Introduction
The ‘tetraphyllidean’ genus Duplicibothrium Williams & Campbell, Reference Williams and Campbell1978 currently includes three described species. Duplicibothrium minutum Williams & Campbell, Reference Williams and Campbell1978 was described from the cownose ray, Rhinoptera bonasus (Mitchell), in the western Atlantic Ocean off coastal Virginia and Rhode Island by Williams & Campbell (Reference Williams and Campbell1978) and Duplicibothrium cairae Ruhnke, Curran, & Holbert, Reference Ruhnke, Curran and Holbert2000 and Duplicibothrium paulum Ruhnke, Curran, & Holbert, Reference Ruhnke, Curran and Holbert2000 were described from the Pacific cownose ray, Rhinoptera steindachneri Eigenmann, in the Gulf of California by Ruhnke et al. (Reference Ruhnke, Curran and Holbert2000). Jensen & Bullard (Reference Jensen and Bullard2010) were the first to generate sequence data for the D1–D3 region of the 28S rDNA gene for members of this genus. Their data provided evidence of two undescribed members of the genus in the Gulf of Mexico. The first, Duplicibothrium n. sp. 1, was based on a unique molecular signature obtained from adult specimens collected from hosts that Jensen & Bullard (Reference Jensen and Bullard2010) originally identified as R. bonasus, but which more recent molecular work (Naylor et al., Reference Naylor, Caira, Jensen, Rosana, White and Last2012; Jones et al., Reference Jones, Hoffmayer and Hendon2017) indicates were actually Rhinoptera brasiliensis Müller. The second, Duplicibothrium n. sp. 2, was based on a unique molecular signature that Jensen & Bullard (Reference Jensen and Bullard2010) obtained from larval specimens collected from the gastropods Neverita duplicata (Say), Solenosteira cancellaria (Conrad), and Nassarius vibex (Say). In addition, Jensen & Bullard (Reference Jensen and Bullard2010) generated sequence data for adult specimens that were morphologically consistent with D. minutum collected from cownose rays now considered to be R. brasiliensis. The molecular signature of these specimens was identical to that of larvae which these authors found in the bivalves Donax variabilis Say and Angulus versicolor (De Kay).
Brooks & Barriga (Reference Brooks and Barriga1995) were the first to note key similarities in proglottid anatomy between Duplicibothrium and the monotypic genera Glyphobothrium Williams & Campbell, 1977 and Serendip Brooks & Barriga, Reference Brooks and Barriga1995, both of which also parasitize members of the genus Rhinoptera Cuvier. These features include a highly digitiform ovary, post-ovarian testes, and lateral fields of vitelline follicles that converge towards the medial line of the proglottid on the dorsal surface. These authors proposed that these three genera compose a clade that is the sister group of Dioecotaenia Schmidt, 1969, the sole genus in the family Dioecotaeniidae Schmidt, 1969. They established the family Serendipidae Brooks & Barriga, Reference Brooks and Barriga1995 for that clade (a name later replaced by Serendipeidae Brooks & Evenhuis, Reference Brooks and Evenhuis1995 [see Brooks & Evenhuis, Reference Brooks and Evenhuis1995] to rectify a homonym). Monks et al. (Reference Monks, Zaragoza-Tapia, Pulido-Flores and Violante-González2015b) expanded Serendip beyond just Serendip deborahae Brooks & Barriga, Reference Brooks and Barriga1995, to include Serendip danbrooksi Monks, Zaragoza-Tapia, Pulido-Flores, & Violante-Gozález, Reference Monks, Zaragoza-Tapia, Pulido-Flores and Violante-González2015. Monks et al. (Reference Monks, Pulido-Flores and Gardner2015a) subsequently argued that morphological similarities between Glyphobothrium and Duplicibothrium justified erection of the family Glyphobothriidae Monks, Pulido-Flores, & Gardner, Reference Monks, Pulido-Flores and Gardner2015 to contain these two genera. However, this proposal was not embraced by Caira et al. (Reference Caira, Jensen, Ruhnke, Caira and Jensen2017) who considered Glyphobothriidae to be a junior synonym of Serendipeidae.
Despite the striking similarities in proglottid anatomy among members of Glyphobothrium, Serendip, and Duplicibothrium, the scoleces of the three genera differ conspicuously from one another. Glyphobothrium bears a globose scolex with four superficial bothridia fused to the outer surface of a sizeable scolex proper and the bothridia each bear three columns of loculi. Serendip bears a scolex consisting of four rounded or triangular bothridia each of which bears radially diverging septa, marginal loculi and a marginal velum. The bothridia of Duplicibothrium are arranged in two dorso-ventral fused pairs that vary in locular configuration among species from one to three columns, with or without a posterior row of elongate loculi. Monks et al. (Reference Monks, Pulido-Flores and Gardner2015a) demonstrated that the bothridia of Glyphobothrium and all three described species of Duplicibothrium also bear apical suckers. This is in contrast to Serendip, both species of which bear bothridia that lack apical suckers (Brooks & Barriga, Reference Brooks and Barriga1995; Monks et al., Reference Monks, Zaragoza-Tapia, Pulido-Flores and Violante-González2015b). In the absence of a rigorous phylogenetic analysis that includes representatives of all three genera, the most appropriate higher classification for these three genera remains unclear.
The present study adds another puzzling element to this situation. Examination of the Lusitanian cownose ray, R. marginata (Geoffroy St. Hilaire), and the African cownose ray, Rhinoptera peli Bleeker, off the coast of Senegal led to the discovery of three new species of Duplicibothrium. Although two of these species bear a scolex that resembles that of the other members of the genus, the third exhibits a scolex that more closely resembles that of Serendip. Somewhat surprisingly, the tree resulting from our phylogenetic analysis of sequence data for the D1–D3 region of the 28S rDNA gene that included adult specimens of nine species with the typical scolex morphology of Duplicibothrium collected from cownose rays around the globe, places the species with the unusual scolex morphology robustly among the species of Duplicibothrium with the typical scolex morphology. At a minimum, this result raises questions regarding the mutual monophyly of Serendip and Duplicibothrium.
