Introduction
Schistosomiasis is a tropical parasitic disease caused by blood-dwelling trematodes of the genus Schistosoma. It is estimated that at least 290.8 million people required preventive treatment in 2018 (WHO, 2020a, b). Chronic schistosomiasis mansoni is associated with peri-portal fibrosis, progressive occlusion of the portal veins and portal hypertension (Barsoum et al., Reference Barsoum, Esmat and El-Baz2013).
Currently, praziquantel (PZQ) is the only drug available for schistosomiasis treatment. However, resistance to treatment has been reported (Fallon & Doenhoff, Reference Keiser, Chollet, Xiao, Mei, Jiao, Utzinger and Tanner1994; Doenhoff et al., Reference Doenhoff, Cioli and Utzinger2008), making it urgent to develop novel chemotherapeutic alternatives. A number of drug re-positioning studies have been carried out to evaluate the anti-schistosomal efficacy of some drugs, including artemether (Utzinger et al., Reference Utzinger, Shuhua, N'Goran, Bergquist and Tanner2001), artemisinin and omega-3 polyunsaturated fatty acids (El-Beshbishi et al., Reference El-Beshbishi, Taman, El-Malky, Azab, El-Hawary and El-Tantawy2013, Reference El-Beshbishi, Saleh, Abd el-mageed, El-nemr, Abdalla, Shebl and Taman2019), ivermectin (Taman et al., Reference Taman, El-Beshbishi, Tantawy, El-Hawary and Azab2014), hydroxyquinoline (El-Shennawy et al., Reference El-Sayed and Allam2007), mefloquine (Keiser et al., Reference Fallon and Doenhoff2009), Synriam (Mossallam et al., Reference Mossallam, Amer and El-Faham2015) and trioxaquines (Portela et al., Reference Portela, Boissier, Gourbal, Pradines, Collière, Coslédan, Meunier and Robert2012). Besides, some newly synthesized compounds have been tested as schistosomicidal agents, including a novel benzimidazole derivative (El Bialy et al., Reference El Bialy, Taman, El-Beshbishi, Mansour, El-Malky, Bayoumi and Essa2013), a newly synthesized quinoline-based compound (PPQ-8) (Taman et al., Reference Taman, Alhusseiny, Saleh, Youssef, Mansour, Massoud and El-Beshbishi2020), novel phenithionate analogues (Zhou & Huang, Reference Zhou and Huang2017) and thiazole derivatives (Pereira et al., Reference Pereira, Silveira, Amaral, Almeida, Oliveira, Lima and Verjovski-Almeida2019).
Benzimidazole is a bicyclic heteroaromatic compound composed of fused benzene and imidazole. The benzimidazole nucleus allows the possibility of substitution at seven different positions. For example, the introduction of a small substituent into the 2-position or 5-position is characteristic for benzimidazole anthelmintics (Singh & Silakari, Reference Singh, Silakari and Silakari2018).
Benzimidazoles are preferred because of their increased stability, bioavailability and significant biological activity. Benzimidazoles have a variety of applications and can serve as anti-microbial, anti-mycobacterial, anti-viral, anti-HIV, anthelmintic, anti-protozoan, anti-diabetic, anti-oxidant, anti-cancer, anti-psychotic, anti-convulsant and analgesic and anti-inflammatory agents (Pullagura et al., Reference Pullagura, Avdhut Kanvinde and Raja2016).
We conducted the current study to evaluate the therapeutic efficacy of a newly synthesized benzimidazole derivative (BTP-OH) in murine schistosomiasis mansoni infection.
Materials and methods
Drugs
PZQ
PZQ (Biltricide, Alexandria Company for Pharmaceuticals and Chemical Industries, Egypt) was used as a reference drug.
