Introduction
The degradation and homogenization of natural habitats are causes of the accelerated loss of biodiversity in the last decades (Brooks et al., Reference Brooks, Mittermeier, da Fonseca, Gerlach, Hoffmann, Lamoreux, Mittermeier, Pilgrim and Rodrigues2006). The intensities of the intermediate disturbances and other environmental fluctuations can reduce the dominance of some species, interfering in the dynamics of the evolutionary and ecological processes of aquatic ecosystems (Reid & Ogden, Reference Reid and Ogden2006), such as floodplains. The damming of rivers interferes in the hydrodynamics of the system of flood and drought pulses, and the connectivity between habitats (Junk et al., Reference Junk, Bayley and Sparks1989; Neiff, Reference Neiff1990; Agostinho et al., Reference Agostinho, Gomes, Veríssimo and Okada2004a, Reference Agostinho, Thomaz and Gomesb).
Due to the increase in the number of dams and the resulting effects on the flooding of the main watercourses in the Paraná Basin, the local physical, chemical and biological characteristics have undergone various changes (Agostinho et al., Reference Agostinho, Bonecker and Gomes2009; Santos et al., Reference Santos, Santana and Ortega2017). The impacts caused by natural flooding oscillations or by anthropic influence cause high environmental modification and contribute to changes in the composition of species (Tombolini et al., Reference Tombolini, Caneva, Cancellieri, Abati and Ceschin2014; Ceschin et al., Reference Ceschin, Tombolini, Abati and Zuccarello2015; Abati et al., Reference Abati, Minciardi, Ciadamidaro, Fattorini and Ceschin2016; Winemiller et al., Reference Winemiller, McIntyre and Castello2016). This change in biodiversity is observed in the ichthyofauna of a specific region and directly reflects on the structure and composition of its parasite communities (Pavanelli et al., Reference Pavanelli, Machado, Takemoto, Guidelli, Lizama, Thomaz, Agostinho and Hahn2004).
The interaction between biotic and abiotic factors in an ecosystem is essential to the composition and structure of parasite communities (Poulin, Reference Poulin2007). In a parasite community, some species undergo substantial changes and this has been explained primarily by parasite life cycles, environmental dynamics and host-specific immune responses (Fallon et al., Reference Fallon, Bermingham and Ricklefs2003; Violante-González et al., Reference Violante-González, Aguirre-Macedo and Vidal-Martinez2008; Nagel et al., Reference Nagel, Robb and Forbes2009; Vital et al., Reference Vital, Varella, Porto and Malta2011). In dams, the distribution of parasites depends on the coverage of upper aquatic vegetation, the extent of shallow areas and the density of bird populations attracted to the reservoir (Iskov, Reference Iskov1976; Morley, Reference Morley2007). The area closest to the dam is generally narrower and deeper, has slower water flow and relatively low temperatures, resulting in a low population density of invertebrates and fish, and consequently few parasites with complex life cycles (Markevich et al., Reference Markevich, Iskov, Koval and Chernogorenko1976; Izyumova, Reference Izyumova1979; Morley, Reference Morley2007). Thus, the definitive host and the intermediate hosts must co-occur in a stable community for the endoparasite to survive (Landsberg et al., Reference Landsberg, Blakesley, Reese, Mcrae and Forstchen1998) and changes in the host fauna can hinder the transmission of endoparasites and, thus, modify parasitic biodiversity (MacKenzie, Reference Mackenzie1999).
