Introduction
Siluriformes is considered one of the world's oldest fish groups (Mo, Reference Mo1991). The biogeographical evolution of this group is undetermined, but it is highly probable that the marine forms evolved from freshwater forms (Nelson, Reference Nelson2006) and carried with them their respective parasites. To date, Siluriformes consists of approximately 39 families and over 6700 valid living species distributed in freshwater, brackish and marine environments in every continent of the world (Eschmeyer et al., Reference Eschmeyer, Fricke and van der Laan2019), except in Antarctica where they have been present in the past (Grande & Eastman, Reference Grande and Eastman1986).
Trinigyrus Hanek, Molnar & Fernando, 1974 comprises gill parasites of loricariid fishes from the Neotropical region (Boeger & Belmont-Jégu, Reference Boeger and Belmont-Jégu1994; Nitta & Nagasawa, Reference Nitta and Nagasawa2016). Currently, the genus includes five species: Trinigyrus hypostomatis Hanek, Molnar & Fernando, 1974, described as a parasite of Hypostomus robinii Valenciennes, 1840, Trinigyrus tentaculoides Kritsky, Boeger & Thatcher, 1986 from Hypoptopoma thoracatum Günther, 1868, Trinigyrus acuminatus Kritsky, Boeger & Thatcher, 1986 from Acanthicus hystrix Spix & Agassiz, 1829, Trinigyrus mourei Boeger & Belmont-Jégu, 1994 parasitizing Squaliforma emarginata (Valenciennes, 1840) [=Hypostomus emarginatus] and Trinigyrus peregrinus Nitta & Nagasawa, 2016 from Pterygoplichthys disjunctivus (Weber, 1991). Most of the species described were found parasitizing fishes from the municipality of Manaus, Amazonas State, Brazil (T. tentaculoides, T. acuminatus and T. mourei). The type species, T. hypostomatis, is naturally distributed in the Talparo River, Trinidad, whereas T. peregrinus was introduced in Okinawa-Jima Island, Japan, with its respective alien host, the vermiculated sailfin catfish P. disjunctivus. The occurrence of T. hypostomatis was also reported in China, parasitizing the gills of the alien fish Hypostomus plecostomus (Linnaeus, 1758) from the Pearl River water system, in the municipality of Guangzhou, Guangdong Province (Li & Huang, Reference Li and Huang2012).
As part of our long-term studies of the biodiversity of fish parasites from the tributaries of the Upper Paraná River basin in Brazil, two new species of Trinigyrus are described from loricariids, supported by morphological and molecular data. New morphological features are added to the diagnosis of the genus. Trinigyrus peregrinus is redescribed based on morphological discrepancies found among the original description and the specimens deposited as holotype and paratypes, as well as new specimens collected for this study. For the first time, gene sequences of Trinigyrus spp. from Brazil were obtained (partial ribosomal 28S and mitochondrial cytochrome c oxidase I (mtCOI)). The phylogenetic relationships among Trinigyrus and other monogenean parasites of siluriforms are also evaluated, including sequences of Hamatopeduncularia spp. parasites of marine siluriforms, which were previously considered as closely related to Trinigyrus spp. by Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986).
Material and methods
Host sampling and parasitological procedures
We collected 276 specimens of loricariids, from which we extracted 1261 monogenean specimens belonging to Trinigyrus. The analysed hosts were as follows: 23 specimens of Hypostomus margaritifer (Regan, 1908), 50 specimens of Hypostomus regani (Ihering, 1905), 50 specimens of Hypostomus strigaticeps (Regan, 1908), 50 specimens of Hypostomus ancistroides (Ihering, 1911) and 23 specimens of a new species belonging to Hypostomus (C.H. Zawadzki, pers. obs., description in progress), all commonly named as ‘suckermouth catfishes’. The Hypostomus spp. specimens were collected between March 2012 and December 2013 in the reservoirs of three small hydroelectric power plants (ANEEL, 2008): Palmeiras (20°32′57.33″S, 47°48′47.26″W), Anhanguera (20°29′38.38″S, 47°51′33.11″W) and Retiro (20°26′12.5″S, 47°53′18.59″W), in the Sapucaí-Mirim River, a tributary of the Grande River (Upper Paraná River basin), municipality of São Joaquim da Barra, São Paulo State, Brazil. Eighty specimens of Pterygoplichthys ambrosettii (Holmberg, 1893), commonly known as ‘airplane catfish’, were collected in the mouth of the Aguapeí River (21°3′36.20″S, 51°45′38.58″W), a tributary of the Paraná River, municipality of Castilho, São Paulo State, from August 2013 to November 2014. Fishes were collected using a nylon monofilament gill net, under the Permanent License for the Collection of Zoological Material (SISBio 13794-1 and IBAMA 577/2015). The specimens were euthanized by a section of spinal cord, stored individually in plastic bags and placed in a Styrofoam box with ice for transportation to the laboratory where they were necropsied.
The gills were removed and analysed fresh when possible or placed in vials containing hot water (~60°C), shaken to detach the monogeneans of the gill filaments and then absolute ethanol was added to produce a final concentration of 70% ethanol (Boeger & Vianna, Reference Boeger, Vianna and Thatcher2006). The monogeneans were collected using a stereomicroscope and some specimens were mounted in Hoyer's medium, Gray and Wess' medium or glycerine and picric acid (GAP) to observe sclerotized structures, whereas others were stained with Gömöri trichrome and mounted in permanent slides using Canada balsam for analysis of the internal organs (Ergens, Reference Ergens1969; Humason, Reference Humason1979; Kritsky et al., Reference Kritsky, Boeger and Thatcher1986). In addition, some specimens of monogeneans obtained from fresh preparation were selected for the molecular analyses (see Molecular analyses section).
Morphometrical and morphological analyses were performed with a computerized image analysis system with differential interference contrast (Leica Application Suite, version 3; Leica Microsystems, Wetzlar, Germany). All measurements are presented in micrometres (μm) and expressed as mean, followed by the range and number of specimens measured (n) in parentheses. Measurements of some sclerotized structures (bar, anchors and male copulatory complex) were performed according to the scheme shown in fig. 1, and others were taken in accordance with Mizelle & Klucka (Reference Mizelle and Klucka1953). Illustrations of the sclerotized structures were obtained with the aid of a camera lucida mounted on a Leica DMLS microscope with phase contrast optics. The prevalence and mean intensity of infestation were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).

Fig. 1. Scheme of measurements for the species of Trinigyrus from this study: (a) anchor length; (b) anchor base width; (c) point length; (d) accessory piece length; (e) male copulatory organ (MCO) length; (f) diameter of the MCO; (g) bar length.
