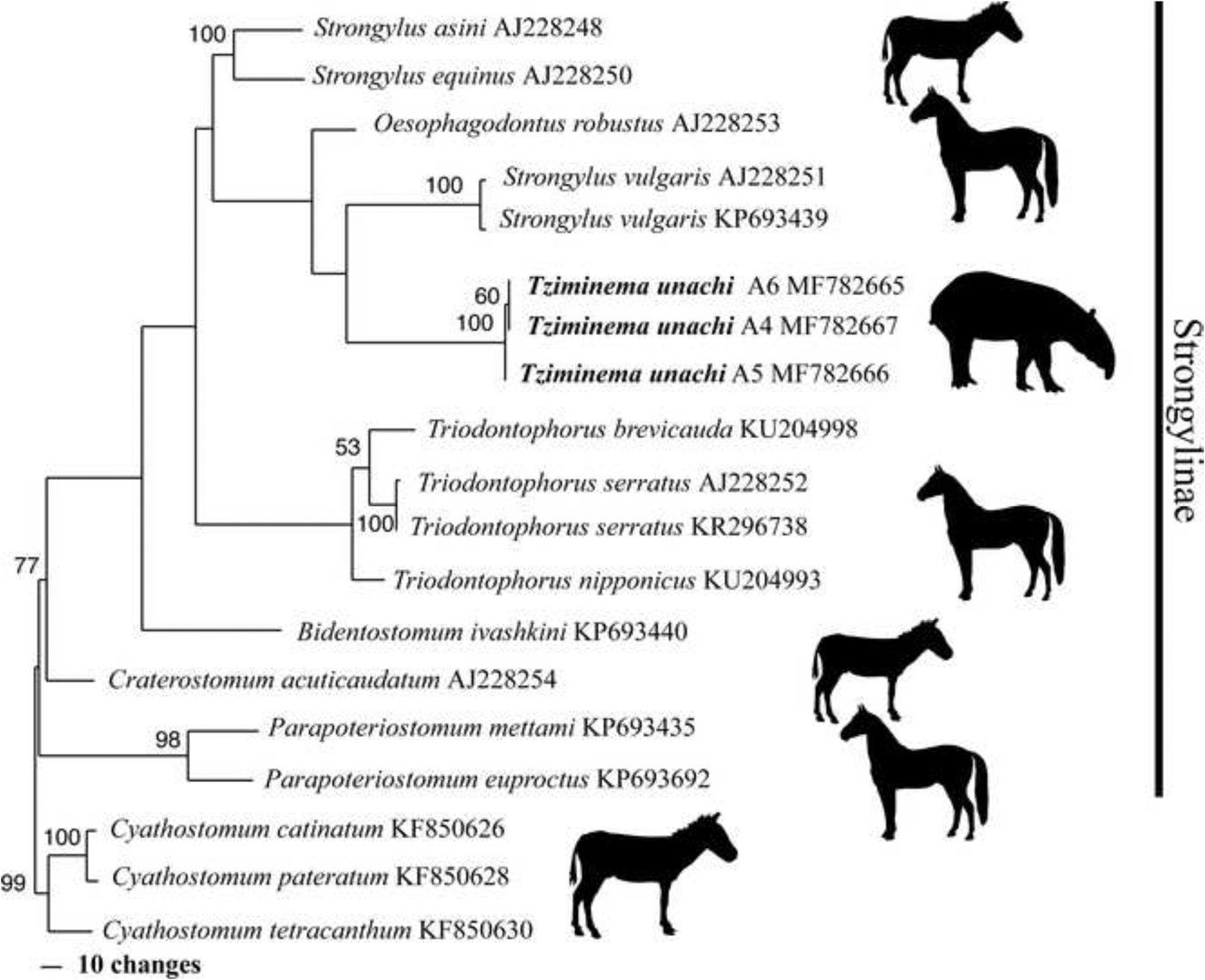
Fig. 4. Strict consensus tree resulting from parsimony analysis of Strongylinae based on partial sequences of ITS1, 5.8S and a partial sequence of ITS2. Branch lengths are proportional to amount of change. Numbers above nodes indicate bootstrap values. GenBank accession codes are indicated after the species’ names. Tziminema unachi n. sp. is in bold.