Materials and methods
Specimen collection
In total, 14 specimens of R. marginata and three specimens of R. peli from fish markets off the coast of Senegal were examined for the new species of Duplicibothrium described here. Specimens of other species of Duplicibothrium included in the molecular phylogenetic analysis were collected from two specimens of R. bonasus off South Carolina, one specimen of Rhinoptera neglecta Ogilby off Australia, one specimen of Rhinoptera jayakari Boulenger off Mozambique, and one specimen of R. brasiliensis off Belize. Each cownose ray was assigned a unique collection code and number, and photographs and measurements were taken. Basic data for each are provided in table 1. More detailed information for these specimens can be accessed in the Global Cestode Database (Caira et al., Reference Caira, Jensen and Barbeau2021) by unique collection code and number (e.g., SE-231). The abdominal cavity of each ray was opened with a mid-ventral incision, and a small sample of liver was taken and preserved in 95% ethanol for molecular verification of host identity. The spiral intestine was then removed and opened with a mid-ventral longitudinal incision. A subset of specimens of each species of cestode found was fixed in 95% ethanol for molecular sequencing; the remaining specimens were fixed in 10% seawater-buffered formalin (9:1) for examination with light and scanning electron microscopy (SEM). The spiral intestine of each ray was subsequently fixed in either 95% ethanol or seawater-buffered formalin. After approximately one week, cestodes and spiral intestines fixed in seawater-buffered formalin were transferred to 70% ethanol for storage. Cestodes and spiral intestines fixed in 95% ethanol were transferred to new 95% ethanol and stored in a 20°C freezer. Spiral intestines were subsequently examined under an Olympus SZ-30 dissecting microscope (Olympus, Center Valley, Pennsylvania), and any additional cestode material was removed and transferred to either 70% or 95% ethanol.
Table 1. Specimens of Rhinoptera examined by species.
Morphological methods
Cestodes were prepared for light microscopy as follows: specimens were hydrated in a graded ethanol series, stained for 20–60 min in a working solution of Delafield's haematoxylin (1:9 mixture of haematoxylin:distilled water), differentiated in tap water, destained in acidic 70% ethanol, neutralized in basic 70% ethanol, dehydrated in a graded ethanol series, cleared in methyl salicylate, mounted in Canada balsam on glass slides under glass coverslips, and left to dry in an oven set to 55°C for 1 wk. Measurements were taken with a Zeiss Axioskop 2 Plus compound microscope (Zeiss, Thornwood, New York) using a SPOT Diagnostic Instrument Digital Camera System and SPOT software (version 4.6; SPOT Imaging Solutions, Sterling Heights, Michigan). Measurements are given in the text as the range (in micrometres unless stated otherwise). In instances in which more than four measurements were taken, the range is following in parentheses by mean, standard deviation, number of specimens measured and total number of measurements in instances in which more than one measurement was made per worm.
One to three scoleces of each of the three new species were prepared for SEM as follows. They were hydrated in a graded ethanol series, transferred to a 1% solution of osmium tetroxide overnight, dehydrated in a graded ethanol series, placed in haexamethyldisilazane in a fume hood for 30 min, and then allowed to air dry. The specimens were then mounted on double-sided PELCO carbon tabs (Ted Pella Inc., Redding, California) on aluminium stubs, sputter-coated with 45 nm of gold/palladium, and examined with an FEI Nova NanoSEM 450 field emission scanning electron microscope (FEI, Hillsboro, Oregon) at the Bioscience Electron Microscopy Laboratory, University of Connecticut (Storrs, Connecticut). Microthrix terminology follows Chervy (Reference Chervy2009).
Museum abbreviations used are as follows: LRP, Lawrence R. Penner Parasitology Collection, University of Connecticut, Storrs, Connecticut, USA; MHNG-INVE, Muséum d'Histroire Naturelle de Genève, Geneva, Switzerland; and USNM, National Museum of Natural History, Smithsonian Institution, Department of Invertebrate Zoology, Washington, DC, USA.
Molecular methods and phylogenetic analysis
Sequence data were generated de novo for the D1–D3 region of the 28S rDNA gene for the following 15 adult specimens of Duplicibothrium. In each case, a hologenophore voucher (sensu Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) was prepared as a whole mount as described above. These consisted of one specimen of D. minutum (KW272) and one specimen of Duplicibothrium n. sp. 2 (KW271) ex R. bonasus (CH-43 and CH-40, respectively); one specimen of Duplicibothrium n. sp. 3 (KW403) ex R. brasiliensis (BE-11); one specimen of Duplicibothrium n. sp. 4 (KW224) and two specimens of Duplicibothrium n. sp. 5 (KW223 and KW226) ex R. jayakari (all from MZ-4); three specimens of Duplicibothrium jeannettae n. sp. (JW186, JW187, and JW558) and one specimen of Duplicibothrium jillae n. sp. (JW553) ex R. marginata (SE-231, SE-231, SE-135, and SE-135, respectively); two specimens of Duplicibothrium n. sp. 6 (KW251 and KW252) ex R. neglecta (CM03-31); two specimens of Duplicibothrium colossum n. sp. (JW190 and JW192) and one specimen of D. jeannettae n. sp. (JW191) ex R. peli (SE-249, SE-251, and SE-249, respectively).
Extraction, amplification, and Sanger sequencing of DNA followed Caira et al. (Reference Caira, Jensen, Hayes and Ruhnke2020). The primer pairs used for amplification were LSU-5 (5′-TAGGTCGACCCGCTGAAYTTA-3′) (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000) and LSU-1500R (5′-GCTATCCTGGAGGGAAACTTCG-3′) (Tkach et al., Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003). The primer pairs used for sequencing were LSU-55F (5′-AACCAGGATTCCCCTAGTAACGGC-3′) (Bueno & Caira, Reference Bueno and Caira2017) and LSU-1200R (5′-GCATAGTTCACCATCTTTCGG-3′) (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000). GenBank numbers for all specimens are provided in fig. 1.

Fig. 1. Phylogenetic tree resulting from maximum likelihood analysis of the D1–D3 region of the 28S rDNA gene for species of Duplicibothrium and Dioecotaenia. Scale bar indicates number of nucleotide substitutions per site within Duplicibothrium; the symbol ‘//’ indicates branches that have been shortened to allow focus on relationships within the genus. Nodes with bootstrap support values ≥90% are indicated with black dots. Taxon labels are presented as cestode and host names followed in parentheses by cestode specimen number, host specimen number, Lawrence R. Penner Parasitology Collection hologenophore accession number (for new sequences only), and GenBank accession number. Newly generated sequences are indicated in boldface type. Adult cestodes collected from definitive hosts are indicated with black host icons; larval specimens collected from intermediate hosts are indicated with grey host icons.