Voucher specimens of the fish hosts were deposited in the Ichthyological Collection of the Limnology, Ichthyology and Aquaculture Research Center (NUP) of the State University of Maringá, Paraná State, Brazil. Holotypes and paratypes of the proposed new species were deposited in the Helminthological Collection of the Oswaldo Cruz Institute (CHIOC), Rio de Janeiro State, Brazil. Additional vouchers were deposited in the Helminthological Collection of the Department of Parasitology, Institute of Biosciences, São Paulo State University – UNESP (CHIBB), in the municipality of Botucatu, São Paulo State, Brazil. For comparative purposes, the slides of the holotypes and paratypes of the following species of Trinigyrus were examined: T. mourei (CHIOC 33052), T. acuminatus (National Institute of Amazon Researches – INPA 110 b–c; T. tentaculoides: INPA 111–112) and T. peregrinus (National Museum of Nature and Science from Japan – NSMT-P1 6196–6203). Additionally, photomicrographs of the holotype of T. peregrinus (NSMT-Pl 6195) and paratypes of species of Trinigyrus deposited in the Smithsonian US National Museum Helminthological Collection (USNM; T. acuminatus: catalogue number 1374541; T. hypostomatis: catalogue number 1368749–50; T. mourei: catalogue number 1374541; T. tentaculoides: catalogue number 1374540) were examined. Scientific names of the hosts follow Froese & Pauly (Reference Froese and Pauly2019).
Molecular analyses
To confirm parasite identity, each specimen was mounted on a slide with glycerine or a drop of water, covered with a coverslip and photographed. Following morphological identification, specimens were removed from the slide and placed into 96% molecular-grade ethanol for molecular analysis. Conspecific specimens (paragenophores, according to Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) were mounted in Gray and Wess' or Hoyer's medium and deposited in CHIBB (556L, 566L and 569–575L). Total genomic DNA was extracted using the Qiagen Dneasy® Blood and Tissue Kit (Qiagen, California, USA) according to the manufacturer's protocol, and adjusted to a final volume of 30 µl. Partial ribosomal (28S, with divergent domains (D1–D3)) and mtCOI genes were amplified according to the procedures of Mendoza-Palmero et al. (Reference Mendoza-Palmero, Blasco-Costa and Scholz2015) and Plaisance et al. (Reference Plaisance, Rousset, Morand and Littlewood2008), respectively. Polymerase chain reaction (PCR) amplifications were performed containing 5 µl of DNA extract, 0.5 µl of each PCR primer and 19 µl of ultrapure water (Sigma, Aldrich, UK), using Ready-to-Go PCR beads (Pure Taq™ Ready-to-Go™ beads, GE Healthcare, Chicago, USA), with a final volume of 25 µl. The thermocycling profile for 28S was an initial denaturation of DNA at 94°C for 3 min, followed by 34 cycles of amplification at 94°C for 30 s, 56°C for 30 s and 72°C for 1.5 min, and a final extension at 72°C for 7 min (Mendoza-Palmero et al., Reference Mendoza-Palmero, Blasco-Costa and Scholz2015); for mtCOI, it was an initial denaturation of DNA at 94°C for 3 min, followed by 35 cycles of amplification at 94°C for 30 s, 44°C for 30 s and 72°C for 2 min, and a final extension at 72°C for 7 min (Plaisance et al., Reference Plaisance, Rousset, Morand and Littlewood2008). Primers used for amplification and sequencing of partial 28S ribosomal DNA (rDNA) fragments were U178 (5′-GCACCCGCTGAAYTTAAG-3′) and L1642 (5′-CCAGCGCCATCCATTTTCA-3′) (Lockyer et al., Reference Lockyer, Olson and Littlewood2003), and L1200R (5′-GCATAGTTCACCATCTTTCGG-3′) for sequencing (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000). For amplification and sequencing of mtCOI, the primers used were COI_Mono_5: 5′-TAATWGGTGGKTTTGGTAA-3′ and COI_Mono_3: 5′-TAATGCATMGGAAAAAAACA-3′ (Plaisance et al., Reference Plaisance, Rousset, Morand and Littlewood2008). PCR products were run on an agarose gel using GelRed™ (Biotium, Hayward, USA), and loading buffer and purified using the QIAquick PCR Purification Kit (Qiagen, California, USA). Automated sequencing in both directions was performed directly on the purified PCR products using the BigDye version 3.1 Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, California, USA). Sequences were read on an Applied Biosystems ABI 3500 DNA genetic analyser. Contiguous sequences were assembled with Sequencher™ version 5.2.4 (Gene Codes, Ann Arbor, Michigan, USA) and submitted to GenBank (accession numbers presented in table 1).
Table 1. Monogeneans included in the phylogenetic analyses. New sequences obtained for the present study are in bold.

a Species used as outgroups.
* Sequences used for the nucleotide divergence (p-distance) analyses using mtCOI (supplementary table S2).
Phylogenetic analyses
Six newly generated sequences of partial genes (three sequences of 28S rDNA and three sequences of mtCOI) were aligned with sequences obtained previously from monogeneans of catfishes (table 1). Murraytrema pricei Bychowsky & Nagibina, 1977 (DQ157672), Pseudorhabdosynochus lantauensis (Beverley-Burton & Suriano, 1981) Kritsky & Beverley-Burton, 1986 (AY553624) and Pseudorhabdosynochus epinepheli (Yamaguti, 1938) Kritsky & Beverley-Burton, 1986 (AY553622) (Diplectanidae) were used as outgroup for the 28S rDNA; and Tetrancistrum nebulosi Young, 1967 (KJ001360) was used as outgroup for the mtCOI. Accession numbers, species and hosts of the sequences used in this study are shown in table 1. Newly obtained sequences from both data sets (28S rDNA and mtCOI) were aligned using MUSCLE implemented in Geneious version 11.1.4 (Kearse et al., Reference Kearse, Moir and Wilson2012) with the extremes of the alignment trimmed. The index of substitution saturation (Iss) was estimated in DAMBE 5 to evaluate the occurrence of substitution saturation (Xia, Reference Xia2013).
Genetic divergence was calculated for partial 28S rDNA and mtCOI genes using the uncorrected p-distances model in MEGA7 software (Kimura, Reference Kimura1980; Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). The alignment of mtCOI was 534 bp, with no stop codons and translation on frame 2, flatworm mitochondrial code.