Fig. 5. Maximum Likelihood tree of Strongylinae based on partial sequences of ITS1, 5.8S and a partial sequence of ITS2 sequences. Numbers above nodes indicate bootstrap values. GenBank accession codes are indicated after the species' names. Tziminema unachi n. sp. is in bold.
Genetic distance using of COI gene sequences within the two samples of T. unachi n. sp. (GenBank accession numbers MF785123 and MF785124) showed low variation (< 0.5%), in contrast with genetic distances recorded for species of other genera of Strongylinae such as Triodontophorus and Strongylus; for these two genera, distances found in all comparisons are larger than 10%. Genetic distances between the new taxon and the unidentified sample of Cyathostominae were higher than 18%.
Discussion
The presence of a subglobular buccal capsule allowed the inclusion of T. unachi n. sp. in the subfamily Strongylinae. The diagnosis of the genera currently included in the subfamily is compatible with the characteristics of the new taxon, supporting the erection of a new genus to accommodate the new species. Molecular data, even fragmentary and independent of the phylogenetic method used, grouped T. unachi n. sp. together with other members of Strongylinae, ratifying its inclusion in the subfamily. The fact that parsimony and ML trees have clear differences, together with the lack of support in most of the nodes in both trees, is probably related to the low taxonomic representation in our analyses and the poor phylogenetic signal of this marker. However, with the current information we can conclude that the new taxon belongs to the Strongylinae, for which a more complete phylogenetic analysis, including more species and markers, is needed to clearly understand the relationships of the species and genera within the group.
The COI genetic distance, taken as indirect evidence, also shows that the new taxon is highly divergent in comparison with the other members of the subfamily, showing values of more than 10% and higher than 18% when comparing with the outgroup. These values, together with the morphological differences and the uncertain phylogenetic position of the new taxon in the parsimony and ML analyses of the nuclear data, support the proposition of a new genus to accommodate the new taxon described here.
The placement of T. unachi n. sp., a parasite of tapirs from the New World, within a clade of parasites of perissodactyls from Asia, strongly suggests a close host–parasite evolutionary relationship between both groups. Recent phylogenetic analyses place the origin of perissodactyls in the early Eocene, about 54.5 million years ago (Rose et al., Reference Rose, Holbrook, Rana, Kumar, Jones, Ahrens, Missiaen, Sahni and Smith2014) and may also indicate that the origin of Strongylinae is much younger than the 205 Myr suggested by Lichtenfels (Reference Lichtenfels, Anderson, Chabaud and Willmott1980). Future work should be designed to include parasites of elephants, ostriches and Australian marsupials assigned to the Strongyliae (Lichtenfels, Reference Lichtenfels, Anderson, Chabaud and Willmott1980) in phylogenetic analyses, in order to establish if they represent cases of more recent host-switching phenomena, taxonomic error or ancient association with only few species of parasites surviving today, as suggested by Lichtenfels (Reference Lichtenfels, Anderson, Chabaud and Willmott1980).
Acknowledgements
Our thanks to Georgina Ortega-Leite for providing important bibliographical references; Alejandro Emmanuel Pérez López for the illustrations; Epigmenio Cruz Aldán and Gabriela Palacios Mendoza for field assistance; Ofelia Delgado, Andrea Jiménez and Laura Marquez of the Laboratorio Nacional de Biodiversidad, Instituto de Biología, UNAM for assisting in the generation of DNA sequences. David Ivan Guadalupe Hernández Mena assisted in the phylogenetic analyses.
Financial support
This work was supported by the Secretaría Técnica de Intercambio Académico, UNAM through the grant Programa Anual de Movilidad Nacional de Académicos, Project 03/Vet/RPR/265/16 ‘Estudios Biomédicos y de Sanidad en Fauna (doméstica, cautiva y silvestre) del Estado de Chiapas, México’ registered by Dirección General de Investigación y Posgrdo, UNACH and the Policlínica y Diagnóstico Veterinario, Tuxtla Gutiérrez, Chiapas, Mexico. A.O.-F. thanks PAPIIT RA202016 for providing funds for molecular work.
Conflict of interest
None.