Compound BTP-OH
Synthesis of compound BTP-OH. The synthetic route started by reacting 4-bromophenol (2) and 5-formylthiophen-2-ylboronic acid (1) to yield 5-(4-hydroxyphenyl)thiophene-2-carbaldehyde (3) was conducted according to the reported method (Costa et al., Reference Costa, Batista, Cardoso, Belsley and Raposo2006). Sodium bisulphite was added to a solution of the three in absolute ethanol and stirred for 30 min. Then, o-phenylenediamine derivative was added; the temperature was raised to 110°C and kept heated under reflux for overnight. The reaction mixture was left to cool and evaporated under reduced pressure. The residue was dissolved in ethyl acetate and washed with water. The organic layer was separated and dried over magnesium sulphate and filtered, then the organic layer was removed under reduced pressure and the crude residue was purified using a chromatography (Hexan/Ethyl acetate, 40:60) solvent system to yield our pure target compound 4-(5-(1H-benzo[d]imidazol-2-yl)thiophen-2-yl)phenol (4), compound BTP-OH (fig. 1).
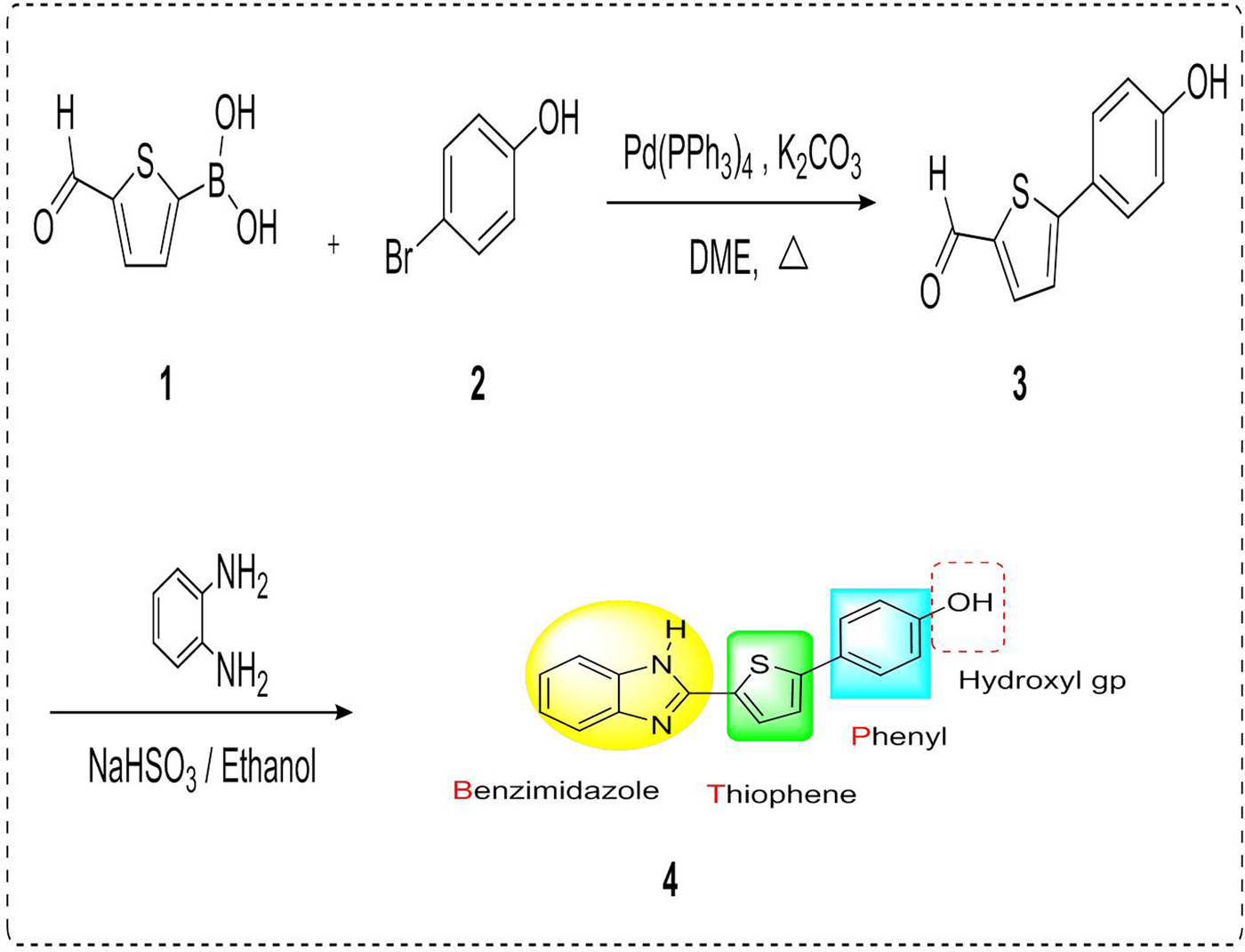
Fig. 1. Structure and synthesis of compound BTP-OH.