The alignment of 28S rDNA gene was 617 bp long and the model of nucleotide substitution selected was GTR + I + G. The most appropriate evolutionary model for maximum likelihood (ML) and Bayesian inference (BI) was selected by JModelTest 2.1.1 programme (Posada, Reference Posada2008) using the Akaike information criterion. ML analysis was performed using the program RAxML version 8 (Guindon & Gascuel, Reference Guindon and Gascuel2003), and BI using MrBayes 3.2 (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003). Bootstrap support values for ML were determined by performing 1000 repetitions. Markov Chain Monte Carlo chains were run for 50 million generations and the log-likelihood scores plotted. The burn-in was set to the first 25% of generations discarded and the consensus tree (majority rules) was estimated using the remaining topologies. MrBayes and RaxML analyses were carried out on the computational resource CIPRES (Miller et al., Reference Miller, Pfeiffer and Schwartz2010). Phylogenetic trees were visualized and edited in FigTree version 1.3.1 (Rambaut, Reference Rambaut2009).
Results
Dactylogyridae Bychowsky, 1933
Trinigyrus Hanek, Molnar & Fernando (1974)
Taxonomic summary
Type species, host and locality. Trinigyrus hypostomatis Hanek, Molnar & Fernando (1974), from H. robinii Valenciennes, 1840, Talparo River, Trinidad.
Other species. Trinigyrus tentaculoides, T. acuminatus, T. mourei, T. peregrinus, Trinigyrus anthus n. sp. and Trinigyrus carvalhoi n. sp.
Diagnosis. Body pyriform, divisible into cephalic region, trunk and haptor (peduncle absent). Tegument thin, smooth. Cephalic lobes, head organs, cephalic glands present. Eyespots absent. Mouth subterminal, midventral; pharynx muscular, glandular; oesophagus short. Two intestinal caeca confluent posterior to gonads; diverticula absent. Gonads intercaecal, overlapping; testis dorsal or dorsoposterior to germarium. Vas deferens looping left intestinal caecum; seminal vesicle as an enlargement of the vas deferens; two prostatic reservoirs. Copulatory complex comprising accessory piece and tubular male copulatory organ (MCO). Weakly sclerotized fringe on wide base of the MCO present or absent. Oviduct short; uterus delicate or well developed; vagina dextral, vaginal tube sclerotized or not; exterior vaginal appendage present or absent; seminal receptacle lying diagonally to the right of midline. Genital pore midventral. Vitelline follicles scattered throughout trunk, absent in region of reproductive organs, coextensive with intestinal caeca. Haptor with ventral anchor/bar complex composed of one pair of anchors, anchors lacking roots distinction (flattened base), one bar, haptoral appendages. Sclerotized basal border on anchor base present or absent. Haptoral glandular reservoirs present (variable number) or absent. Hooks similar in shape and size, proximal dilation of shank; four pairs of hook-bearing appendages: two bilateral pairs bearing hooks pairs 2, 7; single posteroventral pair branched, bearing hook pairs 3, 4; pair of posterodorsal appendages bearing hook pair 6; hook pairs 1, 5 sessile. Sclerotized basal border on the base of anchor present or absent; double filament of anchor. Egg operculate, ovate, with a long, delicate and convoluted filament at proximal pole.
Remarks
The generic diagnosis of Trinigyrus is presented, adding new features for placement of the new species in the genus. The new features include the presence of a sclerotized basal border on the base of anchor, and a weakly sclerotized fringe on the wide base of the MCO of T. peregrinus, T. anthus n. sp. and T. carvalhoi n. sp., which was not described earlier in other congeners. Trinigyrus peregrinus is redescribed based on morphological discrepancies found among the original description presented by Nitta & Nagasawa (Reference Nitta and Nagasawa2016) and the specimens deposited as holotype and paratypes, as well as specimens newly collected for this study.
Trinigyrus anthus n. sp.
Taxonomic summary
Type host. Hypostomus regani (Ihering, 1905) (Siluriformes: Loricariidae) (NUP 15217).
Other hosts. Hypostomus strigaticeps (Regan, 1908) (NUP 14990), H. margaritifer (Regan, 1908) (NUP 15216) and Hypostomus sp. (NUP14997).
Site in host. Gills.
Type locality. Sapucaí-Mirim River (20°29′38.38″S, 47°51′33.11″W), municipality of São Joaquim da Barra, São Paulo State, Brazil.
Prevalence (P) and mean intensity of infestation (MII). Hypostomus regani: P = 60%, MII = 16.2 ± 3.3 (1.0–73.0); H. strigaticeps: P = 64%, MII = 11.6 ± 2.7 (1.0–79.0); H. margaritifer: P = 17.4%, MII = 3.2 ± 1.3 (1.0–7.0); Hypostomus sp.: P = 13%, MII = 3.7 ± 2.2 (1.0–8.0).
Type material. Holotype CHIOC (39249), paratypes CHIOC (39250–39253), vouchers CHIBB (554–560L).
Representative DNA sequences. 1519-bp-long sequence of the 28S rDNA gene – GenBank accession number MN947622; 780-bp-long sequence of the mtCOI gene – GenBank accession number MN916719.
ZooBank registration. urn:lsid:zoobank.org:act:44F08315-A52C-4C43-911D-3C014F42CCBA, according to the regulations of the International Code of Zoological Nomenclature (ICZN, 2012).
Etymology. The specific epithet is from the Latin and is derived from the flower-petal-like fringe on the wide base of the MCO (anthus = flower).