Prochilodus lineatus (Valenciennes, 1837), popularly known as curimba, is a species of fish that has always presented high biomass in the upper Paraná River floodplain (Gubiani et al., Reference Gubiani, Gomes, Agostinho and Okada2007). Their diet consists of inorganic detritus and particles of organic matter that comprise the bottom body of aquatic environments, in addition to algae and benthic macroinvertebrates that are found in this environment (Fugi et al., Reference Fugi, Hahn and Agostinho1996; Lopes et al., Reference Lopes, Benedito-Cecilio and Martinelli2007). Considered medium-sized, their migratory habits are dependent on the flood pulses of floodplains because they migrate upstream during floods to spawn in tributaries (Lizama et al., Reference Lizama, Takemoto and Pavanelli2005; Oyakawa et al., Reference Oyakawa, Menezes, Shibatta, Lima, Langeani, Pavanelli, Nielsen, Hilsdorf, Bressan, Kierulff and Sugieda2009; Piana et al., Reference Piana, Cardoso, Dias, Gomes, Agostinho and Miranda2017). After hatching, the larvae drift downstream, reaching floodplain lakes where they develop into the juvenile and adult stages, grow and mature until the flood pulses again when they are apt to reproduce. After the closing of the Engineer Sérgio Motta Dam in Porto Primavera (state of São Paulo, Brazil) in November 1998, which caused the interruption of the critical phase of the floods in this floodplain (Agostinho et al., Reference Agostinho, Gomes, Veríssimo and Okada2004a, Reference Agostinho, Thomaz and Gomesb), the life cycle of the species underwent considerable changes, since the number of individuals in their populations has steadily decreased (Rosa & Lima, Reference Rosa, Lima, Bressan, Kierulff and Sugieda2008; Oyakawa et al., Reference Oyakawa, Menezes, Shibatta, Lima, Langeani, Pavanelli, Nielsen, Hilsdorf, Bressan, Kierulff and Sugieda2009).
In this study, it is expected that the impacts caused by environmental disturbances will be directly reflected in the composition of the parasite populations; thus, we evaluated the change in the structure of the P. lineatus endoparasite community between two periods sampled 15 years apart in the upper Paraná River floodplain.
Material and methods
Study area, host and parasite sampling