Comparable sequence data were obtained from GenBank for a total of 30 specimens and included in the analysis. These consisted of the following 26 specimens representing three species of Duplicibothrium and one species of Dioecotaenia reported in the Gulf of Mexico by Jensen & Bullard (Reference Jensen and Bullard2010): four larvae of Dupl. minutum from D. variabilis (GQ470134, GQ470135, GQ470139, and GQ470142), two larvae of Dupl. minutum from A. versicolor (GQ470137 and GQ470141), and two adults of Dupl. minutum from R. brasiliensis (GQ470133 and GQ470140); seven adults of Duplicibothrium n. sp. 1 from R. brasiliensis (GQ470125–GQ470130, and GQ470132); four larvae of Duplicibothrium n. sp. 2 from N. duplicata (GQ470146–GQ470148, and GQ470151), four larvae of Duplicibothrium n. sp. 2 from S. cancellaria (GQ470144, GQ470145, GQ470149, and GQ470150), and one larva of Duplicibothrium n. sp. 2 from N. vibex (GQ470152), as well as two adults of Dioe. campbelli from R. brasiliensis. Also included were sequence data for the following four specimens from Healy et al. (Reference Healy, Caira, Jensen, Webster and Littlewood2009): one specimen of Dupl. colossum n. sp. (FJ177135, originally identified as Duplicibothrium n. sp.), one specimen of Dupl. jeannettae n. sp. (FJ177136, originally identified as Duplicibothrium cf. minutum), one specimen of Caulobothrium pedunculatum Coleman, Beveridge, & Campbell, 2019 (FJ177105, originally identified as Caulobothrium n. sp. 5), and one specimen of Caulobothrium opisthorchis Riser, 1955 (FJ177106). The latter two species served as outgroups.
Sequences were initially aligned and trimmed in Geneious Prime 2019.1.3 3 (Biomatters Inc., Newark, New Jersey). They were then realigned using PRANK (Löytynoja & Goldman, Reference Löytynoja and Goldman2010) on the Guidance Server (guidance.tau.ac.il) using the default settings, but with the ‘+F flag’ removed. The best-fitting model of evolution was determined using jModelTest v.2.1.10 (Guindon & Gascuel, Reference Guindon and Gascuel2003; Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) based on evaluation of 88 models on the CIPRES Science Gateway (Miller et al., Reference Miller, Pfeiffer and Schwartz2011). Sample-size corrected Akaike information criterion values were used to evaluate goodness of fit. An MLA was conducted using Garli v. 2.01 (Zwickl, Reference Zwickl2006) on the CIPRES Science Gateway. Default Garli configuration settings were used with the following exceptions: the starting tree topology was set to ‘random’; the number of attachment branches evaluated per terminal was set to 90 (i.e., twice the number of terminals in the matrix); and the number of independent search replicates was set to 100. Based on the results of the jModelTest analysis, GTR + I + G was employed as the model of evolution. Bootstrap (BS) values resulting from 1000 BS replicates were also generated with Garli v. 2.01 on the CIPRES Science Gateway using the configuration settings specified above. Bootstrap values were displayed on the best maximum likelihood (ML) tree using SumTrees v. 4.0.0 (Sukumaran & Holder, Reference Sukumaran and Holder2015) implemented in DendroPy v. 4.0.3 (Sukumaran & Holder, Reference Sukumaran and Holder2010).
Results
Molecular phylogenetic analysis
The tree resulting from our ML phylogenetic analysis is shown in fig. 1. The analysis yielded a strongly supported clade (BS 97%) consisting of all specimens identified as belonging to Duplicibothrium for which data were obtained from GenBank as well all specimens for which sequence data were generated de novo here. As a result of the placement of the specimens of the three species from cownose rays from Senegal – including the species with the unconventional scolex morphology – robustly within this clade, all three of these species are assigned to the genus Duplicibothrium and are described as new below.
Duplicibothrium jeannettae n. sp. (figs 2 and 3a–h)

Fig. 2. Line drawings of Duplicibothrium jeannettae n. sp.: (a) scolex (paratype, National Museum of Natural History, Smithsonian Institution, Department of Invertebrate Zoology, Washington, DC, USA (USNM) No. 1660858); (b) terminal proglottid (paratype, USNM No. 1660858), ventral view; (c) detail of terminal genitalia (paratype, LRP No. 10773); (d) whole worm (holotype, Muséum d'Histoire Naturelle de Genève, Geneva, Switzerland No. PLAT-0138886 ), dorsal view.

Fig. 3. Scanning electron micrographs of Duplicibothrium jeannettae (a–h) and Duplicibothrium jillae (I–L): (a) scolex of D. jeannettae n. sp.; small letters indicate locations of details in micrographs c–h; (b) detail of apical loculus of D. jeannettae n. sp.; (c) papilliform filitriches on loculus in medial column of loculi on distal surface of bothridium of D. jeannettae n. sp.; (d) papilliform filitriches on medial loculus in posterior-most row of loculi on distal surface of bothridium of D. jeannettae n. sp.; (e) papilliform filitriches on proximal surface of posterior region of bothridium of D. jeannettae n. sp.; (f) papilliform filitriches on anterior region of cephalic peduncle of D. jeannettae n. sp.; (g) boundary between papilliform and scutellate regions of cephalic peduncle of D. jeannettae n. sp.; (h) densely arranged capilliform filitriches arranged as scutes on more posterior region of cephalic peduncle of D. jeannettae n. sp.; (i) scolex of Duplicibothrium jillae n. sp.; small letters indicate locations of details in micrographs j–l; (j) papilliform filitriches on apical sucker in anterior loculus on distal surface of bothridium of D. jillae n. sp.; (k) papilliform filitriches on medial loculus on distal surface of bothridium of D. jillae n. sp.; and (l) densely arranged capilliform filitriches arranged as scutes on strobila of D. jillae n. sp.
ZooBank number for species: 61396B96-11B7-4001-AA1B-E41CFDA3ED8F
Based on seven mature and one immature worms, and two scoleces examined with SEM. Worms weakly craspedote, euapolytic, 3.3–4.6 (4 ± 0.5; 7) mm long; maximum width at level of scolex. Proglottids 11–22 (17 ± 4; 7) in total number. Scolex consisting of four bothridia arranged in two dorso-ventral fused pairs (figs 2a and 3a) and elongate cephalic peduncle (fig. 2c). Bothridia lacking apical sucker, pyriform, 366–484 (422 ± 39; 8, 14) long, 180–229 (207 ± 15; 8, 15) wide, with 31 loculi; loculi arranged as single apical loculus followed by two lateral columns of seven loculi, one medial column of six loculi, one row of five square to wider than long loculi, and one row of five longer than wide loculi (fig. 2a). Cephalic peduncle 774–1920 (1298 ± 387; 8) long, 103–134 (116 ± 12; 8) wide. Distal (fig. 3c, d) and proximal (fig. 3e) bothridial surfaces covered with papilliform filitriches; anterior-most regions of cephalic peduncle covered with papilliform filitriches (fig. 3f, g), remainder of length of cephalic peduncle and strobila scutellate; scutes consisting of densely arranged capilliform filitriches (fig. 3h).