Description
Based on eight specimens mounted in Gray and Wess' medium, two specimens in Hoyer's medium and three specimens stained with Gömöri's trichrome (fig.2a‒f). Body pyriform, 604 (426–781; n = 13) long, 191 (126–327; n = 13) wide. Two terminal cephalic lobes, with three bilateral pairs of well-developed head organs; cephalic glands inconspicuous. Pharynx muscular 47 (36–55; n = 9) in diameter. MCO 65 (54–74; n = 13) long, and delicate tube, slightly curved, base with a flower-petal-like fringe, non-articulated with accessory piece. Accessory piece 68 (54–89; n = 13) long, rod-shaped, tapering discretely in proximal portion, slightly recurved distally, serving as guide of distal portion of MCO. Gonads intercaecal. Testis dorsal to germarium, elongated 97 (95–99; n = 2) long, 39 (35–43; n = 2) wide. Vas deferens looping left intestinal caecum. Germarium 193 (143–282; n = 4) long, 87 (42–140; n = 4) wide. Oviduct, ootype and uterus not observed. Conspicuous glands in middle part of body (possibly surrounding ootype region). Egg ovate, 60 (49–72; n = 2) long and 34 (30–39; n = 2) wide, with a proximal filament, long, delicate and convoluted filament. Vagina dextral, non-sclerotized; sac-like seminal receptacle. Haptor 98 (56–174; n = 13) long, 292 (208–443; n = 13) wide, an expanded portion of the body, variable according to disposition of haptoral appendages. Ten haptoral appendages relatively long, bearing hook pairs 2, 3, 4, 6 and 7; hook pairs 1 and 5 sessile. Anchors 54 (41–60, n = 13) long, lacking roots, base 16 (14–19; n = 13) wide; conspicuous sclerotized basal border on base of the anchor; short shaft, elongate point 31 (27–35; n = 13) long, recurved tip; anchor filament double. Bar M-shaped, 207 (172–267; n = 13) long, longitudinal groove along its length and pointed ends. Hooks similar, 13 (11–15; n = 43) long, with recurved shaft, shank proximally dilated, weakly sclerotized; erect thumb. Filamentous hooklet loop approximately two-thirds of shank length. Four pairs of hook-bearing appendages: two bilateral pairs, bearing hook pairs 2, 7; single posteroventral pair branched, bearing hook pairs 3, 4; pair of posterodorsal appendages bearing hook pair 6; hook pairs 1, 5 sessile.

Fig. 2. Trinigyrus anthus n. sp. of Hypostomus regani (Ihering, 1905) from the Sapucaí-Mirim River, São Paulo State, Brazil: (a) entire body, ventral view (composite); (b) male copulatory complex, ventral view; (c) hook; (d) bar; (e) anchor; (f) egg.
Remarks
Trinigyrus anthus n. sp. shares the morphological features of the genus, like one pair of anchors, one bar M-shaped, haptoral appendages, vagina aperture dextral and overlapping gonads. The proposed new species differs from other congeners mainly by the shape of MCO, which is a delicate tube with base containing a flower-petal-like fringe. The accessory piece of the new species is similar in shape of that observed in T. tentaculoides but differs in its length (larger in new species – see table 2; see Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986) for details on T. tentaculoides). The haptoral appendages of the new species are conspicuous, when compared with the type species T. hypostomatis and T. acuminatus; however, they are smaller than T. tentaculoides, according to the description of Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986). The bars of T. anthus n. sp. and T. tentaculoides are morphologically different, including the absence of a flat posteromedial projection (present in T. tentaculoides), and the accentuated M-shaped bar observed in the new species. Moreover, the anchors of the new species present a base with a subrectangular shape versus a ‘tear-drop’ shape in T. tentaculoides. The seminal receptacle of T. anthus n. sp. was often filled with spermatozoa, as described for T. acuminatus and T. tentaculoides by Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986).
Table 2. Morphometric comparison of species of Trinigyrus from loricariid fish.

a Hanek et al. (Reference Hanek, Molnar and Fernando1974).
b Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986).
c Boeger & Belmont-Jégu (Reference Boeger and Belmont-Jégu1994), except the bar length and accessory piece length, which were obtained from the analysis of the slides deposited in museums.
d Nitta & Nagasawa (Reference Nitta and Nagasawa2016). Measurements of the anchors, copulatory complex and bars correspond to fig. 1.
Trinigyrus carvalhoi n. sp.
Taxonomic summary
Type host. Hypostomus ancistroides (Ihering, 1911) (Siluriformes: Loricariidae) (NUP 15003).
Site in host. Gills.
Type locality. Sapucaí-Mirim River (20°29′38.38″S, 47°51′33.11″W), municipality of São Joaquim da Barra, São Paulo State, Brazil.
Prevalence and mean intensity of infestation. P = 34%, MII = 5.0 ± 0.9 (1.0–14.0).
Type material. Holotype CHIOC (39254), paratypes CHIOC (39255–39259), vouchers CHIBB (561–568L).
Representative DNA sequences. 1380-bp-long sequence of the 28S rDNA gene – GenBank accession number MN947608; 757-bp-long sequence of the mtCOI gene – GenBank accession number MN922321.
ZooBank registration. urn:lsid:zoobank.org:act:9D20E486-2B1C-4023-A976-C5D8B7C73BFB, according to the regulations of the International Code of Zoological Nomenclature (ICZN, 2012).
Etymology. The species is named after Edmir Daniel Carvalho (in memoriam), professor and researcher at the São Paulo State University, Institute of Biosciences, Campus of Botucatu, São Paulo State, Brazil, who dedicated his life to the study of ecology and environmental impacts on rivers and reservoirs caused by anthropic actions, and actively participated in the development of our project.
Description
Based on nine specimens mounted in Gray and Wess' medium, two in Hoyer's medium, three mounted in GAP and one stained with Gömöri's trichrome (fig. 3a‒e). Body pyriform, stout, 525 (432–661; n = 8) long, 151 (88–203; n = 8) wide. Two terminal cephalic lobes, head organs, cephalic glands poorly developed. Pharynx subspherical, muscular, 43 (35–46; n = 3) in diameter. MCO 61 (54–66; n = 9) long, counterclockwise C-shaped, measuring 19 (19–20; n = 5) in diameter; weakly sclerotized fringe surrounding wide base of MCO with presence of lateral flap, non-articulated with accessory piece. Accessory piece 52 (46–58; n = 9) long, C-shaped, relatively robust. Gonads intercaecal. Testis dorsoposterior to germarium, elongated 47 (33–57; n = 3) long, 16 (n = 1) wide. Vas deferens looping left intestinal caecum. Germarium 96 (68–150; n = 4) long, 43 (30–60; n = 3) wide. Egg elliptical 63 (58–69; n = 4) long, 40 (36–46; n = 4) wide, with proximal filament, delicate and convoluted. Vagina dextral, non-sclerotized; sac-like seminal receptacle. Haptor 97 (81–123; n = 6) long, 246 (179–288; n = 6) wide, an expanded portion of the body, with haptoral appendages relatively long. Anchors 49 (44–51; n = 9) long, lacking roots, base 13 (11–14; n = 9) wide, elongate; conspicuous sclerotized basal border on base of the anchor; short shaft, elongate point 29 (27–31; n = 9) long, recurved tip; anchor filament double. Bar M-shaped, 195 (182–218; n = 9) long, longitudinal groove along its length, pointed ends. Hooks similar, 14 (13–15; n = 16) long, shank proximally dilated, weakly sclerotized, erect thumb. Filamentous hooklet loop approximately half the shank length. Four pairs of hook-bearing appendages: two bilateral pairs bearing hooks pairs 2, 7; single posteroventral pair branched, bearing hook pairs 3, 4; pair of posterodorsal appendages bearing hook pair 6; hook pairs 1, 5 sessile.

