Introduction
Helminth infections are among the main causes of economic losses for livestock producers. These infections are not limited to one geographical area; they are widespread throughout the world and they exist in different climates, including tropical, subtropical and temperate regions, posing dangers to livestock animals (Morphew et al., Reference Morphew, Wright, LaCourse and Brophy2011).
Gastrointestinal parasites are potential causes of economic loss to livestock products all over the world (Gasser et al., Reference Gasser, Bott, Chilton, Hunt and Beveridge2008). Marshallagia marshalli lives in the sheep abomasum and belongs to the Trichostrongylidae family. Ruminants are commonly affected by Marshallagia spp., which are very prevalent, and there are more than ten species in the genus Marshallagia. Among them, M. marshalli has the widest distribution and is mainly found in tropical and subtropical regions. The prevalence of M. marshalli is higher is goat, sheep and wild ruminants, and leads to several symptoms such as weight loss, diarrhoea, constipation, loss of appetite and even death (Eslami et al., Reference Eslami, Meydani, Maleki and Zargarzadeh1979).
Conventional chemical anthelmintics are among the first methods used to overcome the harmful effects of helminths. However, their high cost makes them undesirable for use in the livestock production industry. Furthermore, farmers cannot afford these compounds due to their scarcity, which makes them even more less desirable. It is also noteworthy that these parasites have become resistant to many of the existing anthelmintics, including imidazothiazole, benzimidazole and ivermectin, which has made them a major concern in the farming industry (Tomar & Preet, Reference Tomar and Preet2017). Therefore, the development of an effective alternative is important, and can be achieved with nanoparticle-based drug formulations (Gopalakrishnan et al., Reference Gopalakrishnan, Ramesh, Ragunathan and Thamilselvan2012; Tomar & Preet, Reference Tomar and Preet2017).
Various scientific fields such as cancer therapy, drug delivery and medicine take advantage of nanoparticles (NPs) (Nair et al., Reference Nair, Sasidharan, Rani and Raina2009). Their nanoscale size and their significant properties have made them suitable for various biomedical applications (Adeyemi & Whiteley, Reference Adeyemi and Whiteley2013). Their ability to produce reactive oxygen species (ROS) turns them into agents to eliminate infectious agents (Butkus et al., Reference Butkus, Labare, Starke, Moon and Talbot2004; Bhardwaj et al., Reference Bhardwaj, Saudagar and Dubey2012). NPs can pass membrane barriers due to their small dimensions and can cause higher reactivity (Adeyemi & Faniyan, Reference Adeyemi and Faniyan2014). There have been numerous successful attempts in producing NPs that are environmentally friendly and effective in eliminating intestinal parasites (Khan et al., Reference Khan, Singh, Ullah, Shoeb, Naqvi and Abidi2015; Rashid et al., Reference Rashid, Ferdous, Banik, Islam, Uddin and Robel2016; Tomar & Preet, Reference Tomar and Preet2017; Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018; Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020).
Copper (Cu) is an element that is widely used all over the world in various industries, such as the electrical sector, and its affordability has led to its widespread adoption. Recently, due to their powerful effect, metal NPs including CuO-NPs have been used in preventing and controlling parasites such as mosquito larva and Giardia deodenalis (Ramyadevi et al., Reference Ramyadevi, Jeyasubramanian, Marikani and Marimuthu2011; Malekifard et al., Reference Malekifard, Tavassoli and Vaziri2020).
Zinc (Zn) is among the elements necessary for human health; however, it is a toxic element for microorganisms (Chitra & Annadurai, Reference Chitra and Annadurai2013). Zn oxide (ZnO) is a mineral that is available in zincite, which is non-toxic and widely used for human skin disorders (Kalpana & Devi Rajeswari, Reference Kalpana and Devi Rajeswari2018). The fact that they are safe for both humans and animals, and also their stability under various conditions, has led to an increasing amount of attention being given to ZnO-NPs (Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018). They have various physiochemical features and serve as antibacterial and antiparasitic agents with a high level of efficacy (Liu et al., Reference Liu, He, Mustapha, Li, Hu and Lin2009; Tomar & Preet, Reference Tomar and Preet2017; Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018).
Due to the ability of metallic NPs to cause oxidative stress and form free radicals inside biological systems (Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020), the current study hypothesized that NPs of Cu oxide (CuO) and ZnO could be used as anthelmintics by inducing DNA damage and oxidative/nitrosative stress. Therefore, we measured several biomarkers of oxidative markers including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), total antioxidant status (TAS), malondialdehyde (MDA), protein carbonylation (PCO), nitric oxide (NO) and DNA damage to elucidate the possible anthelminthic mechanisms. The current study was also designed to assess the anti-helminthic effects of ZnO-NPs and CuO-NPs by measuring various parameters such as egg hatching, motility of larvae and adult worms, along with intensity protein profile in adult M. marshalli following in vitro treatment with the CuO-NPs and ZnO-NPs.
Materials and methods
Chemicals
Unless stated otherwise, Sigma Chemical Co. (St Louis, MO, USA) was the manufacturer of chemicals used in this study.
NPs
CuO-NPs (stock no. us3070; size = 10–40 nm) and ZnO-NPs (stock no. us3590; size = 10–30 nm) were obtained from a commercial supplier (purchased from Iranian Nanomaterials Pioneers Company, NANOSANY, Mashhad, Iran). The obtained NPs were originally produced by US Research Nanomaterials, Inc, USA. To have a homogenous suspension, the obtained NPs were first dispersed in ultrapure water and then sonicated at 100 W and 40 kHz for 40 min. The ZnO-NPs were then serially diluted in sterile ultrapure water and additionally sonicated for 40 min. During dilution, small magnetic bars were put in the suspensions so that the particles were not aggregated or deposited.
CuO-NP and ZnO-NP suspension preparation
Previously described procedures were used to prepare the nanoparticle suspensions with different concentrations (Khan et al., Reference Khan, Singh, Ullah, Shoeb, Naqvi and Abidi2015). CuO-NP and ZnO-NP stock suspensions were prepared in phosphate-buffered saline (PBS) (pH = 7.4). To prevent agglomeration and achieve a uniform dissolution, a sonicator probe (Branson Sonifier, Danbury, USA) was used to sonicate the solution intermittently for 10 min at 30 W. By diluting the stock solution, the final concentrations of CuO-NPs (1, 4, 8, 12 and 16 ppm) and ZnO-NPs (1, 4, 8, 12 and 16 ppm) were prepared (Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018).
Collection and diagnosis of adult M. marshalli samples
For the purpose of the study, the abomasa with high nematode infection were collected from 20 sheep farms in local abattoirs. A total number of 50 abomasum samples were obtained from newly slaughtered sheep of different breeds, including Haraki, Makoui and Ghezel, and sent to the laboratory of parasitology, Faculty of Veterinary Medicine, Urmia University, in order to isolate and characterize the adult M. marshalli. The obtained worms were separated based on gender and were identified under a light microscope following washing in triplicate with tap water. Subsequently, they were rinsed and placed in PBS (pH = 7.4). They were stored at 37 ± 2°C until the initiation of the analysis. The previously described method was used to mount and identify male and female nematodes based on the morphologic characterization (Lichtenfels & Pilitt, Reference Lichtenfels and Pilitt1989; Hoberg et al., Reference Hoberg, Abrams, Pilitt and Jenkins2012).