The upper Paraná River floodplain is situated between the Engineer Sérgio Motta Dam (North) and Itaipu Reservoir (South) and is the last remaining free-flowing stretch of this river (230 km long) within Brazil (fig. 1). This river stretch is regulated by the cascade of dams upstream (e.g., Muniz et al., Reference Muniz, García-Berthou, Ganassin, Agostinho and Gomes2021), but receives large tributaries and still has marked water level variations, although not as intense and as frequent as before the dams were built (Gubiani et al., Reference Gubiani, Gomes, Agostinho and Okada2007). The collections occurred in two periods, where Period 1 corresponds to the collections in the years 2000 and 2001, and Period 2 corresponds to the collections in the years 2016 and 2017.
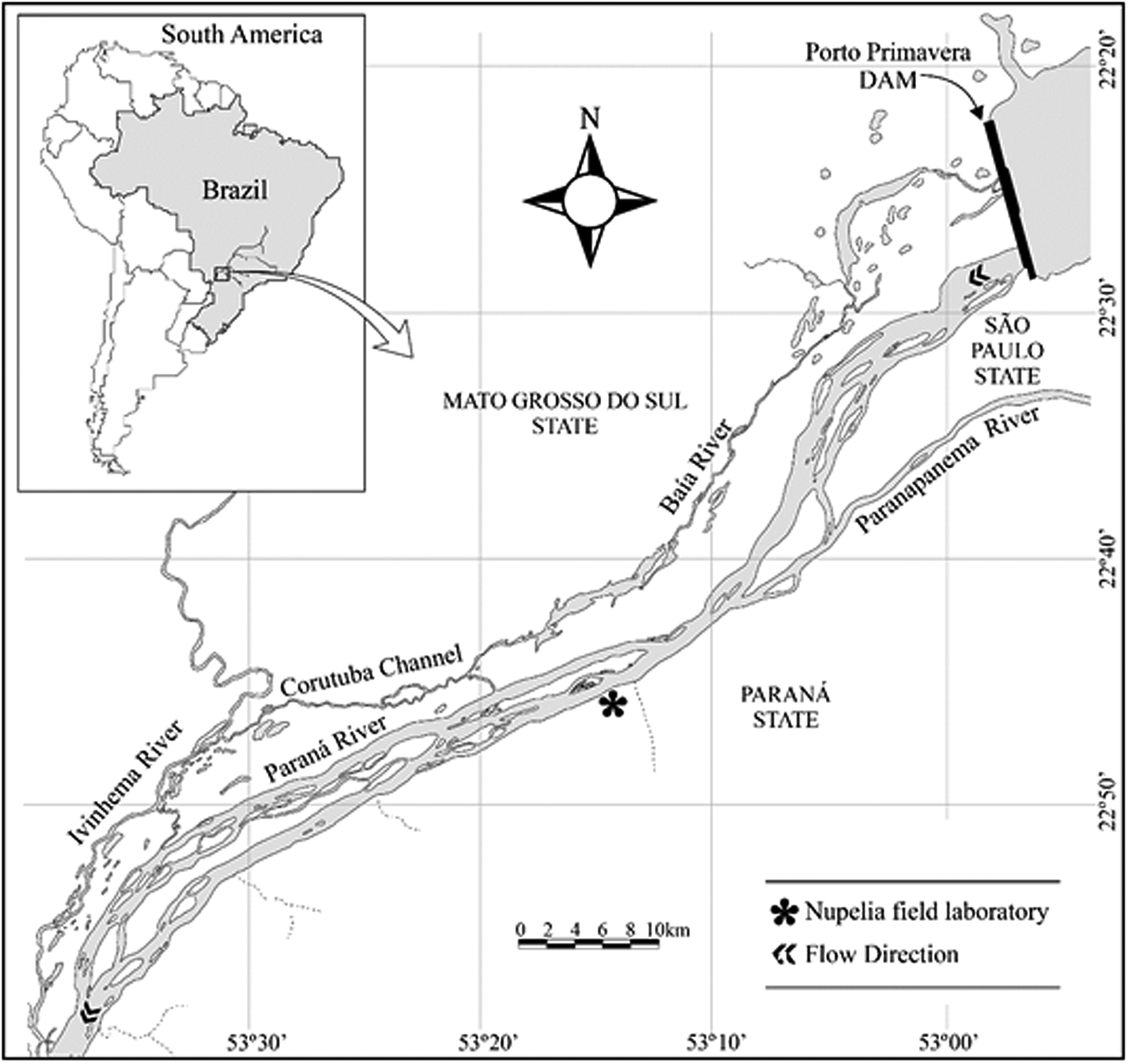
Fig. 1. Upper Paraná River floodplain. From Jaime Luiz Lopes Pereira.
Specimens of Prochilodus lineatus were collected using gillnets of different mesh sizes (2.4, 3, 4, 5, 6, 7, 8, 10, 12, 14 and 16 cm between opposite knots on 20 m long nets) for both periods. The nets were deployed for 24 h and checked at 8:00 h, 16:00 h and 22:00 h. It is fundamental to clarify that the sampling effort for fish collections was standardized and is part of the Long Term Ecological Research Program (LTER) that has been monitoring the upper Paraná river floodplain since 2000 (LTER PIARP- site 06). The captured fish were anaesthetized with 5% benzocaine, euthanized according to the LTER PIARP-06 protocol, measured, weighed and eviscerated. Each fish was identified based on specialized literature. The intestine was collected and screened in the laboratory, and the sampled endoparasites were collected, fixed (Eiras et al., Reference Eiras, Takemoto and Pavanelli2006) and identified according to Moravec (Reference Moravec1998), Vidal-Martínez et al. (Reference Vidal-Martínez2000), Thatcher (Reference Thatcher2006) and Kohn et al. (Reference Kohn, Fernandes and Cohen2007). A total of 149 specimens of P. lineatus were collected in Period 1 and 40 specimens in Period 2 in the floodplain (table 1).
Table 1. Biometric data of the hosts Prochilodus lineatus collected during the two sampling periods in the upper Paraná River floodplain.

To test the sufficiency of samples (number of hosts collected), the species accumulation curve was performed for both periods (fig. 2) using the iNEXT package (Hsieh et al., Reference Hsieh, Ma and Chao2016). The number of hosts collected, even in Period 2, was sufficient to obtain a representative sample of the parasite species present in the infra-communities and both curves showed a trend towards stability.

Fig. 2. Species accumulation curve for the parasitic community of Prochilodus lineatus in the upper Paraná River floodplain. (Period 1: 2000–2001 and Period 2: 2016–2017).