Fig. 3. Trinigyrus carvalhoi n. sp. (composite) of Hypostomus ancistroides (Ihering, 1911) from the Sapucaí-Mirim River, São Paulo State, Brazil, showing: (a) male copulatory complex, dorsal view; (b) bar; (c) anchor; (d) hook; (e) egg.
Remarks
Although the copulatory complex of this new species resembles in shape that of T. peregrinus and T. mourei, the MCO of T. mourei is the shortest and more robust among them (median values: 36 to T. mourei, 61 to T. carvalhoi n. sp., 60 and 72 to T. peregrinus from Japan and Brazil, respectively). Differences in shape of the MCO are also present: J-shaped in T. mourei, one counterclockwise circle in T. peregrinus (see Redescription section) and C-shaped (curved, but not forming a circle) in T. carvalhoi n. sp. The sclerotized fringe on the wide base of the MCO present in T. carvalhoi n. sp. and T. peregrinus (more evident in T. carvalhoi n. sp.) is not present in T. mourei. Comparatively, the accessory piece of T. carvalhoi n. sp. is shorter than that of T. peregrinus (52 (46–58) versus 71 (61–77), respectively).
The base of the anchor of T. carvalhoi n. sp. is slightly more elongate than that observed in T. peregrinus from Brazil, and more similar to that described in T. mourei, although differences in measurements can be verified (see table 2). In contrast, the sclerotized basal border on the base of the anchor is more evident in T. peregrinus when compared to T. carvalhoi n. sp., and apparently absent in T. mourei. Trinigyrus carvalhoi n. sp. and T. peregrinus can be distinguished from each other based on the sclerotization of the vagina (absent in the new species), differences in size and shape of the eggs (63 (58–69) × 40 (36–46), elliptical in T. carvalhoi n. sp. versus 57 × 28 (27–29), ovate in T. peregrinus), differences in size of the haptoral bar (195 (182–218) in T. carvalhoi n. sp. versus 321 (229–358) in T. peregrinus) and differences in total body size (525 (432–661) in T. carvalhoi n. sp. versus 757 (540–963) in T. peregrinus).
Trinigyrus peregrinus Nitta & Nagasawa, 2016
Taxonomic summary
Type host. Pterygoplichthys disjunctivus (Weber, 1991) (Siluriformes: Loricariidae).
Other host. Pterygoplichthys ambrosettii (Holmberg, 1893) (Siluriformes: Loricariidae).
Site in host. Gills.
Type locality. Hija River, Misato, Okinawa city, Japan.
Other localities. Sembaru Reservoir, Sembaru, Nishihara town, Okinawa-jima Island, Okinawa Prefecture, Japan (Nitta & Nagasawa, Reference Nitta and Nagasawa2016), and Aguapeí River, municipality of Castilho (Paraná River basin), São Paulo State, Brazil (21°3′36.20″S, 51°45′38.58″W) (present study).
Specimens studied. Holotype NSMT-Pl 6195, paratypes NSMT-P1 6196–6203 and newly collected specimens from P. ambrosettii from the Aguapeí River.
Prevalence and mean intensity of infestation. P = 12% and MII = 29.4 ± 24.0 (1.0–246.0) from hosts from the Aguapeí River.
Material deposited (present study). Vouchers CHIOC (39260–39263) and CHIBB (569–577L).
Representative DNA sequences. 954-bp-long sequence of the 28S rDNA gene – GenBank accession number LC104308 (Nitta & Nagasawa, Reference Nitta and Nagasawa2016); and 1514-bp-long sequence of the 28S rDNA gene – GenBank accession number MN944890 (present study); 746-bp-long sequence of the mtCOI gene – GenBank accession number MN913212 (present study).
Redescription
Based on 12 specimens mounted in Hoyer's medium, one in GAP medium and two specimens stained with Gömöri's trichrome (fig. 4a‒f). Body robust, pyriform, 757 (540–963; n = 12) long, 197 (139–294; n = 12) wide. Two terminal cephalic lobes poorly developed. Pharynx subspherical, muscular, 59 (50–72; n = 6) in diameter. Oesophagus short. MCO 72 (67–78; n = 12) long, forming one counterclockwise circle measuring 23 (22–23; n = 4) in diameter; weakly sclerotized fringe surrounding wide base of MCO with presence of lateral flap, non-articulated with accessory piece. Accessory piece 71 (61–77; n = 12) long, robust, C-shaped. Vitelline follicles scattered throughout trunk, absent in region of reproductive organs, coextensive with intestinal caeca. Testis dorsal to germarium, elongated 75 (n = 1) long, 22 (n = 1) wide. Vas deferens looping left intestinal caecum. Seminal vesicle elongated, as an enlargement of the vas deferens; two prostatic reservoirs subovate. Germarium 145 (97–193; n = 2) long, 63 (40–85; n = 2) wide. Conspicuous glands in middle part of body (possibly surrounding ootype region). Egg ovate, 57 (n = 2) long, 28 (27–29; n = 2) wide, with proximal filament, delicate and convoluted. Vagina dextral, sclerotized tube; sac-like seminal receptacle. Haptor 189 (109–374; n = 12) long, 389 (282–519; n = 10) wide, an expanded portion of the body, with measurements varying according to arrangement of haptoral appendages; variable number of glandular reservoirs, conspicuous in stained specimens. Ventral anchor/bar complex composed of one pair of anchors, one bar, haptoral appendages relatively long and robust. Anchors 60 (54–65, n = 12) long, base 20 (16–24; n = 12) wide, lacking roots; conspicuous sclerotized basal border on anchor base; short shaft, elongate point with 32 (31–35; n = 12) long and sharply recurved tip; anchor filament double. Bar M-shaped 321 (229–358; n = 12) long, longitudinal conspicuous groove along its length, pointed ends. Hooks similar, 14 (13–15; n = 25) long, with shank proximally dilated, weakly sclerotized; delicate, erected thumb. Filamentous hooklet loop approximately two-thirds of the shank length. Four pairs of hook-bearing appendages: two bilateral pairs, bearing hook pairs 2, 7; single posteroventral pair branched, bearing hook pairs 3, 4; pair of posterodorsal appendages bearing hook pair 6; hook pairs 1, 5 sessile.