Collection and extraction of M. marshalli eggs
The rectally collected faecal samples underwent a larval motility test (LMT) and an egg hatch test (EHT). Sheep populations residing in Urmia, Iran, were used for collecting fresh faecal samples. The samples were collected no longer than one hour after defecation. A portable cooler with an average temperature of 2°C was used for storing the samples, and they were transferred to Urmia University within 3 h after collection. The obtained samples were refrigerated at 1°C for 1–3 days until use.
The technique used by Aleuy et al. (Reference Aleuy, Hoberg, Paquette, Ruckstuhl and Kutz2019) was used to extract M. marshalli eggs from the faeces. A plastic container was used to mix 10 g of faeces obtained from at least five animals and the resulting mixture was representative of the egg sample of the host population. A little tap water was poured into a plastic bag and a sub-sample containing 30 g of faeces was placed inside it. A homogenized sample was obtained by sealing and massaging the plastic bag by hand. A 300-μm sieve was used to pass the homogenized material. The sieve was rinsed with tap water afterwards. The obtained sediments from sieving were placed in a few 3 L beakers. The same sediments were passed through a 63-μm sieve one more time. Tap water was used to wash the resulting material in 250-ml plastic bottles. They were centrifuged for 10 min at 400 g. The pellet was obtained by removing the supernatant, and a 13% salt solution was added after being vortexed. The obtained slurry underwent centrifugation process for 10 min at 400 g. The same 63-μm sieve was used to collect the eggs. Distilled water was used to wash the eggs off the sieves, and they were stored in a plastic petri dish (Aleuy et al., Reference Aleuy, Hoberg, Paquette, Ruckstuhl and Kutz2019).
A dissecting microscope was used to identify M. marshalli eggs under 30–40× magnification and the identified eggs were placed in a separate dish (Aleuy et al., Reference Aleuy, Hoberg, Paquette, Ruckstuhl and Kutz2019). Compared to strongyles eggs, it was easier to distinguish M. marshalli due to their larger size. They also have an elongated shape and the well-developed morula helps one distinguish them (Aleuy et al., Reference Aleuy, Hoberg, Paquette, Ruckstuhl and Kutz2019).
EHT
EHT was conducted based on a modified version of that used by Tomar & Preet et al. (Reference Tomar and Preet2017). About 100–200 eggs dissolved in 0.5 ml of water were poured into the 5-ml test tubes during the experiment. Different concentrations of CuO-NPs and ZnO-NPs were made using 0.5 ml of distilled water. They reached the test volume of 1 ml by the addition of the water containing the eggs. The incubation process was carried out for 24 h at 27°C (Tomar & Preet, Reference Tomar and Preet2017). Distilled water and Albendazole (0.55 mg/mL) were respectively selected as negative and positive controls (Tariq et al., Reference Tariq, Chishti, Ahmad and Shawl2008). The experiment was repeated three times for each concentration. The results of each experiment were recorded as the percentage of egg hatch inhibition. A dissecting microscope was used to count the unhatched eggs and hatched larvae under 40× magnification. Colour change to a darker tone and lack of movement were used as criteria for confirming the viability of M. marshalli eggs.
Third-stage larvae (L3) motility test
A modified version of the method suggested by Aleuy et al. (Reference Aleuy, Hoberg, Paquette, Ruckstuhl and Kutz2019) was used to evaluate the motility of L3 in various concentrations of NPs. The development of M. marshalli to L3 was monitored by placing 200 eggs in distilled water in a petri dish and maintaining the temperature of the petri dish at 30°C (Aleuy et al., Reference Aleuy, Hoberg, Paquette, Ruckstuhl and Kutz2019). The eggs were then incubated for 24 h, and the morphological changes and developmental stages in the eggs and larvae were recorded. Furthermore, the changes were recorded every 24 h until all the eggs had developed to L3. The criteria used by Cruz et al. (Reference Cruz, Allanson, Kwa, Azizan and Izurieta2012) was used for describing the developmental stages. There was no need for any type of medium for the larvae to survive and develop at the time of hatching during the experiment due to the independency of M. marshalli to external nutrition in the process of development from egg to L3 stage (Aleuy et al., Reference Aleuy, Hoberg, Paquette, Ruckstuhl and Kutz2019).
Then, 50 μL of suspension containing 50 L3 and different concentrations of CuO-NPs and ZnO-NPs were added to microdilution plates. The resulting plates were left incubating at 27°C for 24 h (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013). Motile and non-motile larvae were counted, and the counting process was focused on sinusoidal movements of the larvae (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013). Two groups of positive and negative controls were also included in the study. Albendazole (0.55 mg/mL) and distilled water were chosen as positive and negative controls, respectively. Light microscopy and moving the microdilution plates were used to further stimulate the smooth sinusoidal movement. The results were obtained after repeating the experiments for three times and were expressed as inhibition percentage of larval motility (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013).
Adult M. marshalli mortality and mobility tests
The method described by Hounzangbe-Adote et al. (Reference Hounzangbe-Adote, Paolini, Fouraste, Moutairou and Hoste2005) was used to conduct adult M. marshalli mortality and mobility tests. Microdilution plates were used to expose adult M. marshalli to different concentrations of CuO-NPs and ZnO-NPs, and each of the plates contained 20 adult motile worm per well/group. Positive (albendazole 0.55 mg/mL) and negative (PBS) controls were included in the assay (Tariq et al., Reference Tariq, Chishti, Ahmad and Shawl2008). An oven with a temperature of 37°C was used for storing the plates, and the parasites’ mortality and mobility were evaluated every 4 h up to 24 h after the onset of the test under experimental conditions. A dissecting microscope was used to count the number of motile (alive) and immotile (dead) worms and they were recorded separately for each concentration. A qualitative scale with five levels was used to evaluate the parasite mobility. The experiment was done in triplicate before reporting the results as mortality percentage. Percent mortality was calculated for each concentration using the following formula (Tomar & Preet, Reference Tomar and Preet2017):
Homogenization of adult M. marshalli for biochemical assessment
The adult M. marshalli were exposed for 24 h and subsequently washed with deionized water. The method described by Hadaś & Stankiewicz (Reference Hadaś and Stankiewicz1998) was used and the worms were cut into small parts prior to homogenization; 0.1 M PBS at an ice-cold temperature (pH = 7.4) was used for the purpose of homogenization. Then, an ice bath was utilized for the sonication of the samples for 3 × 1 min at intervals of 30 s. The supernatants were collected with centrifugation at 9000 ×g for 15 min at the temperature of 4°C. The resulting supernatants were kept at −20°C.