Assessment of compound BTP-OH toxicity. Five Swiss albino female mice were daily administered a high dose of compound BTP-OH (600 mg/kg) for five days, and another group of five non-treated mice were used as controls. The mortality rate, clinical signs (as ruffled fur and inactivity), body weights and food consumptions were determined. All mice were euthanized at day seven, and a histopathological examination of major organs (liver, brain, kidney and spleen) was carried out.
Animals, parasites and infection
All animal studies were approved by the Medical Experimental Research Center (MERC), Faculty of Medicine, Mansoura University, Mansoura, Egypt, based on the institutional and national regulations for animal experimentation.
A total of 40 laboratory-bred Swiss albino female mice (CD-I strain, aged 6–8 weeks and weighing 20–25 g) were used. Mice were subcutaneously infected with Schistosoma mansoni cercariae Egyptian strain (60 ± 10 cercariae), freshly shed from infected Biomphalaria alexandrina snails, purchased from Schistosome Biological Supply Center, Theodor Bilharz Research Institute, Giza, Egypt.
Animal groups. Mice were randomly divided into four groups, each comprising ten mice at the beginning of the study:
Group I: infected, non-treated (n = 10).
Group II: infected and treated with PZQ at 42 days post infection (PI) as 500 mg/kg/day for two successive days (n = 10).
Group III: infected and treated with BTP-OH at 49 days PI as 150 mg/kg (n = 10).
Group IV: infected and treated with BTP-OH at 49 days PI as 300 mg/kg (n = 10).
Mice were kept at the MERC, Faculty of Medicine, Mansoura University, Mansoura, Egypt, in an air-conditioned animal house, at 20–22°C, with 12 h light and 12 h dark cycle, and maintained on a standard commercial pellet diet and normal drinking water ad libitum.
Cremophor El 2% and dimethyl sulphoxide were used as solvents for PZQ and BTP-OH, respectively. Drugs were administered by oral gavage using a mouse-feeding needle, in a volume of 200 μl/mouse.
Mice in all groups were euthanized ten weeks PI.
Parasitological studies
Adult worm burden
Adult worms were recovered from porto-mesentric vessel perfusates after euthanasia for subsequent counting (Smithers & Terry, Reference Smithers and Terry1965).
Tissue egg load
Weighted portions from the liver and large intestine were used to assess the number of eggs following potassium hydroxide digestion. The egg load per gram of tissue was estimated (Cheever, Reference Cheever1968).
Oogram pattern
Segments from the middle part of the small intestine (each about 1 cm long) were separated to determine the different egg developmental stages (immature, mature and dead) (Pellegrino et al., Reference Pellegrino, Oliveira, Faria and Cunha1962).
Histopathological study
Liver portions from euthanized mice were fixed in 10% formalin and processed to paraffin blocks. Sections were cut (5 μm thick), and then stained with haematoxylin and eosin to study histopathological changes. Lobular inflammation was categorized based on inflammatory foci at 200× magnification as follows: (0 = none; 1 = 1–2/200×; 2 = up to 4/200×; 3 = >4/200×) (Tandra et al., Reference Tandra, Yeh, Brunt, Vuppalanchi, Cummings, Unalp-Arida, Wilson and Chalasani2011). Focal necrosis in liver cells around the central vein away from granuloma was scored as follows: none (0%); minimal (1–10%); mild (11–30%); moderate (31–60%); and marked (>60% of liver cells were affected) (Suzuki & Toledo-Pereyra, Reference Suzuki and Toledo-Pereyra1993).