Immature proglottids wider than long, becoming longer than wide with maturity. Mature proglottids one (n = 7) in number; terminal mature proglottid (fig. 2b) 514–656 (572 ± 55; 7) long, 186–316 (265 ± 45; 7) wide, length:width ratio 1.7–2.8 (2.2 ± 0.4; 7):1. Testes 21–30 (25 ± 3; 6) in total number, arranged in two irregular columns extending throughout proglottid length, including dorsal to ovary, in two irregular layers, oblong, 30–65 (45 ± 11; 6, 24) long, 31–79 (62 ± 14; 6, 24) wide. Vas deferens minimal, coiled medial and posterior to cirrus sac. Cirrus sac pyriform, slightly angled anteriorly (fig. 2c), 68–96 (78 ± 11; 6) long, 28–56 (47 ± 13; 6) wide; containing coiled cirrus; cirrus armed with spinitriches. Genital pores irregularly alternating, submarginal, 82–90% (88 ± 3; 6) of proglottid length from posterior end of proglottid. Vagina extending from ovarian bridge along midline of proglottid to anterior margin of cirrus sac, then along anterior margin of cirrus sac to open into common genital atrium anterior to cirrus. Ovary terminal in position, highly digitiform, 155–304 (237 ± 51; 6) long, 121–205 (181 ± 31; 6) wide. Vitellarium follicular; ventral vitelline follicles arranged in two lateral bands of multiple columns of follicles extending throughout length of proglottid, interrupted by terminal genitalia, not interrupted by ovary, converging posterior to ovary; dorsal vitelline follicles arranged in two extensive lateral fields converging on mid-line of proglottid; vitelline follicles 8–16 (12 ± 3; 6, 24) long, 10–36 (24 ± 7; 6, 24) wide. Uterus median, ventral, sacciform, extending from ovarian bridge to level of cirrus sac. Excretory ducts four in number, arranged in one dorsal and one ventral pair. Gravid proglottids not observed.
Taxonomic summary
Type host. Lusitanian cownose ray, R. marginata (Geoffroy St. Hilaire) (Myliobatiformes: Rhinopteridae Jordan & Evermann) (host specimen Nos.: SE-84, SE-135, SE-137, SE-138, SE-139, SE-231).
Additional host. African cownose ray, R. peli Bleeker (Myliobatiformes: Rhinopteridae) (host specimen Nos.: SE-249, SE-254).
Site of infection. Spiral intestine.
Type locality. St. Louis (16°01′28″N, 16°30″33″W), Senegal, Atlantic Ocean.
Additional localities. Diogue (12°34′30″N, 16°45′2″W) and Joal (14°10′30″N, 16°51′12″W), Senegal, Atlantic Ocean.
Prevalence of infection. Six of 14 (42.9%) in R. marginata; two of three (66.7%) in R. peli.
Etymology. This species is named after the first author's mother in appreciation of her steadfast and enthusiastic support of his pursuit of scientific endeavours.
Specimens deposited. Holotype (MHNG-INVE No. PLAT-0138886) and one paratype (MHNG-INVE No. PLAT-0138887); three paratypes (USNM Nos. 1660858–1660860); three paratypes (LRP Nos. 10773–10775), two paratype SEM strobila vouchers (LRP Nos. 10776, 10777), scoleces prepared for SEM retained with J. N. Caira (JNC) at the University of Connecticut.
Sequence data. GenBank accession OK358919 (hologenophore, SE-249 [JW191], LRP No. 10414); GenBank accession OK358920 (hologenophore, SE-231 [JW186], LRP No. 10415); GenBank accession OK358921 (hologenophore, SE-135 [JW558], LRP No. 10416); GenBank accession OK358922 (hologenophore, SE-231 [JW187], LRP No. 10417); GenBank accession FJ177136 (hologenophore, SE-254, LRP No. 3928).
Remarks
Duplicibothrium jeannettae n. sp. conspicuously differs from D. paulum in that it is a larger worm (3.3–4.6 mm vs. 0.7–2.9 mm in total length [TL]) that possesses, rather than lacks, a cephalic peduncle. It differs from D. minutum in that the anterior region of its bothridia bear both longitudinal and transverse septa, rather than only transverse septa. It further differs from this species in that the posterior-most region of each bothridium bears a row of five, rather than seven, loculi. This new species most closely resembles D. cairae but differs from this species in that the posterior-most region of each bothridium bears a row of five, rather than seven, loculi. In addition, this new species generally has fewer proglottids (11–22 vs. 20–35) than D. cairae.