Antioxidant enzymes activity assessment
An automated biochemistry analyser (BT1500, Rome, Italy) was used to conduct the biochemical analyses. Spectrophotometrical analysis was used to measure the activity of antioxidant enzymes. A commercial standard kit (Randox Laboratories Ltd., Crumlin, UK) was used to determine the SOD activity according to the xanthine–xanthine oxidase assay (McCord & Fridovich, Reference McCord and Fridovich1969). SOD activity was recorded at the wavelength of 505 nm via a standard curve. Disappearance of hydrogen peroxide (H2O2) was used to measure the CAT activity. Supplementation with 3.4 ml of 30% v/v H2O2 started the reaction, and the change in absorbance at 240 nm was checked for 30 s using a blank with PBS (pH = 7.4, 0.1 M) rather than using the substrate (Kotze & McClure, Reference Kotze and McClure2001). A GSH-Px detection kit (Ransel, RanDox Co., UK) was used to evaluate GSH-Px activity and the method described by the manufacturer was followed for the measurement. The reduction of absorbance was spectrophotometrically measured using a blank at 340 nm (Nazarizadeh & Asri-Rezaie, Reference Nazarizadeh and Asri-Rezaie2016). Due to the fact that the units are classified based on the protein content of the parasite homogenate, the Lowry colorimetric method was used to measure the protein level of the supernatant, and the bovine serum albumin was used as the standard (Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018).
Assessment of lipid peroxidation, TAS and NO content
A slightly modified version of the method described by Buege & Aust (Reference Buege and Aust1978) was used to measure MDA as a biomarker of lipid peroxidation. To this end, one volume of homogenate was blended thoroughly with two volumes of a stock solution of 15% v/v trichloroacetic acid, 0.375% v/v thiobarbituric acid and 0.25 mol l-1 hydrochloric acid. Following the heating and cooling periods, the resulting solution was centrifuged at 1000 rpm for 10 min in order to obtain a clear solution. The resulting solution was used for the absorbance analysis and the absorbance at 535 nm was read and the MDA content was calculated using 1.56 × 105 mol-1 cm-1 as molar absorbance coefficient. MDA content was recorded as nmol per mg protein; 2, 2′azino-di-[3-ethylbenzthiazoline sulfonate] (Randox Laboratories Ltd., Crumlin, UK) was used as the substrate for calculating TAS and the result was recorded in micromoles per milligram of protein. Griess reaction method was used for the measurement of the total nitrate/nitrite content of the samples that had parasites (Green et al., Reference Green, Wagner, Glogowski, Skipper, Wishnok and Tannenbaum1982). A 96-well plate was used for mixing 100 μl of supernatant with 100 μl of Griess reagent. The absorbance was recorded at 570 nm after 10 min (Ding et al., Reference Ding, Nathan and Stuehr1988). The NO content of the samples was recorded as nmol per mg of protein in samples.
The method used by Levine (Reference Levine1985) was used for the measurement of PCO. The measurement was conducted by 2,4-dinitrophenylhydrazine, hydrochloric acid, ethanol–ethyl acetate (1:1, v/v) and guanidine hydrochloride solution (6 M). The molar extinction coefficient of 2,4-dinitrophenylhydrazine (ɛ = 2.2 × 104 cm/M) was used for the calculation of carbonyl content.
DNA damage assessment
A slightly modified version of the alkaline comet assay used by Singh et al. (Reference Singh, McCoy, Tice and Schneider1988) was chosen for the analysis of the DNA damage of M. marshalli. The non-invasive extrusion method suggested by Eyambe et al. (Reference Eyambe, Goven, Fitzpatrick, Venables and Cooper1991) was used for obtaining the coelomocytes of the worms following incubation. Visual inspection was the basis for scoring the comets and they were classified based on the DNA amounts in the tails (Azqueta et al., Reference Azqueta, Meier, Priestley and Collins2011). The images were grouped based on the fluorescence intensity (using a fluorescence microscope; Eclipse Ts2R, Nikon, Tokyo, Japan) in the comet tail and they were given a value of 0, 1, 2, 3 or 4. Arbitrary units of 0–400 were used for the total scores (Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020).
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE)
Trichloroacetic acid (TCA)/acetone (10% w/v) was used to precipitate the parasite somatic proteins in a 1:1 ratio over night at the temperature of −20°C and then centrifuged at 10,000 ×g at 4°C for 30 min. The pellet was washed with acetone after discarding the supernatants four times, and the dye-binding method was used to estimate the protein content (Spector, Reference Spector1978). The method described by Shirvan et al (Reference Shirvan, Movassaghi, Khakzad and Abd El Hameed2016) was used to conduct SDS-PAGE. The samples were mixed with loading buffer (0.05 M Tris, pH 6.8, containing 5% (w/v) SDS, 20% (v/v) glycerol, 0⋅01% (w/v) bromophenol blue and 10 mm Dithiothreitol (DTT)) and boiling water was used for heating for 5–10 min (Shirvan et al., Reference Shirvan, Movassaghi, Khakzad and Abd El Hameed2016). The samples that were prepared in advance were placed in the wells and an appropriate voltage was applied to the gel. The proteins were separated using the electrophoretic method. The molecule weight of the protein bands of M. marshalli were measured and the obtained weights were compared to the existing molecular weight marker.
Statistical analysis
SPSS software (version 26, Chicago, IL, USA) was used for statistical analyses. Levene's test was used for testing the homogeneity of variances. One-way and two-way analysis of variance along with Bonferroni post-hoc test were used to compare the analysed parameters between control and treated groups. The data were presented as the mean ± standard deviation and the values lower than 0.05 (P < 0.05) were considered significant.
Results
Physicochemical characterization of CuO-NPs and ZnO-NPs
The crystalline nature of CuO-NPs was determined by X-ray diffraction (XRD) pattern. The XRD pattern of CuO-NPs was obtained at room temperature using a PANalytical X'Pert ProTM X-ray diffractometer, which had a nickel filter by the use of Cu Kα (l = 1.54056 A°) radiations as X-ray source. The Transmission electron microscopy (TEM) diameter of CuO-NPs was calculated as 20 nm on average (fig. 1). Furthermore, TEM characterization from ZnO-NPs using an X-ray diffractometer showed that the highest points of diffraction could be indexed to the hexagonal phase of ZnO-NPs with a crystallite size of 212 Å. The ball-like structure of ZnO-NPs was revealed by a transmission electron microscope and the diameters of the ball-like structures were 20–30 nm (fig. 1).