Granuloma count and diameter were determined (in three successive microscopic fields of serial tissue sections, >250 μm apart). Granuloma diameter was measured using an ocular micrometre; non-confluent, lobular granulomas containing a single egg in their centres were measured.
Scanning electron microscopy (SEM)
Adult worms recovered from non-treated as well as BTP-OH-treated groups and PZQ-treated were rinsed twice in phosphate-buffered saline, and fixed in glutaraldehyde phosphate 2.5% for 4 h at room temperature, followed by sequential dehydration through incubation for 30 min in increasing concentrations of ethanol (Buchter et al., Reference Buchter, Hess, Gasser and Keiser2018). Worms were processed for examination using SEM (model JSM-6510LV, JEOL, Peabody, MA, USA).
Statistical analysis
Data were analysed using Statistical Package for Social Sciences (SPSS) software (SPSS Inc., Chicago, USA), version 22. Data were presented as mean ± standard deviation. Analysis of variance followed by post-hoc testing using Fisher's least significant difference were used to compare the means of more than two groups. The percentage of reduction was calculated using the equation: (mean value of untreated group-mean value of treated group) × 100/mean value of untreated group. The results were considered significant when P-values were <0.05.
Results
Assessment of compound BTP-OH toxicity
The mice group given a high dose of BTP-OH showed no mortality, no weight loss and normal food consumption, compared to the control group. Macroscopic and microscopic examination of liver, brain, kidney and spleen revealed no pathological changes, compared to the control group.
Assessment of parasitological criteria
Adult worm burden
The administration of PZQ or BTP-OH as 150 or 300 mg/kg significantly reduced male (by 75%, 42.67% and 61.08%, respectively), female (by 71.45%, 48.94% and 68.13%, respectively) and total worm count (by 75.21%, 42.42% and 62.28%, respectively), compared with the infected non-treated group (table 1).
Table 1. Effect of compound BTP-OH on adult worm burden in Schistosoma mansoni-infected mice.

NNumber of mice dead. R%, percentage of reduction compared to infected non-treated group.
Values are presented as mean ± standard deviation.
* Significant difference from infected non-treated group at P < 0.0001.
** Significant difference from both BTP-OH-treated groups at P < 0.01.
*** Significant difference from BTP-OH (150 mg/kg)-treated group at P < 0.01.
Tissue egg load
Significant reductions in hepatic egg load were documented in response to PZQ, BTP-OH (150 mg/kg) and BTP-OH (300 mg/kg) (71.22%, 42.12% and 66.04%, respectively), compared with the infected untreated group. In addition, the same dosing regimens induced significant reductions in intestine egg load (65.61%, 35.48% and 49.81%, respectively), compared with infected untreated mice (table 2).
Table 2. Effect of compound BTP-OH on hepatic and intestinal egg load in Schistosoma mansoni-infected mice.

NNumber of mice died.
Values are expressed as means ± SD. Values enclosed in parentheses indicate the percentage of reduction compared with infected non-treated group.
* Significant difference from infected non-treated group at P < 0.0001.
** Significant difference from both BM3-5-treated groups at P < 0.01.
*** Significant difference from BTP-OH (150 mg/kg)-treated group at P < 0.05.
Oogram pattern
Mice given PZQ showed a shift in the oogram pattern (significant reduction in immature eggs as well as mature eggs, and significant increase in dead eggs). BTP-OH administered as 150 and 300 mg/kg significantly decreased the percentage of immature eggs and significantly increased the percentage of dead eggs, compared with infected non-treated mice (table 3).
Table 3. Effect of compound BTP-OH on oogram pattern in Schistosoma mansoni-infected mice.

NNumber of mice dead. R%, percentage of reduction compared to infected non-treated group.
Values are presented as mean ± standard deviation.
* Significant difference from infected non-treated group at P < 0.05.