Data analysis
To assess whether individuals showed differences in weight and length between periods, we used the analysis of covariance (ANCOVA). We used the weight and length of individuals as the response variable and covariate in the model, respectively. The period was used as a fixed factor. At ANCOVA we tested the parallelism (homogeneous slope) assumption of standard ANCOVA through the interaction between period and length (García-Berthou & Moreno-Amich, Reference García-Berthou and Moreno-Amich1993).
In addition, the relative condition factor (Le Cren, Reference Le Cren1951) was performed for each analysed fish and the weight/length ratio was estimated using the equation (Wt = a * Ltb), where Wt = weight, a = intercept, Lt = total length, and the exponent b is derived from the weight-to-length ratio. The a and b values were used to calculate the expected weight (We = a * Ltb) and then the relative condition factor (Kn) was calculated as the ratio of the observed weight to the theoretically expected weight (Kn = Wt/We). To assess the differences in condition factor between periods and parasitized and non-parasitized fish, the non-parametric Kruskal–Wallis (H) analysis was performed.
To describe the structure and quantitative analysis of the parasites found, we used the parasitic indices described by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Four descriptors of the parasite infra-communities were calculated: abundance (the sum of all parasites); richness (number of species); species diversity (calculated using the Shannon index); and evenness (calculated using the Pielou index). As the parasite descriptors did not present normal distribution, we used non-parametric tests: the Mann–Whitney test to compare parasite descriptors between periods and Spearman's correlations to correlate parasitological variables and fish body length.
As an exploratory method, a non-metric multidimensional scaling (NMDS) (vegan package) was performed based on the Bray–Curtis dissimilarity matrices, in two dimensions, to visualize the differences in the parasite community between the sampled periods (Period 1 and Period 2). To detect significant differences in the parasite community between the periods a multivariate permutational analysis of variance (PERMANOVA) with 999 permutations was applied (vegan package) (Anderson, Reference Anderson2005). We used the indicator species analysis (IndVal) to determine the representative parasite species in each sample period (indicspecies package) (Dufrêne & Legendre, Reference Dufrêne and Legendre1997). The level of statistical significance adopted was P ≤ 0.05. All of these statistical procedures were performed using R 3.2.4 software (R Development Core Team, 2017).
Results
In the ANCOVA performed for the host weight and length data, we found a significant effect of the period on the weight–length relationship, being that the individuals collected in Period 1 showed greater investment in weight and length, compared to individuals collected in Period 2 (table 2) (fig. 3). Regarding the Kn, the mean of the values was similar between the periods (Period 1 Kn = 1.007 ± 0.17; Period 2 Kn = 1.007 ± 0.11), and there were no significant differences (H = 1.31; P = 0.201) between the parasitized individuals and non-parasitized in both periods (Period 1: non-parasitized Kn = 0.98 ± 0.13; parasitized Kn = 1.03 ± 0.17/Period 2: non-parasitized Kn = 0.96 ± 0.16; and parasitized Kn = 1.01 ± 0.1).

Fig. 3. Weight–length relationship of the host Prochilodus lineatus in the periods (Period 1: 2000–2001 and Period 2: 2016–2017) in the upper Paraná River floodplain.
Table 2. Summary of the analysis of covariance of the weight–length relationship of Prochilodus lineatus between the periods (Period 1: 2000–2001 and Period 2: 2016–2017) in the upper Paraná River floodplain.

*statistically significant correlations (P ≤ 0.05) are indicate in boldface type.
A total of 15 species of endoparasites were found: 11 in Period 1 and 9 in Period 2 (table 2), but only Neoechinorhynchus curemai, Saccocoelioides sp. 1, Saccocoelioides magnorchis, Saccocoelioides nanii, and Saccocoelioides saccodontis occurred in both periods. In Period 1, S. magnorchis and S. nanii were the most prevalent species among hosts. In Period 2, N. curemai was the most prevalent species among the hosts.
The abundance (U = 2566.5; P = 0.16) of the infra-communities did not differ between periods. However, species richness (U = 2072; P = 0.001) and diversity (U = 2457.5; P = 0.04) were higher in Period 1, and equitability (U = 2425.5; P = 0.02) was higher in Period 2 (table 4). Due to length being significantly correlated with fish weight (fig. 3), we considered it for correlations with parasite descriptors. In Period 1, we observed that the correlation of descriptors (richness, diversity and equitability) increased with length (table 4), though the correlation of abundance with length increased in Period 2 (table 3).