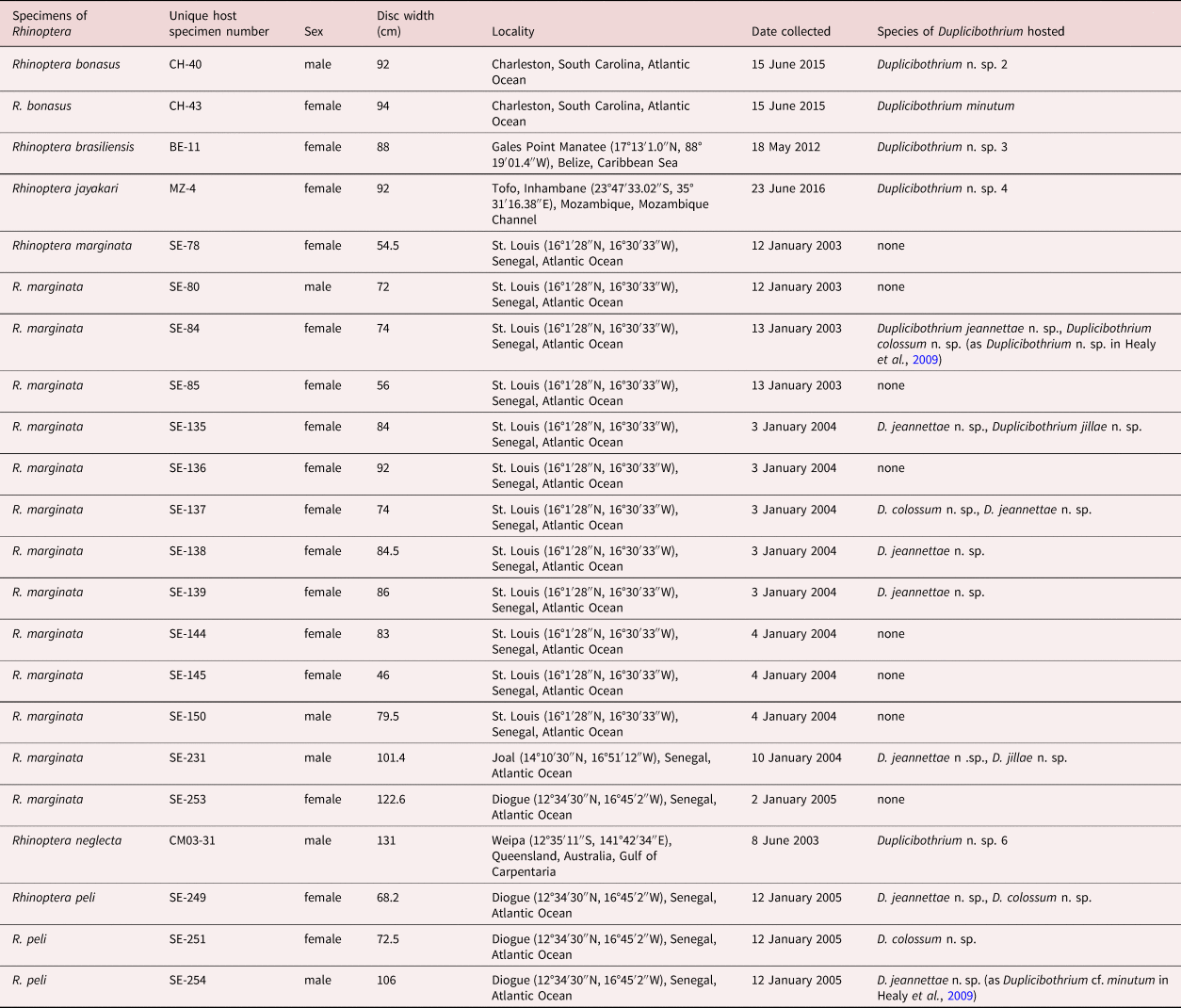
Duplicibothrium jillae n. sp. (figs 3i–l and 4)
Fig. 4. Line drawings of Duplicibothrium jillae n. sp. (holotype, Muséum d'Histoire Naturelle de Genève, Geneva, Switzerland PLAT-0138888): (a) scolex; (b) terminal proglottid, ventral view; (c) detail of terminal genitalia; and (d) whole worm, ventral view.
ZooBank number for species: F0FA1E09-5FBE-4772-AB99-AAAC7E0492C8
Based on four mature worms, and one scolex examined with SEM. Worms weakly craspedote, euapolytic, 0.7–1.0 mm long; maximum width at level of scolex. Proglottids two to four in total number. Scolex consisting of four bothridia arranged in two dorso-ventral fused pairs (figs 3i and 4a). Bothridia oval, 292–363 (325 ± 26; 3, 6) long, 111–126 (117 ± 7; 3, 6) wide, with apical sucker and 59 loculi; loculi arranged as single apical loculus bearing sucker, followed by two lateral columns and one medial column of 18 loculi and five posterior loculi (figs 3i and 4a). Distal (fig. 3j, k) and proximal bothridial surfaces covered with papilliform filitriches; strobila scutellate; scutes consisting of densely arranged capilliform filitriches (fig. 3l). Cephalic peduncle absent (fig. 4d).
Immature proglottids wider than long, becoming longer than wide with maturity. Mature proglottids one (n = 4) in number; terminal mature proglottid (fig. 4b) 218–482 long, 79–126 wide, length:width ratio 1.72–6.1:1. Testes 23–28 in number, arranged in two irregular columns extending throughout proglottid length including dorsal to ovary, in two irregular layers, oblong, 11–30 (19 ± 6; 3, 12) long, 12–36 (24 ± 8; 3, 12) wide. Cirrus sac pyriform, slightly angled posteriorly (fig. 4d), 33–36 long, 28–37 wide; containing coiled cirrus. Cirrus weakly developed in terminal proglottids, armed with spinitriches. Genital pores submarginal, 80–86% of proglottid length from posterior end of proglottid, irregularly alternating. Vagina extending from ovarian bridge along midline of proglottid to anterior margin of cirrus sac then along anterior margin to open into genital atrium anterior to cirrus. Ovary terminal in position, highly digitiform, 82–84 long, 70–74 wide. Vitellarium follicular; ventral vitelline follicles arranged in two lateral bands of multiple columns of follicles extending throughout length of proglottid, interrupted by terminal genitalia, not interrupted by ovary, converging posterior to ovary; dorsal vitelline follicles arranged in two extensive lateral fields converging on mid-line of proglottid; vitelline follicles 3–5 (4 ± 1; 1, 5) long, 5–8 (6 ± 1; 1, 5) wide. Uterus median, ventral, sacciform, extending from ovarian bridge to level of cirrus sac. Excretory ducts four in number, arranged in one dorsal and one ventral pair. Gravid proglottids not observed.
Taxonomic summary
Type and only known host. Lusitanian cownose ray, R. marginata (Geoffroy St. Hilaire) (Myliobatiformes: Rhinopteridae) (host specimen Nos.: SE-135, SE-231).
Additional hosts. None.
Site of infection. Spiral intestine.
Type locality. Joal (14°10′30″N, 16°51′12″W), Senegal, Atlantic Ocean.
Additional localities. St. Louis (16°01′28″N, 16°30′33″W), Senegal, Atlantic Ocean.
Prevalence of infection. Two of 14 (14.3%).
Etymology. This species is named after Dr. Jill Wegryzn in recognition of her contributions to, and interest in, advancing the genomics and transcriptomics of elasmobranch cestodes.
Specimens deposited. Holotype (MHNG-INVE No. PLAT-1038888); two paratypes (USNM Nos. 1660861, 1009862); one paratype (LRP No. 10778), paratype scolex prepared for SEM retained with JNC at the University of Connecticut.
Sequence data. GenBank accession OK358931 (hologenophore, SE-135 [JW553], LRP No. 10425).
Remarks
Duplicibothrium jillae n. sp. differs from D. cairae, D. jeannettae and D. minutum in that it lacks, rather than possesses, a cephalic peduncle. In addition, it is a smaller worm (0.7–1 mm vs. 3.6–9.8 mm, 3.3–4.6 mm, and 2.5–6.5 mm in TL) with fewer proglottids (2–3 vs. 20–35, 11–22, and 6–14) than D. cairae, D. jeannettae and D. minutum, respectively. This new species most closely resembles D. paulum, but generally bears fewer proglottids (2–3 vs. 3–11) and features a posterior row of five, rather than three, loculi on its bothridia. In addition, it exhibits a narrower terminal proglottid (79–126 vs. 133–263).