** Significant difference from both BTP-OH-treated groups at P < 0.0001.
*** Significant difference from BTP-OH (150 mg/kg)-treated group at P < 0.05.
Histopathological studies and hepatic granuloma count and diameter
Liver sections from infected untreated mice showed liver tissue exhibiting extensive interstitial inflammatory cellular infiltrate with some necrotic hepatocytes, together with obvious granulomatous reaction, showing central living bilharzial ova with intact shell surrounded by inflammatory cells and fibrosis (fig. 2a). Examination of liver sections of PZQ-treated mice revealed granulomatous inflammatory reaction with central degenerated bilharzial ova surrounded by inflammatory cells and fibrosis. The surrounded liver tissue is more or less normal, with mild inflammatory cells (fig. 2b).

Fig. 2. Histopathological study of liver sections of mice infected with Schistosoma mansoni and euthanized ten weeks PI (Hematoxylin and Eosin ×200). (a) Liver tissue from infected non-treated mice showing extensive interstitial inflammatory cellular infiltrate with some necrotic hepatocytes, together with obvious granulomatous reaction, showing central living bilharzial ova with intact shell surrounded by inflammatory cells and fibrosis. (b) Liver tissue showing granulomatous inflammatory reaction with central degenerated bilharzial ova surrounded by inflammatory cells and fibrosis. The surrounded liver tissue is more or less normal, with mild inflammatory cells. (c) Liver tissue showing mild inflammatory changes; the granuloma is smaller in size, the bilharzial ova is dead and cells are significantly less inflammatory. (d) Liver tissue appears more or less normal apart from an area of focal necrosis with lymphocyte infiltrate around degenerated liver cells, which is assumed to be a site of previous granulomatous reaction. Arrow points to the ova.
The administration of BTP-OH as 150 mg/kg ameliorated liver pathology, with less inflammatory cells, and a small number and size of granulomas encircling partially degenerated ova (fig. 2c). With BTP-OH at 300 mg/kg, liver tissue appears more or less normal apart from an area of focal necrosis with lymphocyte infiltrate around degenerated liver cells, which is assumed to be a site of previous granulomatous reaction (fig. 2d). Mice administered PZQ showed the highest significant reduction in hepatic granuloma count (by 51.23%), with no significant reduction in hepatic granuloma diameter, compared to the infected untreated group. BTP-OH as 150 or 300 mg/kg significantly decreased hepatic granuloma count (by 33.87% and 44.77%, respectively), and hepatic granuloma diameter (by 39.23% and 49.40%, respectively), compared to infected non-treated mice (table 4).
Table 4. Effect of compound BTP-OH on granuloma count and diameter in Schistosoma mansoni-infected mice.

NNumber of mice died.
Values are expressed as means ± SD. Values enclosed in parentheses indicate the percentage of reduction compared with infected non-treated group.
* Significant difference from infected non-treated group at P < 0.0001.
** Significant difference from both BTP-OH-treated groups at P < 0.05.
*** Significant difference from PZQ-treated group at P < 0.0001.
**** Significant difference from BTP-OH (150 mg/kg)-treated group at P < 0.05.
SEM
The SEM examination of untreated adult worms recovered from porto-mesentric vessels perfusates revealed normal tegumental ultrastructure (tubercles, spines and inter-tubercular ridges), as well as normal oral and ventral suckers (fig. 3a, b).

Fig. 3. SEM of adult male schistosomes recovered from the studied groups. (a, b) Worms recovered from non-treated mice showing normal tegumental tubercles and normal suckers. (c, d) BTP-OH at a dose of 150 mg/kg showed erosions at the anterior end, deformities at the region of the ventral sucker and tegumental swelling. (e, f) Increasing BTP-OH dose to 300 mg/kg led to the appearance of blebs, vesicle formation, ulcerations, fissuring and destruction of the tegument.
The administration of BTP-OH at a dose of 150 mg/kg resulted in erosions at the anterior end, deformities at the region of the ventral sucker and tegumental swelling (fig. 3c, d). Increasing BTP-OH dose to 300 mg/kg led to the appearance of blebs, vesicle formation, ulcerations, fissuring and destruction of the tegument (fig. 3e, f). Adult S. mansoni treated with PZQ showed ulceration, loss of tubercles and spines (fig. 4a, b).