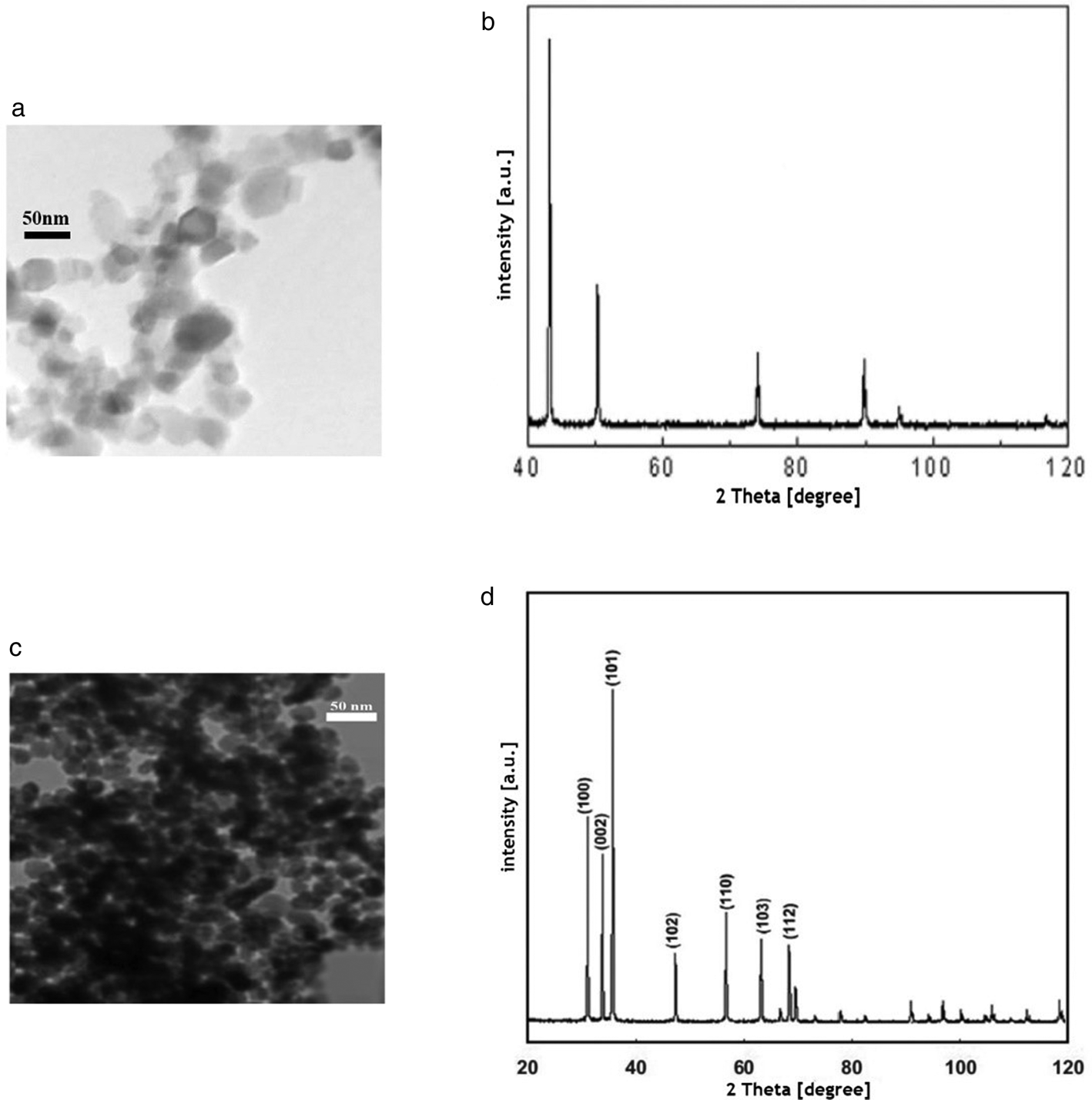
Fig. 1. (A) TEM image of CuO-NPs; (B) X-ray diffraction (XRD) of CuO-NPs; (C) TEM image of ZnO-NPs; (D) XRD of ZnO-NPs.
EHT
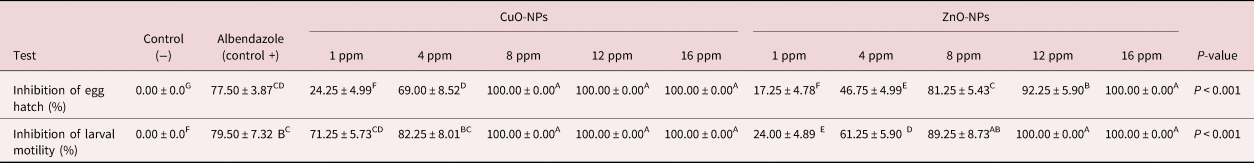
The results obtained from the EHT shown in table 1 depict a significant activity of CuO-NPs and ZnO-NPs; 8, 12 and 16 ppm CuO-NPs and 16 ppm ZnO-NPs had higher inhibition percentages (100%). The mean rate of inhibition for negative controls was measured as 0.00% (fig. 2).
Table 1. Inhibitory effect of CuO-NPs and ZnO-NPs on larval motility and egg hatch tests against Marshallagia marshalli.
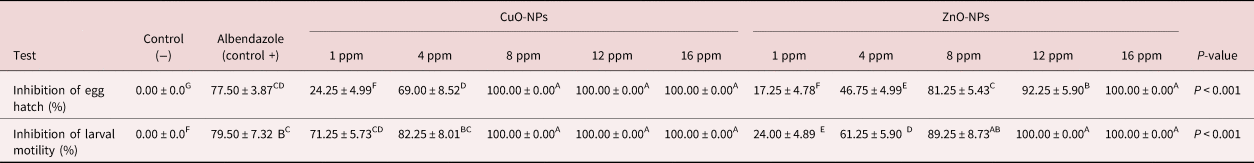
Different superscripts (A–F) within the same row indicate a significant effect.
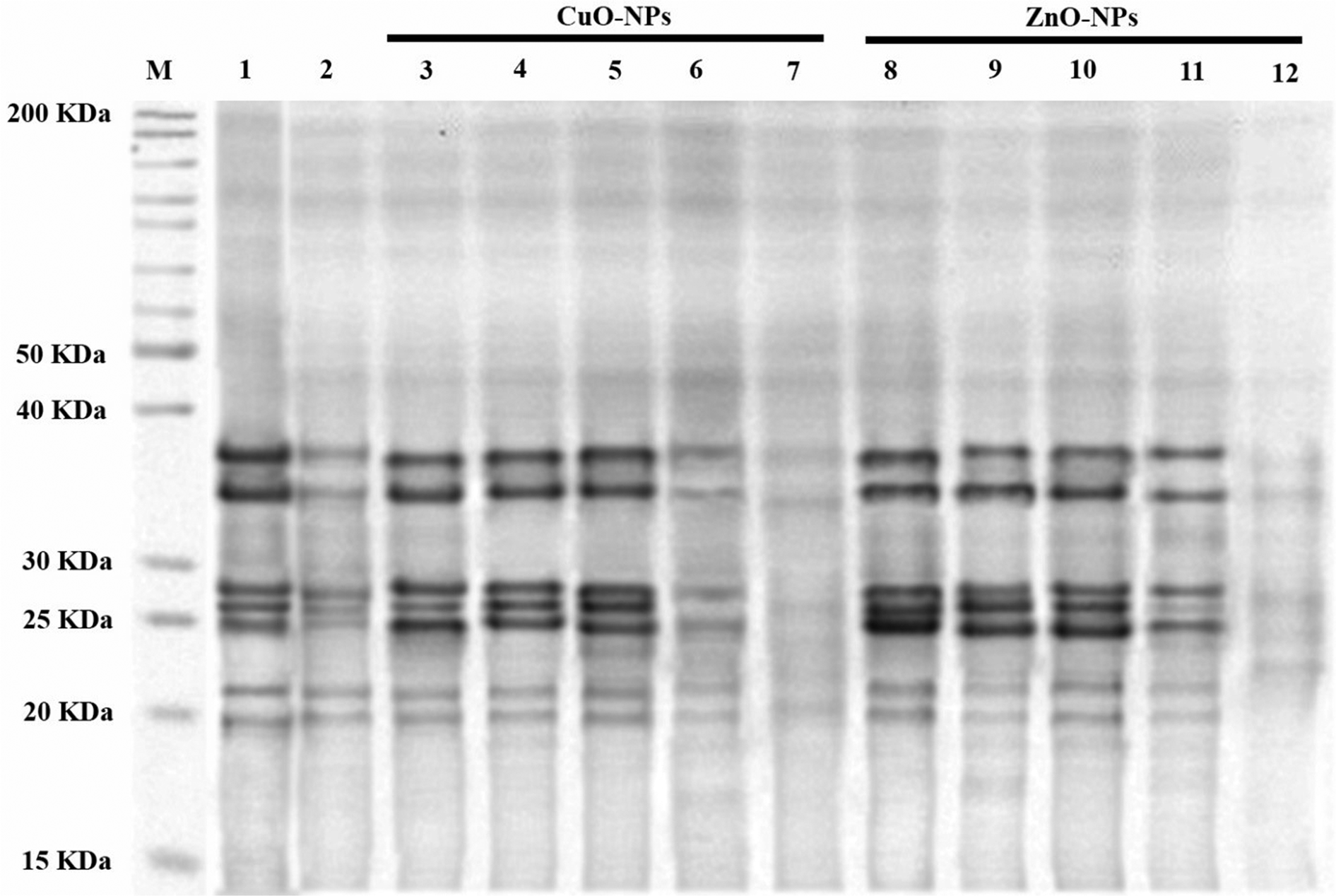
Fig. 2. Change in polypeptide profile expression: the polypeptide profile of TCA/acetone (10%) precipitated somatic proteins following SDS-PAGE. Lane 1: marker; lane 2: control negative; lane 3: control positive; lanes 4–13 are the protein profiles of the worms treated with 1, 4, 8, 12 and 16 ppm CuO-NPs and 1, 4, 8, 12 and 16 ppm ZnO-NPs, respectively.