Duplicibothrium colossum n. sp. (figs 5 and 6)

Fig. 5. Line drawings of Duplicibothrium colossum n. sp.: (a) scolex (holotype, Muséum d'Histoire Naturelle de Genève, Geneva, Switzerland No. PLAT-0138884); (b) terminal proglottid (paratype, National Museum of Natural History, Smithsonian Institution, Department of Invertebrate Zoology, Washington, DC, USA (USNM) No. 1660863), ventral view; (c) detail of terminal genitalia of free proglottid (paratype, Lawrence R. Penner Parasitology Collection, University of Connecticut, Storrs, Connecticut, USA No. 10783); and (d) whole worm (paratype, USNM No. 1660863), ventral view.

Fig. 6. Scanning electron micrographs of Duplicibothrium colossum n. sp.: (a) scolex; small letters indicate locations details in micrographs d–f; (b) detail of apical sucker and two of four flanking loculi; small letter indicates location of detail in micrograph c.; (c) papilliform filitriches on apical sucker on distal surface of bothridium; (d) papilliform filitriches on elongate loculus on distal surface of bothridium; (e) papilliform filitriches on proximal surface of bothridium; and (f) densely arranged capilliform filitriches arranged as scutes on cephalic peduncle.
ZooBank number for species: FA70131B-ED72-4B56-B193-44D166D0FC06
Based on nine mature and one immature worm, one free proglottid and three scoleces examined with SEM. Worms weakly craspedote, euapolytic, 11.2–29.4 (19.4 ± 5.6; 9) mm long; maximum width at level of scolex. Proglottids 85–139 (109 ± 17; 9) in total number. Scolex consisting of four bothridia arranged in two dorso-ventral fused pairs (figs 5a and 6a) and elongate cephalic peduncle (fig. 5d). Bothridia each with apical sucker, oval, wider than long, 425–688 (537± 63; 10, 40) long, 684–1208 (945 ± 145; 10, 40) wide, with 13 loculi; loculi arranged as four small loculi flanking apical sucker on each side and single posterior row of five elongate loculi (figs 5a and 6a); bothridial margins with thin velum (fig. 6a). Cephalic peduncle 2633–4643 (3501 ± 649; 10) long, 246–425 (322 ± 60; 10) wide. Distal (fig. 6c, d) and proximal (fig. 6e) bothridial surfaces covered with papilliform filitriches; cephalic peduncle (fig. 6f) and strobila scutellate; scutes consisting of densely arranged capilliform filitriches.
Immature proglottids wider than long, becoming longer than wide with maturity. Mature proglottids one (n = 9) in number; terminal mature proglottid (fig. 5b) 561–834 (709 ± 92; 9) long, 379–679 (512 ± 77; 9) wide, length:width ratio 1.1–1.9 (1.5 ± 0.3; 9):1. Testes 34–49 (34 ± 7; 6) in number, arranged in four to six irregular columns extending throughout proglottid length including dorsal to ovary, in two irregular layers, oblong, 40–87 (62 ± 11; 6, 24) long, 36–67 (54 ± 8; 6, 24) wide. Cirrus sac pyriform, slightly angled posteriorly (fig. 5d), 71–118 (94 ± 19; 5) long, 62–112 (82 ± 19; 5) wide; containing coiled cirrus; cirrus armed with spinitriches. Genital pores submarginal, 81–88% (85 ± 3; 7) of proglottid length from posterior end of proglottid, irregularly alternating. Vagina extending from ovarian bridge along midline of proglottid to anterior margin of cirrus sac then along anterior margin to open into genital atrium anterior to cirrus. Ovary terminal in position, highly digitiform, 233–312 (279 ± 32; 6) long, 296–371 (326 ± 26; 6) wide. Vitellarium follicular; ventral vitelline follicles arranged in two lateral bands of multiple follicles extending throughout length of proglottid, encroaching towards middle of proglottid anterior to ovary, not interrupted by terminal genitalia or ovary; dorsal vitelline follicles arranged in single extensive dorsal field, interrupted by ovary; follicles 8–16 (12 ± 3; 6, 24) long, 10–36 (24 ± 7; 6, 24) wide. Uterus median, ventral, sacciform extending from anterior margin of ovary to level of cirrus sac. Excretory ducts four in number, arranged in one dorsal and one ventral pair. Gravid proglottids not observed.
Taxonomic summary
Type host. African cownose ray, R. peli Bleeker (Myliobatiformes: Rhinopteridae). (host specimen Nos.: SE-249, SE-251).
Additional host. Lusitanian cownose ray, R. marginata (Geoffroy St. Hilaire) (Myliobatiformes: Rhinopteridae) (host specimen Nos.: SE-84, SE-137).
Type locality. Diogue (12°34′30″N, 16°45′2″W), Senegal, Atlantic Ocean.
Additional localities. St. Louis, (16°01′28″N, 16°30′33″W), Senegal, Atlantic Ocean.
Site of infection. Spiral intestine.
Prevalence of infection. Two of 14 (14.3%) in R. marginata; 2 of 3 (66.7%) in R. peli.
Etymology. The name of this species is derived from the Latin collosum – a thing of immense size – in recognition of the large size of this species relative to its congeners.
Specimens deposited. Holotype (MHNG-INV No. PLAT-0138884) and one paratype (MHNG-INV No. PLAT-0138885); four paratypes (USNM Nos. 1660863–1660866); five paratypes (including one free proglottid, LRP Nos. 10779–10783), paratype scoleces prepared for SEM retained with JNC at the University of Connecticut.
Sequence data. GenBank accession OK358923 (hologenophore, SE-249 [JW190], LRP No. 10418); GenBank accession OK358924 (hologenophore, SE-251 [JW192], LRP No. 10419); GenBank accession FJ177135 (hologenophore, SE-84, LRP No. 3918).
Remarks
This new species is easily distinguished from all five of its described congeners in that its bothridia are wider than long, rather than longer than wide, and in the configuration of its bothridial loculi. Rather than an anterior loculus and/or apical sucker followed by one to three columns of loculi, which in some cases is followed by a posterior-most row of five to seven loculi, the bothridia of D. colossum n. sp. each bear an apical sucker flanked by four small loculi on each side which are then followed by a row of five large, elongate loculi that occupy the majority of the surface of the bothridium. Duplicibothrium colossum n. sp. is also a larger worm than D. cairae, D. jeannettae, D. jillae, D. minutum and D. paulum (11.2–24.4 mm vs. 3.6–9.8 mm, 3.3–4.6 mm, 0.7–1 mm, 2.5–6.5 mm, and 0.7–2.9 mm in TL, respectively).