Fig. 4. Scanning electron microscopy of adult male schistosomes recovered from infected mice treated with PZQ showed smooth surface with loss of tubercles and spines (a), ulceration and sloughing of the tegument (b).
Discussion
Schistosomiasis is a tropical parasitic disease, which represents a major health burden. Hepatosplenic schistosomiasis is associated with hepatocellular failure and fatal complications (Da Silva, Reference Da Silva1992).
Previously, BTP-Iso, a benzimidazole-related compound, was tested as an anti-schistosomal agent on adult S. mansoni and its snail host B. alexandrina (El Bialy et al., Reference El Bialy, Taman, El-Beshbishi, Mansour, El-Malky, Bayoumi and Essa2013; Taman et al., Reference Taman, El-Beshbishi, Bardicy, Tadros, Ayoub, Mansour and El-Bialy2016). In the current study, we evaluated the effect of a new benzimidazole compound, BTP-OH, on mice infected with S. mansoni through a number of parasitological parameters, and histopathological and SEM studies.
In our study, the administration of PZQ and BTP-OH as two treatment regimens (150 and 300 mg/kg) caused significant reductions in male, female and total worm count, compared to the infected non-treated control. Worm reduction may be attributed to tegumental destruction and subsequent elimination by the host immune system.
We observed that better activity was induced by the higher dose of BTP-OH, in terms of worm reductions, and schistosomicidal efficacy was higher in females when using BTP-OH at both doses, while PZQ caused more reduction in male burden. These findings run parallel to the results previously reported by El Bialy et al. (Reference El Bialy, Taman, El-Beshbishi, Mansour, El-Malky, Bayoumi and Essa2013), who documented more reduction in female worm burden, in response to another benzimidazole-derived compound (BTP-Iso).
Drugs affecting female schistosomes are of interest due to their role in the reduction of disease spread and morbidity.
Concerning tissue egg load, all dosing regimens significantly reduced hepatic and intestinal egg load, compared to non-treated mice. This decrease in egg load might be due to the anti-schistosomal activity of the drugs and reduction in female worms.
In the current study, the reduction in the percentage of immature eggs in response to PZQ is caused by the eradication of adult worms and cessation of oviposition.
The increase in the percentage of mature eggs following BTP-OH administration denotes interference with egg laying because of the schistosomicidal efficacy of the compound, especially affecting female worms, since Schistosoma eggs are laid immature and mature after six days (Pellegrino et al., Reference Pellegrino, Oliveira, Faria and Cunha1962).
The administration of PZQ was documented to alleviate liver pathology (Berhe et al., Reference Berhe, Myrvang and Gundersen2008). The improved liver pathology seen in response BTP-OH might be due to the significant reductions in worm burdens and egg loads.
The histopathological changes reported in our study were similar in both untreated and PZQ-treated mice (moderate lobular inflammation and moderate focal necrosis), while administration of BTP-OH alleviated liver pathology, as shown by the presence of mild lobular inflammation and mild focal necrosis.
For granulomas parameters, the PZQ dosing regimen was associated with the highest reduction in granuloma count, but caused non-significant reduction in granuloma diameter, compared to the infected non-treated group. The significant reduction in granuloma count is caused by the eradication of adult worms and subsequent reduction in hepatic egg load. The non-significant reduction in granuloma diameter in the PZQ-treated group may be related to the persistent inflammation and non-reduced inflammatory cellular infiltrate. Similar findings were also reported by Alhusseiny et al. (Reference Alhusseiny, El-Beshbishi, Hashim, El-Nemr and Handoussa2017). In contrast, Abdel-Hafeez et al. (Reference Abdel-Hafeez, Ahmad, Abdulla, Aabdel-Wahab and Mosalem2012) reported less reduction in granuloma count, and a higher reduction in granuloma diameter, in response to PZQ therapy.