LMT
As shown in table 1, the CuO-NPs left at 8, 12 and 16 ppm, and ZnO-NPs at 12 and 16 ppm, inhibited the motility rate of L3 by 100%. The positive controls led to inhibition equalling 79.50%, while the motility inhibition rate in the negative controls was 0.00%.
Adult worm mobility test
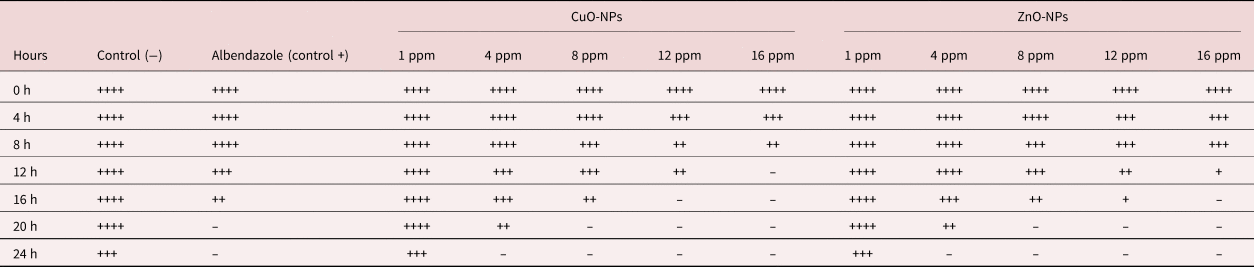
Exposure over 24 h to various concentrations of CuO-NPs and ZnO-NPs has led to the significant inhibition of mobility in adult worms, and the rate of inhibition was higher in comparison with negative controls (table 2). It should be noted that the rate of inhibition was dependent on the exposure time and dose of NPs. In the present study, 16 ppm of CuO-NPs entirely inhibited the mobility of adult worms during the initial 12 h of observation. The same effects were observed for the 12 and 16 ppm of CuO-NPs and 16 ppm of ZnO-NPs during the 16 h of observation (table 2).
Table 2. The effect of various concentrations and incubation time of CuO-NPs and ZnO-NPs on the mobility of Marshallagia marshalli.
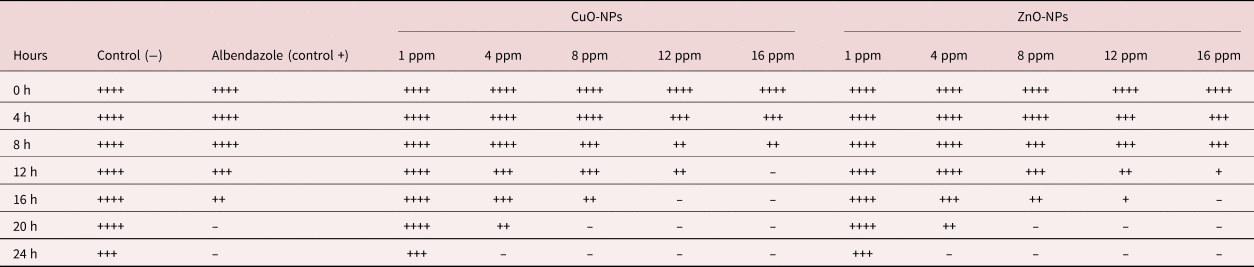
++++, high; +++, moderate; ++, low; +, very low; –, no motility.
Adult worm mortality test
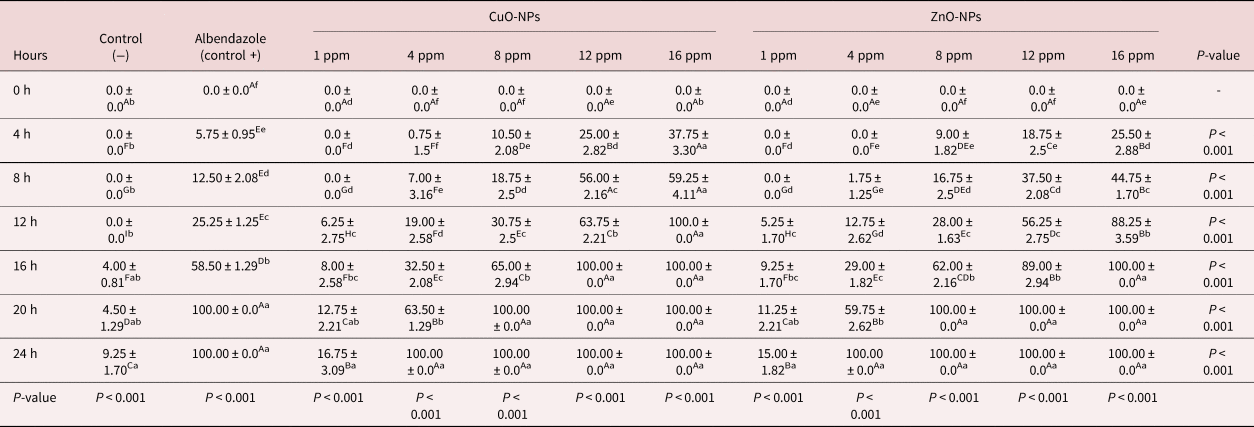
As shown in table 3, an increase in exposure time and ZnO-NP and CuO-NP concentration has led to destroying the adult worms accordingly. Incubation of the adult worms with the lower concentrations (1 and 4 ppm) did not show any detrimental effects on the parasites within the first interval (4 h). It should be noted that the other higher concentrations could destroy the adult worms within four hours. In the present study, 100% mortality was observed with the highest concentration (16 ppm) of ZnO-NPs during the initial 12 h of observation. The same effects were observed for the 12 and 16 ppm of CuO-NPs and 16 ppm of ZnO-NPs during the 16 h of observation. As shown in table 3, a complete mortality effect was noted in positive controls during 20 h after the initiation of observation; however, it differed from that observed for the negative controls, in which about 9.25% of the worms had motility after 24 h.