Morphological similarities between D. colossum n. sp. and Serendip, which include scolex configuration and relatively large TL (11.2–29.4 mm in D. colossum n. sp. vs. up to 60 mm in S. deborahae and 11–23.3 mm in S. danbrooksi), prompts us also to distinguish this new species from both members of Serendip. Duplicibothrium colossum n. sp. differs from S. danbrooksi and S. deborahae in that its bothridia are oval, rather than triangular, and in that the four septa of the posterior row of large loculi are parallel to one another, rather than bifurcating and/or radially diverging from the anterior margin of the bothridium. Furthermore, D. colossum n. sp. bears an apical sucker on each bothridium, whereas this feature appears to be lacking from both species of Serendip.
The diagnosis of Duplicibothrium of Ruhnke et al. (Reference Ruhnke, Curran and Holbert2000) is emended below to accommodate new information for members of this genus provided here as well as by Monks et al. (Reference Monks, Pulido-Flores and Gardner2015a).
Duplicibothrium Williams & Campbell, Reference Williams and Campbell1978 revised
Synonyms. None.
Diagnosis. Serendipeidae. Worms protandrous; proglottids euapolytic, weakly craspedote. Scolex with four bothridia, each with, or occasionally without, apical sucker; dorsal and ventral bothridia fused into two pairs. Bothridial surfaces divided into loculi by horizontal septa, longitudinal septa, or a combination of both types of septa, or occasionally with four small loculi flanking apical sucker on each side and single posterior row of elongate loculi a series of posterior. Cephalic peduncle present or absent. Surfaces of scolex with papilliform filitriches; spinitriches lacking; strobila, and cephalic peduncle when present, scutellate. Testes extending into post-ovarian field, in two irregular dorso-ventral fields. Genital pores in anterior fourth of proglottid, submarginal, irregularly alternating. Vagina opening into genital atrium anterior to cirrus. Ovary digitiform, radiating from central isthmus. Uterus ventral, sacciform, extending to cirrus sac. Vitellarium follicular; in two ventral lateral bands; converging medially in dorsal field. Parasites of cownose stingrays (Rhinopteridae); cosmopolitan in distribution.
Type species. Duplicibothrium minutum Williams & Campbell, Reference Williams and Campbell1978
Additional species. Duplicibothrium cairae Ruhnke, Curran, & Holbert, Reference Ruhnke, Curran and Holbert2000; D. colossum n. sp.; D. jeannettae n. sp.; D. jillae n. sp.; D. paulum Ruhnke, Curran, & Holbert, Reference Ruhnke, Curran and Holbert2000.
Remarks
The establishment of the Glyphobothriidae by Monks et al. (Reference Monks, Pulido-Flores and Gardner2015a) was based on morphological similarities shared by species of Duplicibothrium and Glyphobothrium that do not appear to be shared by species of Serendip – the only genus in the Serendipeidae – such as, for example, the presence, rather than lack, of an accessory sucker on the bothridium, and bothridial septa that extend radially, rather than laterally and/or horizontally. However, the remarkable resemblance between the scolex and strobila of D. colossum and those of both species of Serendip provides morphological evidence of affinities between Duplicibothrium and Serendip that are not shared by Glyphobothrium. The mutual monophyly, and thus validity, of these three genera remain to be confirmed, ideally by molecular phylogenetic work that includes representation of all three groups.
Discussion
Increased global sampling efforts in combination with the application of molecular methods have advanced our understanding of the taxonomy and distribution of species of Rhinoptera substantially over the last decade. Most relevant here, Last et al. (Reference Last, White, Jones, Last, White, de Carvalho, Séret, Stehmann and Naylor2016) resurrected the names R. marginata and R. peli for species with unique molecular signatures that occur off the coast of Senegal (referred to as Rhinoptera sp. 1 and Rhinoptera cf. bonasus, respectively in the molecular work of Naylor et al., Reference Naylor, Caira, Jensen, Rosana, White and Last2012). The northernmost extent of the distribution of R. brasiliensis has been expanded to include the Gulf of Mexico. As a result, the latter species is now considered to co-occur with R. bonasus in this body of water (Jones et al., Reference Jones, Hoffmayer and Hendon2017). This discovery has important implications for our understanding of the host associations of species of Duplicibothrium in the Gulf of Mexico, especially given the close morphological similarities between R. bonasus and R. brasiliensis. When Jensen & Bullard (Reference Jensen and Bullard2010) conducted their work, R. bonasus was widely accepted as the only cownose ray species known to occur in the Gulf of Mexico (e.g., McEachran & de Carvalho, Reference McEachran, de Carvalho and Carpenter2002; Neer & Thompson, Reference Neer and Thompson2005) and thus all cownose rays from which they collected cestodes were identified as R. bonasus. However, sequence data generated by Naylor et al. (Reference Naylor, Caira, Jensen, Rosana, White and Last2012) led to questions about those identifications because all six of the cownose ray specimens from Jensen & Bullard (Reference Jensen and Bullard2010) included in the analysis of Naylor et al. (Reference Naylor, Caira, Jensen, Rosana, White and Last2012) differed substantially in sequence from confirmed specimens of R. bonasus. This led these authors to assign the provisional name Rhinoptera cf. steindachneri to the specimens from the Gulf of Mexico. Subsequent work by Jones et al. (Reference Jones, Hoffmayer and Hendon2017) suggests these specimens are actually R. brasiliensis. The names employed here (and in fig. 1) reflect the revised identities of these specimens of Rhinoptera. One of the main implications of this change is that it now appears that D. minutum parasitizes both R. bonasus and R. brasiliensis.
Overall, our results expand the known host associations of Duplicibothrium beyond R. bonasus, R. brasiliensis and R. steindachneri to include four of the five other species of Rhinoptera recognized by Last et al. (Reference Last, White, Jones, Last, White, de Carvalho, Séret, Stehmann and Naylor2016) (i.e., R. jayakari, R. marginata, R. neglecta and R. peli). The striking similarities between the species described as Echeneibothrium javanicum Shipley & Hornell, Reference Shipley and Hornell1906 by Shipley & Hornell (Reference Shipley and Hornell1906) and Duplicibothrium – a fact noted previously by Euzet (Reference Euzet, Khalil, Jones and Bray1994) and Ruhnke et al. (Reference Ruhnke, Curran and Holbert2000) – suggests that Rhinoptera javanica Müller & Henle will likely be added to this host list once specimens of Duplicibothrium from this host can be examined in more detail. If this is the case, one or more species of Duplicibothrium is now known from all eight valid species of Rhinoptera.