Remarks
Trinigyrus peregrinus was described by Nitta & Nagasawa (Reference Nitta and Nagasawa2016) for dactylogyrids from the gills of P. disjunctivus native to South America that were introduced to Japan. The morphology of the holotype (NSMT-Pl 6195) and all paratypes of T. peregrinus deposited in the National Museum of Nature and Science in Japan (NSMT-P1 6196–6203) corresponded to that of the species of Trinigyrus found in this study from P. ambrosettii in Brazil. However, several discrepancies were found among the original description (text and drawings) and the examined specimens.
Some morphological characteristics of T. peregrinus that are not represented in Nitta & Nagasawa (Reference Nitta and Nagasawa2016) were detected in the holotype and paratypes, and also in the specimens collected in Brazil. These characteristics include the presence of a weakly sclerotized fringe on the wide base of the MCO; sclerotized vaginal tube; conspicuous glands in the middle part of the body (possibly surrounding ootype region); overlapping gonads (testis dorsal to germarium as opposed to posterior to ovary in Nitta & Nagasawa, Reference Nitta and Nagasawa2016); glandular reservoirs in the haptor (visible in the stained specimens); conspicuous sclerotized basal border on the base of the anchor; bar with longitudinal groove along its length; and hooks with shank proximally dilated. The discrepancies among the specimens described by Nitta & Nagasawa (Reference Nitta and Nagasawa2016) and the specimens analysed in the present study can also be made when comparing the line drawings (fig. 4a–f) of the present study, and consulting Nitta & Nagasawa (Reference Nitta and Nagasawa2016). Differences in size of some structures between T. peregrinus, P. disjunctivus and P. ambrosettii are presented in table 2.
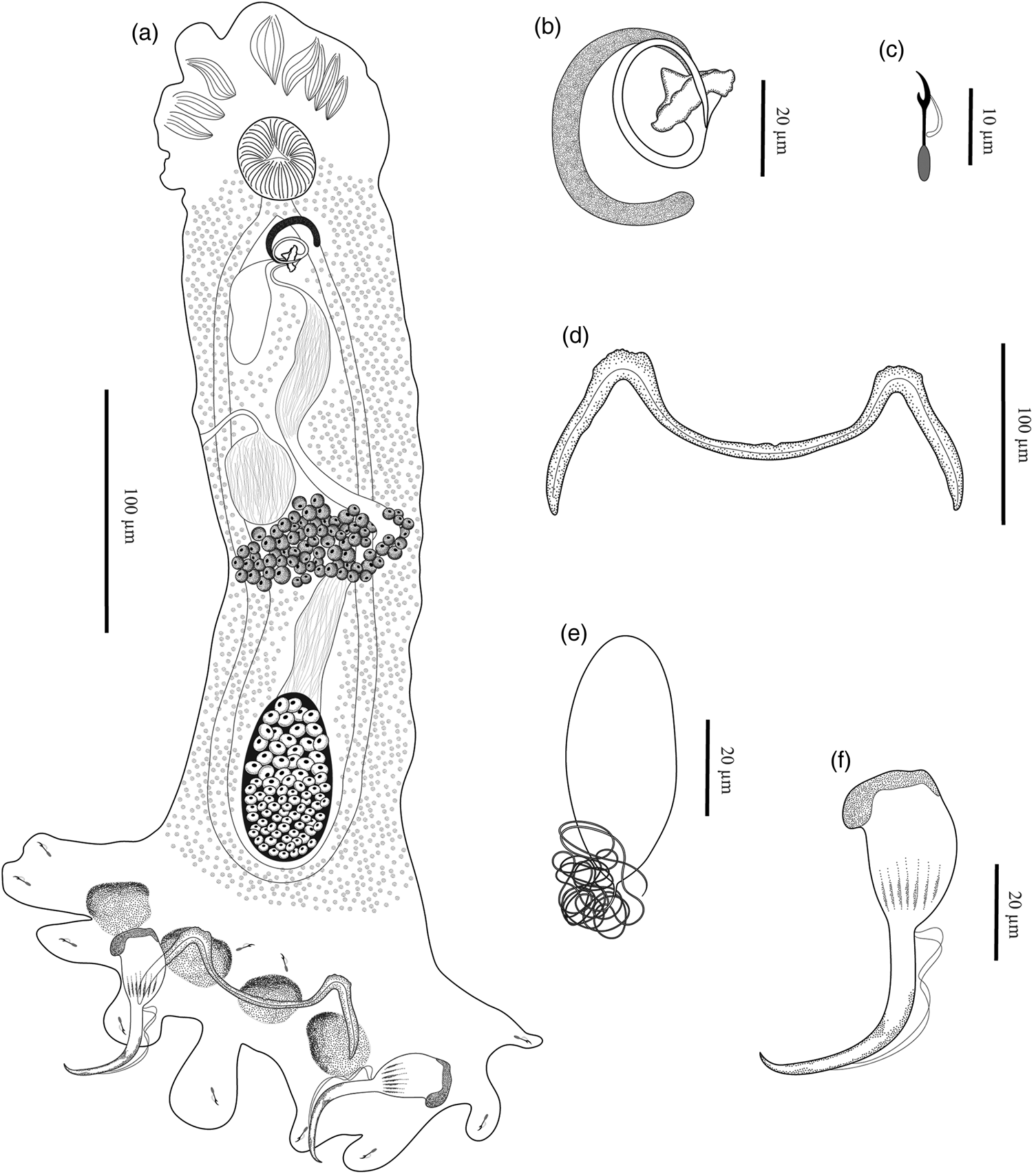
Fig. 4. Trinigyrus peregrinus Nitta & Nagasawa, 2016 of Pterygoplichthys ambrosettii (Holmberg, 1893) from the Aguapeí River, São Paulo State, Brazil, showing: (a) entire body, ventral view (composite); (b) male copulatory complex, dorsal view; (c) hook; (d) bar; (e) egg; (f) anchor.
The two sequences of the partial ribosomal 28S of T. peregrinus, one from P. disjunctivus introduced to Japan and one from P. ambrosettii from Brazil, showed genetic divergence of 6 bp (1% for 28S rDNA analysis). Although discrepancies were observed among the line drawings of T. peregrinus specimens represented by Nitta & Nagasawa (Reference Nitta and Nagasawa2016) and those of the present study, when analysing the museum's paratypes and considering the new molecular data, it was concluded that these discrepancies are a consequence of incongruities in the graphic representativeness of the specimens. This fact reinforces the importance of consulting specimens deposited in collections during the course of taxonomic studies, minimizing the possibility of making incorrect and incomplete descriptions.