Table 3. The effect of various concentrations and incubation time of CuO-NPs and ZnO-NPs on the mortality of Marshallagia marshalli.
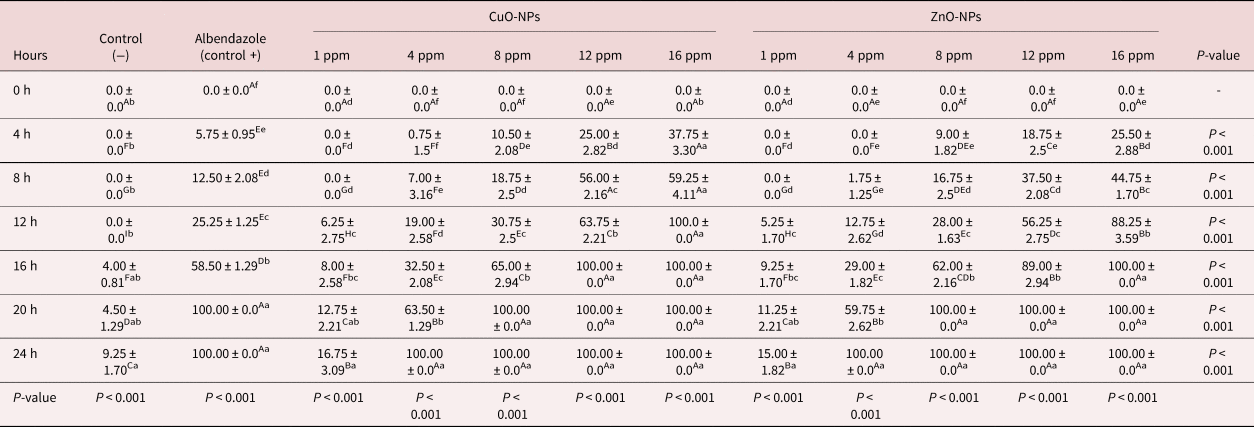
Different superscripts (a–f) within the same row indicate a significant toxicity effects of different concentrations of NPs within each exposure time. Different superscripts (A–I) within the same column indicate a significant toxicity effects of each concentration of NPs during the different exposure times.
Assessment of oxidative/nitrosative stress parameters
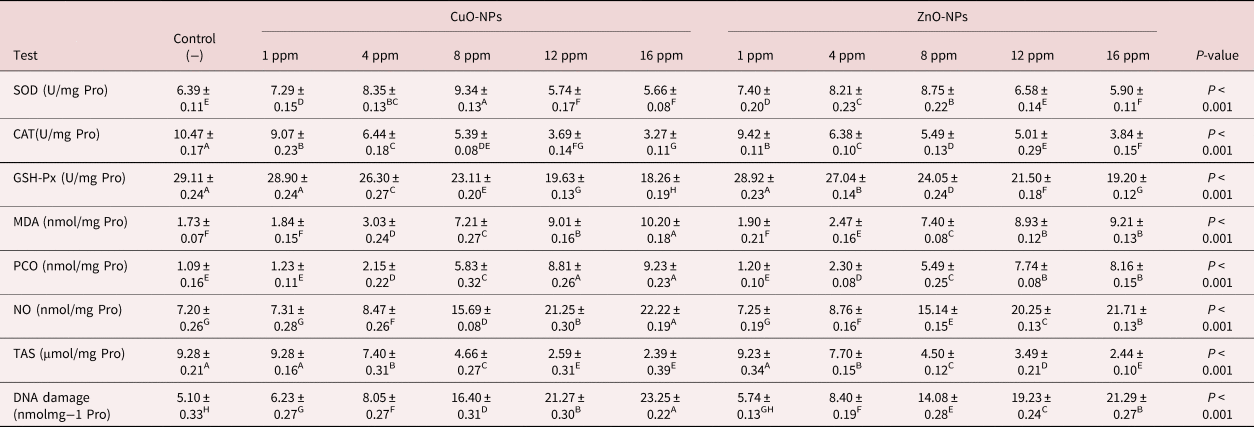
Following 24 h of incubation, oxidative/nitrosative stress indices were evaluated in the homogenate of the parasite. It could be seen that almost all oxidative/nitrosative stress biomarkers showed significant changes after incubation with two metal NPs with various concentrations; however, the lowest concentration (1 ppm of CuO-NPs and ZnO-NPs) did not have any considerable influence on the indicators in comparison with the control Petri dishes. SOD activity was increased in lower concentrations (1, 4, 8 ppm). In other concentrations including 12 ppm of ZnO-NPs, SOD activity was constant and was significantly reduced in the highest concentrations, as shown in table 4. It should be also noted that the highest concentrations (16 and 12 ppm of CuO-NPs and 16 ppm of ZnO-NPs) reduced the CAT activity (table 4). As shown in table 4, the activity of GSH-Px was significantly reduced after being exposed to different concentrations of metal NPs. Increasing concentrations also led to a gradual increase in MDA content. As presented in table 4, the highest concentration (16 ppm of CuO-NPs) increased MDA levels by about five times in comparison with the negative controls. However, increasing CuO-NP and ZnO-NP concentrations significantly suppressed TAS content. The addition of 12 and 16 ppm of CuO-NPs and 16 ppm of ZnO-NPs significantly reduced TAS content by up to three times (table 4). An increase was also noted in levels of PCO and NO after incubation with 16 and 12 ppm of CuO-NP and 16 ppm of ZnO-NP concentrations, as shown in table 4. Marshallagia marshalli DNA damage was evaluated in tail DNA and the results are presented in table 4. The concentration of the NPs influenced the DNA damage in comparison with the negative controls and, as shown in table 4, the highest concentration of CuO-NPs (16 ppm) increased the damage about four-fold in comparison with the negative controls.
Table 4. The effect of various concentrations of CuO-NPs and ZnO-NPs on oxidative/nitrosative stress parameters and DNA damage after 24 h.