On the other hand, the significant reductions in granuloma diameter in response to both BTP-OH doses can be explained by the anti-inflammatory activity of benzimidazole (Bukhari et al., Reference Bukhari, Lauro, Jantan, Fei Chee, Amjad, Bifulco, Sher, Abdullah and Rahman2016). However, an immunomodulatory effect after treatment cannot be excluded, since cytokines are believed to modulate the granuloma size and to play a fundamental role in the pathology of schistosome infection (Aly et al., Reference Aly, Hendawy, Ali, Hassan and Nosseir2010).
The ultrastructural changes assessed through SEM may help to provide insight into the mechanism of action of BTP-OH in S. mansoni infection. The compound induced tegumental erosions and blebs, which, in turn, interferes with parasite defence against the host immune system, since the tegument is important for the success of infection and adult survival inside the host (Skelly & Wilson, Reference Skelly and Wilson2006). In addition, the tegument has a vital role in worm nutrition and sensation.
Compound BTP-OH seems to be more lipophilic based on the calculated log partition coefficient, which equals 5.04. So, it is expected to penetrate plasma membranes and the tegument.
Similar ultrastructural changes were also described following the administration of schistosomicidal agents, including peeling of the tegument, damaged oral sucker and reduced and disorganized tubercles, in response to imidazolidine derivatives (Albuquerque et al., Reference Albuquerque, Pitta, Irmão, Peixoto, Malagueño, Santana, Lima, Galdino and Pitta2007) and triclabendazole (El-Sayed & Allam, Reference El-Shennawy, Mohamed and Abass1997; Mansoury, Reference Mansoury1997).
Benzimidazoles function through binding to β-tubulin, causing depolymerization of cytoplasmic microtubules and disturbance of the microtubule-based process in helminthic parasites as a suppression of mitosis in vitelline and spermatogenic cells (Lacey, Reference Lacey1988).
Herein, the mechanism of action of BTP-OH is not fully elucidated. However, from the obtained data, we can hypothesize that BTP-OH can work via different mechanisms.
First, by causing the destruction of the tegument (based on SEM results), it can interfere with the tegument structure and functions leading to interference with worm nutrition, with subsequent elimination of the worm by the immune system.
Second, since BTP-OH is structurally related to benzimidazoles, the mode of action could be alike. Both inhibition of mitosis and spermatogenic cells and the benzimidazoles antimicrotubular effect, which block the transport of tegumental secretory bodies could have lethal effect on schistosomes.
Third, since BTP-OH causes disruption of the tegumental coat, it can deeply penetrate into the internal structures to bind another target, such as muscles of the parasite, or cause disturbance in the normal physiological and biochemical processes of the worm.
Besides schistosomiasis, a number of neglected tropical diseases (NTDs) affect more than one billion people and represent major health problems in underdeveloped countries, in conditions of poverty, lack of adequate sanitation and abundance of infectious vectors, domestic animals and livestock. Owing to the broad-spectrum activity of benzimidazoles, it would be of interest to test BTP-OH on some NTDs that are prevalent in these areas, where patients infected with Schistosoma may be harbouring other parasitic infection and BTP-OH may act as double-edged weapon targeting schistosomes and other parasitic infections.
In conclusion, the BTP-OH compound exhibited anti-schistosomal activity, shown by its effect on adult burden and tissue egg load. In addition, BTP-OH resulted in the amelioration of histopathological changes, a decrease in granuloma count and diameter, and caused tegumental deformities. Accordingly, our results point to the BTP-OH compound as a new anti-schistosomal agent, but further studies are needed to explore the full mechanisms of action and to recognize its effects on the juvenile stages and other human schistosomes. Also, it would be of interest to test if the BTP-OH could affect the levels of inflammatory and liver fibrosis markers with amelioration of the liver pathology.
Financial support
None.
Conflicts of interest
None.
Ethical standards
All experimental procedures were performed following the institutional guidelines of the Ethical Committee of Medical Research, Faculty of Medicine, Mansoura University, in accordance with the National Institute of Health guide for the care and use of laboratory animals..