Phylogenetic relationships
The BLAST search performed using each of the generated sequences of the new species did not match any other monogenean sequences available in GenBank. The estimates for evolutionary divergences with the partial 28S rDNA gene were compared using the sequences of species of Trinigyrus with 38 other sequences of dactylogyrids and the three sequences of diplectanids used as outgroup retrieved from GenBank, with data varying from 1 to 38% (see supplementary table S1). The genetic divergences among the new species and T. peregrinus varied from 2 to 3% (6–18 bp), among Trinigyrus spp. and Heteropriapulus spp. ranged from 16 to 17% (96–105 bp), and from Trinigyrus spp. and Unilatus unilatus Mizelle & Kritsky, 1967 varied from 13 to 15% (83–96 bp). See supplementary table S1 for information on the genetic divergence values among Trinigyrus spp. using the 28S rDNA gene and each species used in the phylogenetic analyses. The estimates for evolutionary divergences using the mtCOI gene were compared using the species of Trinigyrus with 14 species of dactylogyrids retrieved from GenBank, with data varying from 7 to 12% (1–132 bp) (see supplementary table S2). The genetic divergences among the new species of Trinigyrus and T. peregrinus varied from 6 to 7% (83–92 bp).
Both ML and BI phylogenetic analyses converged in similar topologies with highly supported nodes, with the two main clades labelled as A and B (fig. 5). The Iss indicated no saturation in either transitions or transversions. Critical index of substitution saturation (Iss.c) values were greater than the Iss values.

Fig. 5. Maximum likelihood topology based on partial 28S ribosomal DNA sequences of monogenean parasites of siluriforms. GenBank accession numbers precede species names. New sequences obtained for the present study are in bold. Murraytrema pricei, Pseudorhabdosynochus lantauensis and Pseudorhabdosynochus epinepheli (Diplectanidae) were used as outgroup. Support values are above nodes: posterior probabilities <0.90 and bootstrap scores <60 are not shown or are represented by a dash. Branch-length scale bar indicates number of substitutions per site (see supplementary table S1).
Clade A is strongly supported from both analyses and is divided into two well-supported subclades (A1 and A2). Clade A1 comprises Ameloblastella spp. (from heptapterids and pimelodids), Vancleaveus janauacaensis Kritsky, Thatcher & Boeger 1986 (from doradids) and Unibarra paranoplatensis Suriano & Incorvaia, 1995 (from pimelodids). Clade A2 comprises species that parasitize exclusively loricariids: U. unilatus, Heteropriapulus spp. and Trinigyrus spp. Species of Trinigyrus formed a lineage sister to Heteropriapulus spp. with high support values (fig. 5).
Clade B is also strongly supported and is divided into two well-supported clades: clade B1 (subdivided into B1′ and B1″) and clade B2 comprising Thaparocleidus spp. (from silurids), which forms the basal group of the main clade B1. Clade B1′ (not supported) comprises Cosmetocleithrum spp. (from doradids) and a closely related clade that includes different species of monogenean parasites of pimelodids from Brazil and Peru (Demidospermus spp., Walteriella spp. and Nanayella spp.). The clade B1″ (not supported) includes Demidospermus spp. (from Brazilian loricariids), Aphanoblastella spp. (from heptapterids) and monogenean parasites of marine catfishes, as Hamatopeduncularia spp. and Chauhanellus boegeri Domingues & Fehlauer, 2006 (from ariids) that are closely related to Schilbetrema sp. from freshwater catfishes (Schilbeidae).
Discussion
The erection of the new species proposed is supported by a combination of the differences observed in the morphological and molecular data among Trinigyrus spp. To date, 26 valid species belonging to four dactylogyrid genera, Demidospermus Suriano, 1983 sensu stricto (five species), Unilatus (six species), Trinigyrus (seven species, including the new species described herein) and Heteropriapulus Kritsky, 2007 (eight species) have been commonly reported from loricariid catfishes in the Neotropical region (natural distribution) and from areas where they were co-introduced with their hosts (Nitta & Nagasawa, Reference Nitta and Nagasawa2016; Acosta et al., Reference Acosta, Franceschini, Zago, Scholz and Silva2017, Reference Acosta, Scholz, Blasco-Costa, Alves and Silva2018; Franceschini et al., Reference Franceschini, Zago, Müller, Francisco, Takemoto and Silva2018 and references therein).
Negrelli et al. (Reference Negrelli, Abdallah and Azevedo2017) reported the occurrence of Trinigyrus sp. parasitizing the gills of Astyanax lacustris (Lütken, 1875) (=Astyanax altiparanae Garutti & Britski, 2000) from the Batalha River, State of São Paulo, Brazil. However, the occurrence of Trinigyrus sp. was not reported by the same research group in a revision about parasites of characiforms from the Batalha River collected at a concomitant period, with the specimens analysed by Negrelli et al. (Reference Negrelli, Abdallah and Azevedo2017) (see Dias et al., Reference Dias, Vieira, Camargo, Silva, Azevedo and Abdallah2017 and references therein). In their checklist, the authors reported the occurrence of Trinibaculum altiparanae (Abdallah, Azevedo & Silva, 2013) instead of Trinigyrus sp. in Astyanax specimens. In both studies, the authors did not deposit voucher specimens in any museum collection as they stated.
According to Boeger & Kritsky (Reference Boeger and Kritsky1993), one pair of ventral anchors in the haptor is a synapomorphy for the Class Monogenea (=Class Monogenoidea), whereas two ventral pairs of anchors in the haptor developed later as a synapomorphy for the Order Dactylogyridea (Boeger et al., Reference Boeger, Domingues and Kritsky1997). The occurrence of a single anchor pair in some dactylogyrid species apparently represents multiple examples of independent and secondary loss of either the ventral or dorsal pairs in the evolutionary history of the Dactylogyridae (see Kritsky & Kulo, Reference Kritsky and Kulo1992; Boeger et al., Reference Boeger, Domingues and Kritsky1997 and references therein), such as that observed in Trinigyrus. Besides the loss of the dorsal anchor/bar complex in Trinigyrus spp., it is possible to identify other derived characters, including loss of eyespots, the presence of confluent intestinal caeca and the development of haptoral appendages (Kritsky et al., Reference Kritsky, Boeger and Thatcher1986; Boeger & Kritsky, Reference Boeger and Kritsky1993).