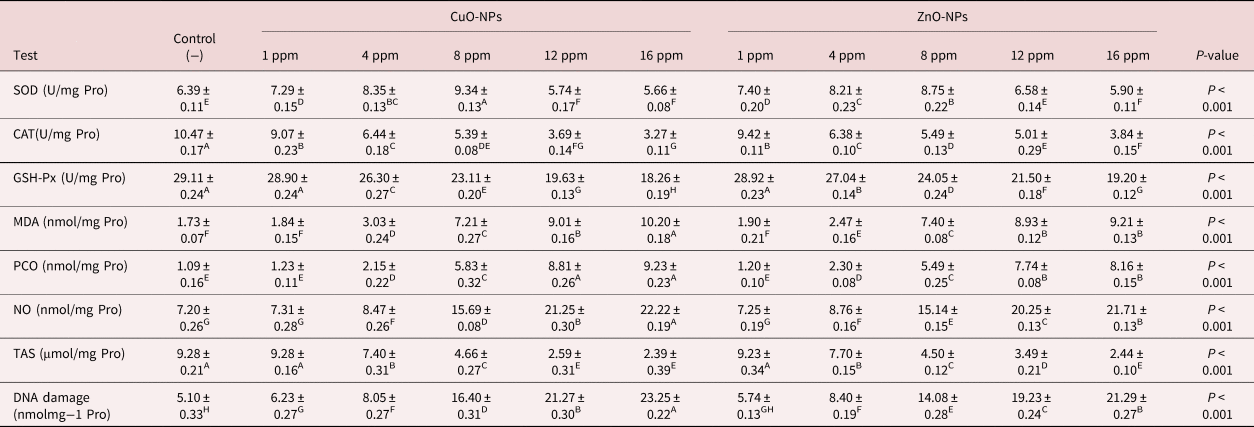
SOD, Superoxide dismutase; CAT, catalase; GSH-xp, glutathione peroxidase; MDA, malondialdehyde; PCO, protein carbonylation; TAS, total antioxidant status; NO, nitric oxide. Different superscripts (A–H) within the same row indicate a significant effect.
Inhibition and under-expression of proteins might lead to low intensity of polypeptide bands
Compared to the control groups, the SDS-PAGE profile of TCA/acetone-precipitated M. marshalli somatic proteins exhibited low-intensity bands of apparent molecular weight (Mr) 20, 22, 25, 27, 28, 35 and 37 kD in 12 and 16 ppm of CuO-NPs and 16 ppm of ZnO-NPs treated M. marshalli. Various factors such as degradation, under-expression or inhibition of the proteins exposed to 12 and 16 ppm of CuO-NPs and 16 ppm of ZnO-NPs might be the reasons for bands with lower intensity (fig. 1).
Discussion
Regarding the oxidative stress induction by CuO-NPs and ZnO-NPs in living organisms (Khan et al., Reference Khan, Singh, Ullah, Shoeb, Naqvi and Abidi2015; Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018; Morsy et al., Reference Morsy, Fahmy, Mohamed, Ali, El-Garhy and Shazly2019; Malekifard et al., Reference Malekifard, Tavassoli and Vaziri2020), the current study aimed to assess oxidative/nitrosative stress biomarkers and DNA damage in adult M. marshalli after exposure to different concentrations of CuO-NPs and ZnO-NPs. Furthermore, egg hatchability, motility of larvae and adult worms, and amounts of DNA damage along with intensity protein profile in adult M. marshalli were semi-quantitatively assessed.
Various in vitro studies have been conducted to assess different parameters such as egg hatching and larval motility (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013) in order to discover new agents as anthelmintic (Costa et al., Reference Costa, Morais, Bevilaqua, Souza and Leite2002; Camurça-Vasconcelos et al., Reference Camurça-Vasconcelos, Bevilaqua, Morais and Vieira2007). In vitro analysis of anthelmintic agents before testing them in vivo is a great strategy for saving time and money, and reduces the number of animals required to develop novel therapeutic agents to treat and control parasites (de Souza Chagas & da Silva Vieira, Reference de Souza Chagas and da Silva Vieira2007; Taíse et al., Reference Taíse, Luciana Ferreira, Almeida and Batatinha2009). The current study indicated that 8, 12 and 16 ppm of CuO-NPs and 16 ppm of ZnO-NPs could lead to egg hatch inhibition, and 8, 12 and 16 ppm of CuO-NPs and 12 and 16 ppm of ZnO-NPs could inhibit larval motility by more than 90%. The present study was consistent with the results of a study by Ferreira et al. (Reference Ferreira, Castro, Chagas, França and Beleboni2013), which showed that the efficacy of anthelmintic agents was proven when they inhibited egg hatching and larval motility by more than 90%. They also showed that in cases where the inhibition was 80–90%, the agent was considered moderately effective (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013).
In the current study, the motility of adult M. marshalli was reduced based on concentration and time. Exposure to the highest concentration (16 ppm) of CuO-NPs for 12 h could entirely destroy the adult M. marshalli. In agreement with our study, Esmaeilnejad et al. (Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018) concluded that exposure to 16 ppm ZnO-NPs could eliminate Haemonchus contortus. Similar results can be found in a study conducted by Dorostkar et al. (Reference Dorostkar, Ghalavand, Nazarizadeh, Tat and Hashemzadeh2017) in which the motility of Toxocara vitulorum was eliminated after 24 h of exposure to 12 ppm concentration of ZnO-NPs. Other studies were focused on the impacts of bio-fabricated silver NPs with two different extracts taken from plants on H. contortus. These studies concluded that biosynthesized NPs had anthelmintic effects on H. contortus (Preet & Tomar, Reference Preet and Tomar2017). Furthermore, it should be noted that CuO-NPs and ZnO-NPs affected three stages of the parasite's life cycle: eggs, larvae and adult parasites. This was very significant due to limiting the resistance of the parasites to treatment and applying anthelmintic action against various stages of worm development (Hounzangbe-Adote et al., Reference Hounzangbe-Adote, Paolini, Fouraste, Moutairou and Hoste2005).
It has been shown that the existence of enzymatic and non-enzymatic antioxidant systems in most parasites may act as a defence mechanism against the oxygen radicals generated by hosts (Chiumiento & Bruschi, Reference Chiumiento and Bruschi2009). In other words, these parasites were protected by antioxidant systems against free radicals generated by hosts. Studies show that even under normal conditions, small amounts of ROS are produced in the parasites (Franklin et al., Reference Franklin, Rogers, Apte, Batley, Gadd and Casey2007). Hence, the antioxidant enzymes residing in the parasites serve a dual purpose and neutralize the oxidant molecules produced both by the host and the parasite. SOD was significantly influenced with in vitro exposure of M. marshalli to different concentrations of CuO-NPs and ZnO-NPs. The activity of this enzyme was increased using lower concentrations of CuO-NPs and ZnO-NPs. These results showed that using lower concentrations of CuO-NPs and ZnO-NPs might increase ROS generation, which could increase the activities of the antioxidant enzyme to deal with the increased oxidative stress. Moderate concentrations of CuO-NPs and ZnO-NPs could lead to a plateau in SOD activity. This might be due to the fact that the detoxification ability of the enzyme reached the highest point, and the enzyme was at work with the highest possible capacity. It seems that similar to H. contortus, M. marshalli had less efficient SOD in comparison with Teladorsagia circumcincta, since SOD activity reached a plateau by the highest concentration of NPs in M. marshalli. The results regarding mortality and mobility also supported this hypothesis (Khan et al., Reference Khan, Singh, Ullah, Shoeb, Naqvi and Abidi2015; Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018; Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020). Several studies have noted that SOD has the highest catalytic efficiency compared to any other enzyme available. Hence, less efficient SOD by M. marshalli could not lead to its standing against oxidative stress.