Our results also greatly expand the number of instances of two or more species of Duplicibothrium parasitizing the same host species, beyond the report by Ruhnke et al. (Reference Ruhnke, Curran and Holbert2000), of D. cairae and D. paulum from R. steindachneri in the Gulf of California. The specimens of R. bonasus from South Carolina hosted D. minutum and Duplicibothrium n. sp. 2 (of Jensen & Bullard, Reference Jensen and Bullard2010). Rhinoptera jayakari hosted Duplicibothrium n. sp. 4 and Duplicibothrium n. sp. 5 off Mozambique. Rhinoptera peli off Senegal hosted D. jeannettae and D. colossum. Rhinoptera marginata off Senegal hosted D. jeannettae, D. colossum and D. jillae. Based on the revised identities of the hosts in the Gulf of Mexico, R. brasiliensis also hosts three species: D. minutum and Duplicibothrium n. sp. 1 (of Jensen & Bullard, Reference Jensen and Bullard2010) in the Gulf and Duplicibothrium n. sp. 3. off Belize. This leads us to believe that examination of a greater number of specimens of R. neglecta is likely to yield at least a second species of Duplicibothrium. These associations also highlight the fact that, unlike their oioxenous congeners, D. jeannettae, D. colossum, and D. minutum each parasitize two species of Rhinoptera. In all three cases, the pairs of Rhinopatera species parasitized are sympatric in distribution. This leads us to wonder if further work might also show this to be true for the other pair of sympatric species, R. javanica and R. jayakari.
This work also extends the geographical distribution of Duplicibothrium to include regions well beyond its current known distribution off the eastern and western coasts of North America. The three new species described here occur off Senegal on the western coast of Africa. Two of the undescribed species included in our molecular analysis (i.e., Duplicibothrium n. sp. 4 and Duplicibothrium n. sp. 5) occur in the western Indian Ocean off Mozambique on the southeastern coast of Africa. The undescribed species Duplicibothrium n. sp. 6 was collected in the Gulf of Carpentaria off Australia. The collection of Duplicibothrium n. sp. 3 off Belize extends the distribution of the genus southward to include the tropical waters off the eastern coast of Central America. If, as a result of the collection of new material from its type locality, E. javanicum is determined to belong to Duplicibothrium, the distribution of the genus would also include the northern Indian Ocean off Sri Lanka.
With respect to life cycle stages, one of the adult forms we found parasitizing R. bonasus had a molecular signature identical to that of the larvae of Duplicibothrium n. sp. 2 that Jensen & Bullard (Reference Jensen and Bullard2010) had found parasitizing a variety of gastropods (Nev. duplicata, Nas. vibex and S. cancellaria) in the Gulf of Mexico. The other adult form we found parasitizing R. bonasus was D. minutum, the larvae of which Jensen & Bullard (Reference Jensen and Bullard2010) had found parasitizing bivalves (A. versicolor and D. variabilis) also in the Gulf. This suggests that although both species of Duplicibothrium co-occur in the same species of definitive host, they may use different groups of molluscs as intermediate hosts. However, substantial additional sampling of a diversity of invertebrates for larvae of Duplicibothrium is required to determine whether this is true in general.
The results of our phylogenetic analysis are interesting given that D. colossum much more closely resembles Serendip than Duplicibothrium in scolex morphology and size. Duplicibothrium colossum grouped robustly (i.e., with a BS support value of >90%) within the clade consisting of the nine other species of Duplicibothrium in the tree resulting from the ML analysis (fig. 1). Furthermore, the three specimens of D. colossum differed from the five specimens of D. jeannettae – a typical member of the genus – by only zero to three base pairs. This leads us to begin to question the validity of Serendip relative to Duplicibothrium – a question that is best resolved by including a representative of one or both species of Serendip in a molecular phylogenetic analysis. However, given Glyphobothrium is the oldest of the three generic names in the Serendipeidae, the relationship of this monotypic genus relative to species of Duplicibothrium and Serendip is equally, and perhaps even more, important to establish before any taxonomic action is taken. The scolex of Glyphobothrium zwerneri Williams & Campbell, 1977 differs from that of members of the other two genera in that it is globular and bears sessile bothridia. In contrast, the proglottids of G. zwerneri closely resemble those of Duplicibothrium and Serendip in possessing a digitiform ovary, post-ovarian testes, and vitelline follicles that encroach on the mid-line of the proglottid. If G. zwerneri is ultimately found to group among species of Duplicibothrium, Glyphobothrium would be the valid generic name. This is another example of discordant phylogenetic signal between scolex morphology and sequence data as reported previously, for example, in the Lecanicephalidea (see Jensen et al., Reference Jensen, Caira, Cielocha, Littlewood and Waeschenbach2016). Moving forward it would be interesting to explore these relationships using additional molecular markers.
A final point worth noting is that the ordinal affinities of the Serendipeidae remain uncertain. Although their affinities with the Dioecotaeniidae seem clear based on morphology (e.g., Ruhnke et al., Reference Ruhnke, Curran and Holbert2000) and preliminary molecular work (Caira et al., Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014 and our results), both families were treated as members of a non-monophyletic ‘Tetraphyllidea’ by Caira et al. (Reference Caira, Jensen, Ruhnke, Caira and Jensen2017). Given the fidelity both families have for species of Rhinoptera and the fact that most species of Rhinoptera have now been examined for these cestodes, little diversity likely remains to be discovered. As a consequence, the challenge of resolving their ordinal affinities will need to focus data for additional molecular markers for known species rather than on denser taxon sampling.
Acknowledgements
We thank Cheikh Tidiane Ba from the Cheikh Anta Diop University and Mady Ndiaye of the University of Dakar for their expert assistance with all aspects of the logistics associated with our fieldwork in Senegal. Tim Ruhnke, Ken Barber and Carrie Fyler also assisted with that fieldwork. We are grateful to Kirsten Jensen for assisting with the collection of tapeworms from cownose rays in Australia, Belize and Mozambique, for providing specimens from South Carolina, as well as for assisting with the content and preparation of fig. 1. Kaylee Herzog and Rachel Guyer assisted with the fieldwork in South Carolina. We are especially grateful to Hannah Ralicki for generating the sequence data for 15 of the specimens of Duplicibothrium included in our phylogenetic analysis.
Financial support
This work was supported with funds from the National Science Foundation (grant numbers 1921404 and 1921411).
Conflicts of interest
Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and international guides on the care and use of animals.