Supported by several shared morphological characters, Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986) proposed the phylogenetic relationship of Trinigyrus with Hamatopeduncularia Yamaguti, 1953, both genera parasitizing freshwater and marine siluriforms, respectively. Although species of Hamatopeduncularia have retained more primitive characteristics when compared to those of Trinigyrus (Kritsky et al., Reference Kritsky, Boeger and Thatcher1986), species of both genera possess haptoral appendages, glandular reservoirs in the haptor (e.g. T. tentaculoides, T. peregrinus and most species belonging to Hamatopeduncularia, such as Hamatopeduncularia arii Yamaguti, 1953, Hamatopeduncularia major Kearn & Whittington, 1994 and Hamatopeduncularia pearsoni Kearn & Whittington, 1994) and a flat posteromedial projection on bar, a common character in some species of Hamatopeduncularia (e.g. Hamatopeduncularia thalassini Bychowsky & Nagibina, 1969 and H. arii), which was also described in T. tentaculoides (Kritsky et al., Reference Kritsky, Boeger and Thatcher1986).
In the present study, phylogenetic analyses based on partial 28S rDNA sequences, considering monogenean parasites of siluriform fishes, showed that Trinigyrus (in the main clade A) and Hamatopeduncularia (in the main clade B) were not closely related, as proposed by Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986). Trinigyrus species clustered together as a sister group to Heteropriapulus spp., and closely related to Unilatus spp., forming a well-supported clade of monogenean parasites of loricariids, specifically fishes belonging to the Hypostominae, from Neotropical freshwater environments (fig. 5). Jogunoori et al. (Reference Jogunoori, Kritsky and Venkatanarasaiah2004) proposed a phylogenetic link among Unilatus, Trinigyrus and Heteropriapulus based only on morphologically shared features. Although Trinigyrus, Unilatus and Heteropriapulus share morphological characters (see Jogunoori et al., Reference Jogunoori, Kritsky and Venkatanarasaiah2004 and references therein), Trinigyrus spp. can be easily recognized because they are the unique representatives of this clade, with a single anchor/bar complex (ventral), bar M-shaped and a redistribution of hooks in haptoral appendages (except the sessile pairs 1 and 5). The phylogenetic relationships among these three genera confirm the phylogenetic link suggested by Jogunoori et al. (Reference Jogunoori, Kritsky and Venkatanarasaiah2004) based on comparative haptoral morphology, but refute the proposal of Kritsky et al. (Reference Kritsky, Boeger and Thatcher1986), once Trinigyrus and Hamatopeduncularia are not closely related.
According to the ‘Fahrenholz rule’, parasites and their hosts speciate in synchrony, with the phylogeny of parasite groups usually corresponding directly to the natural relationships of their hosts, including the closeness of the phylogenetic relationships among them, since the majority of hosts are susceptible to a specific group of these parasites (Eichler, Reference Eichler1948; Kritsky et al., Reference Kritsky, Boeger and Thatcher1986; Kritsky & Kulo, Reference Kritsky and Kulo1992; Thatcher, Reference Thatcher2006; Braga et al., Reference Braga, Araújo and Boeger2014). Considering that siluriforms from the Neotropical region, specifically, do not represent a monophyletic group (Sullivan et al., Reference Sullivan, Lundberg and Hardman2006; Braga et al., Reference Braga, Araújo and Boeger2014), monophyly is also not observed in some groups of monogeneans that parasitize fishes belonging to this order, such as Demidospermus spp. (Mendoza-Palmero et al., Reference Mendoza-Palmero, Blasco-Costa and Scholz2015, Reference Mendoza-Palmero, Mendoza-Franco, Acosta and Scholz2019; Acosta et al., Reference Acosta, Scholz, Blasco-Costa, Alves and Silva2018, Reference Acosta, Mendoza-Palmero, da Silva and Scholz2019; Franceschini et al., Reference Franceschini, Zago, Müller, Francisco, Takemoto and Silva2018).
So far, monophyly is proposed for some genera of monogenean parasites of siluriforms, such as Heteropriapulus, Ameloblastella, Aphanoblastella (Acosta et al., Reference Acosta, Mendoza-Palmero, da Silva and Scholz2019; Mendoza-Palmero et al., Reference Mendoza-Palmero, Mendoza-Franco, Acosta and Scholz2019) and herein the monophyly is also suggested for Trinigyrus. Thereby, the strongly supported clade comprising the species of Trinigyrus, Unilatus and Heteropriapulus, which parasitize only loricariids belonging to the Suborder Loricarioidei (the deepest group of catfish from the Neotropical region – see Kappas et al., Reference Kappas, Vittas, Pantzartzi, Drosopoulou and Scouras2016), enable us to propose that these genera of monogeneans may share an ancient history with their respective hosts.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X20000097.
Acknowledgements
We are most grateful to Edmir Daniel Carvalho (in memoriam), Sandro Geraldo de Castro Britto, Diogo Freitas Souza and Marcos Gomes Nogueira for their logistical support in the collection of hosts; Aline Gouveia de Souza Lins for support in the laboratory analysis; Cláudio Henrique Zawadzki and Walter Antonio Pereira Boeger for taxonomic suggestions; Ummey Shameem, from the Department of Zoology, Andhra University, for kindly providing information about the sequences of Hamatopeduncularia spp. from India used in the present study. We are also very thankful to Célio Ubirajara Magalhães Filho from INPA, Marcelo Knoff from CHIOC and Toshiaki Kuramochi from NSMT for lending us the paratypes of Trinigyrus analysed in this study. Thanks are extended to Masato Nitta for providing the photomicrographs of the holotype of Trinigyrus peregrinus, and to Freya Goetz and Anna Phillips for providing the photomicrographs of the paratypes of Trinigyrus deposited in the USNM. We would also like to thank the Duke Energy, Central Elétrica Anhanguera (CELAN) and the Companhia Energética de São Paulo (CESP) for their logistical support for the sampling expeditions.
Financial support
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES), São Paulo Research Foundation (FAPESP) and Pro-Rectory of Research (PROPe – UNESP) for financial and scientific support, and the post-graduate scholarships granted to M.I.M. (grant number AUX-PE-PNPD 3005/2010; the Young Researcher Program PROPE-UNESP 02/2016; FAPESP: 2017/16546-3); L.F. (FAPESP: 2012/07850-7, 2015/11543-0; CAPES/PNPD 17/2016); A.A.A. (FAPESP: 2012/22895-7, 2015/22382-8) and A.C.Z. (FAPESP: 2011/23588-8, 2015/11542-4, 2016/07829-9). R.J.S. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (309125/2017-0; PROTAX: 440496/2015-2) and FAPESP (2016/50377-1). We would also like to thank the Teaching and Extension Research Support Foundation (FUNEP) (1.01852/2011), Duke Energy and Central Elétrica Anhanguera (CELAN) for their financial support for the sampling expeditions to the Sapucaí-Mirim River..
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.