This study showed that using higher concentrations of CuO-NPs and ZnO-NPs could lead to CAT activity reduction. The study conducted by Khan et al. (Reference Khan, Singh, Ullah, Shoeb, Naqvi and Abidi2015) also showed significant inhibition of SOD and CAT activities in Gigantocotyle explanatum incubated with ZnO-NPs (Khan et al., Reference Khan, Singh, Ullah, Shoeb, Naqvi and Abidi2015). It seems probable that the enzyme was overwhelmed due to higher levels of oxidative stress, or it was likely that free radicals attacked the functional site of CAT and led to denaturation. On the other hand, activity of CAT was readily suppressed in M. marshalli in comparison with H. contortus, similar to T. circumcincta, which shows its low level of efficiency (Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018; Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020). The effects of exposing earthworms to ZnO-NPs had the same effect in CAT activity pattern (Hu et al., Reference Hu, Li, Cui, Li, Chen and Yang2010).
The reduction in GSH-Px activity after exposure to the higher concentrations (12 and 16 ppm) of CuO-NPs and ZnO-NPs could be due to the destruction of antioxidant enzymes or the depletion of minerals or vitamins (Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020). As shown by Baghbani et al. (Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020), in the excessive production of ROS and other free radicals, they attack and damage protein molecules and antioxidant enzymes, thereby reducing their activities.
Evaluating the antioxidant ability of tissues and organs can reveal some hints on the potential of the body to repel oxidative stress (Tiwari et al., Reference Tiwari, Chakraborty, Dhama, Wani, Kumar and Kapoor2014). Hence, the capacity of antioxidants were evaluated along with their synergistic interaction to create a balance between oxidants and antioxidants in vivo (Ghiselli et al., Reference Ghiselli, Serafini, Natella and Scaccini2000). The results indicated the role of CuO-NPs and ZnO-NPs in inducing oxidative stress. It was observed that lipids and proteins were attacked by free radicals and ROS, leading to an increase in MDA and PCO levels. On the contrary, the oxidative damage was reduced by consuming all antioxidants, decreasing TAS levels. These findings were in agreement with previous reports (Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018; Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020).
The current study showed that culture supernatants in CuO-NPs and ZnO-NPs had higher NO levels in comparison with controls. As shown by the study conducted by Soneja et al. (Reference Soneja, Drews and Malinski2005), NO was able to react with various other oxidative molecules including molecular oxygen, ROS, transition metals and thiols to create different reactive nitrogen species; hence, it was able to induce nitrosative stress (Soneja et al., Reference Soneja, Drews and Malinski2005). It is also able to use different pathways to react with DNA. After production, the conversion of NO to nitrous anhydride and/or peroxynitrite causes nitrosative deamination of DNA bases such as guanine and cytosine (Burney et al., Reference Burney, Caulfield, Niles, Wishnok and Tannenbaum1999). This oxidative/nitrosative stress is able to target various biological systems, causing severe damage to biomolecules. As shown by Baghbani et al. (Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020), NO can lead to irreversible changes and destroy any bio-structure even if used in higher concentrations. Having the same results for both of the stress parameters, this shows a close nexus of oxidative and nitrosative rationale in the direct antifilarial effect. Studies have shown that NO acts as a host defence against various pathogens inside the cells (Rajan et al., Reference Rajan, Porte, Yates, Keefer and Shultz1996). The same study also showed that the existence of interaction between the intermediates of reactive oxygen and nitrogen causes synergy between the oxidative burst and NO-mediated effects (Rajan et al., Reference Rajan, Porte, Yates, Keefer and Shultz1996). The current study indicated that the increase in the concentrations of CuO-NPs and ZnO-NPs raised the levels of NO in worms. In agreement with other studies, our study showed that exposure to ZnO-NPs could increase NO levels of nematodes (Esmaeilnejad et al., Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018; Baghbani et al., Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020). Furthermore, the increase in oxidants including chemical mediators elaborated by the cells recruited to mount inflammatory response appears to play a key role in the mediation of direct antimicrobial effect (Chen et al., Reference Chen, Ike and Fujita2002). NO plays a significant role in various physiological pathways; however, its higher reactivity can damage surrounding tissues and cells.
Analysing the oxidative DNA damage both quantitatively and qualitatively in living organism can help with the assessment of genotoxic influences. Reinecke & Reinecke (Reference Reinecke and Reinecke2004) suggested using comet assay as a biomarker of genotoxic influences on invertebrates. The results of the comet assay in our study indicated that the damage to M. marshalli DNA occurred in a concentration-dependent manner. We used 16 ppm of CuO-NPs to treat M. marshalli and it was revealed that this concentration caused structural damage of whole DNA. Our results were in agreement with the study conducted by Hu et al. (Reference Hu, Li, Cui, Li, Chen and Yang2010), showing that exposing Eisenia fetida to ZnO-NPs at 5 g/kg can lead to DNA damage in comparison with control values. Furthermore, the studies conducted by Esmaeilnejad et al. (Reference Esmaeilnejad, Samiei, Mirzaei and Farhang-Pajuh2018) and Baghbani et al. (Reference Baghbani, Esmaeilnejad and Asri-Rezaei2020) concluded that H. contortus and T. circumcincta showed DNA damage while being exposed to ZnO-NPs.
ROS is constant under normal conditions; however, several factors such as drugs, stress and disease can increase ROS levels. ROS mainly targets DNA, proteins and lipids (Marnett, Reference Marnett2000; Stadtman & Levine, Reference Stadtman and Levine2000). The study conducted by Shirvan et al. (Reference Shirvan, Movassaghi, Khakzad and Abd El Hameed2016) used SDS-PAGE to show the protein profile of M. marshalli. The results of SDS-PAGE in the present study showed under-expression of low-intensity protein bands in worms treated with CuO-NPs and ZnO-NPs. In agreement with our study, the study conducted by Khan et al. (Reference Khan, Singh, Ullah, Shoeb, Naqvi and Abidi2015) indicated that the exposure of G. explanatum to ZnO-NPs could lead to low intensity of protein bands following SDS-PAGE. It should be noted that further studies are needed to prove these observations.
In conclusion, the results of our study indicated the anthelmintic effects of CuO-NPs and ZnO-NPs on M. marshalli through oxidative/nitrosative damage to biomolecules. The results also showed that the effects were dependent on the concentrations and higher concentrations (12 and 16 ppm of CuO-NPs, 16 ppm of ZnO-NPs) could suppress M. marshalli's antioxidant systems and damage lipids, proteins and DNA. The present study was conducted under experimental conditions; however, the in vivo effects of CuO-NPs and ZnO-NPs on parasites require further investigation. Furthermore, there is a need for dose-dependent studies in order to obtain the most efficient dose with the lowest level of undesirable in vivo effects, which remains to be investigated in future studies.
Acknowledgements
This paper has been extracted from Dr Tahereh Shafienejad Jalali's Doctor of Veterinary Medicine thesis carried out at Urmia University, and the authors would like to sincerely thank the members of the Faculty of Veterinary Medicine and Urmia University Research Council for the approval and support of this research.
Financial support
This work was supported by the Research Council of Urmia University.
Conflicts of interest
None.
Ethical standards
This study was approved by the Animal Ethics Committee in Urmia University, Urmia, Iran (IR-UU-AEC-75/AD/3-2021) and conducted under the regulations of this committee.
Author contributions
F.M., B. E. and S.A.R. contributed to the conception, design, data collection, statistical analysis and drafting of the manuscript. T.Sh.J, F.M., B.E. and S.A.R. contributed to the conception, design and supervision of the study and drafting of the manuscript. All authors approved the final version for submission.