Table 3. Species of parasites, periods (Period 1: 2000–2001 and Period 2: 2016–2017) and their parasitological indices found in Prochilodus lineatus in the upper Paraná River floodplain.

Table 4. Mean (± SD), minimum and maximum (Min-Max) values of parasite infra-community descriptors of Prochilodus lineatus between the periods (Period 1: 2000–2001 and Period 2: 2016–2017) in the upper Paraná River floodplain, Spearman rank correlation coefficients (rs) between these descriptors and total host body length (cm) and statistically significant values (P).

*statistically significant correlations (P ≤ 0.05) are indicate in boldface type.
An NMDS ordered the variability of the species composition of parasites between the periods: even with the overlaps and sharing of species (fig. 4), the periods showed differences in the parasite infra-communities (PERMANOVA: F = 15.21; P= < 0.05). IndVal showed that S. margnorchis (P = 0.001) and Saccocoelioides nanni (P = 0.001) were the indicator species in Period 1 and Colocladorchis ventrastomis (P = 0.001), Neoechinorhynchus prochilodorum (P = 0.001) and S. saccodontis (P = 0.02) in Period 2.

Fig. 4. Non-metric multidimensional scaling (NMDS) showing the variability in the composition of parasite species in Prochilodus lineatus between the periods (Period 1: 2000–2001 and Period 2: 2016–2017) in the upper Paraná River floodplain.
Discussion
The results of this study showed that there was a change in the structure of the parasitic community of P. lineatus, as some species showed higher prevalence in Period 1, and others showed a decrease in prevalence in Period 2, such as S. magnorchis. Some species were recorded only in one of the periods, such as N. prochilodorum and C. ventrastomis in Period 2. Such variations seem to result from factors linked to environmental disturbances occurring in the floodplain, which directly affect the host and, consequently, the parasitic community.
The formation of a new dam destabilized the aquatic environment for several years which has serious implications for the aquatic wildlife of the affected area (Morley, Reference Morley2007). With the construction of the Porto Primavera dam, the floodplain suffered changes in the structure and dynamics of aquatic communities that were influenced by the flood regime because the flow of the Paraná River redistributed and altered the hydrological regime (Agostinho et al., Reference Agostinho, Bonecker and Gomes2009). Although changes in ecological conditions in dammed rivers depend on several factors (e.g., water quality, geomorphology and sediments) that are varied and complex, some changes that occur both upstream and downstream of dams are acute and irreversible (Arantes et al., Reference Arantes, Fitzgerald, Hoeinghaus and Winemiller2019).
Santos et al. (Reference Santos, Santana and Ortega2017), studying a series of reservoirs in three different basins, demonstrated the role of dams as environmental filters, reducing the abundance of fish species. The reduction in the average size of migratory species in the Paraná, São Francisco, Iguaçu and Paranapanema rivers, which are rivers that have a cascade of reservoirs, is associated with characteristics that convey tolerance or vulnerability to the new ecological conditions of the impacted system (Arantes et al., Reference Arantes, Fitzgerald, Hoeinghaus and Winemiller2019). As the curimba is a long-distance migratory species, gonad maturation, spawning, development and larval growth are strictly related to flooding. Moreover, recruitment success depends on the timing and duration of floods (Gomes & Agostinho, Reference Gomes and Agostinho1997). Lopes et al. (Reference Lopes, Peláez, Dias, Oliveira, Rauber, Gomes and Agostinho2020) reported in a floodplain study with curimba, smaller body size in a dammed river (Paraná River) when compared to a river without a dam (Ivinhema River).
For parasites, body size and host weight play an important role in determining host susceptibility to parasite infection, as host body size is considered a representation of the number of resources available (i.e., habitat area and nutrients or energy) for exploitation by the parasite (Luque et al., Reference Luque, Mouillot and Poulin2004; Poulin et al., Reference Poulin, Guilhaumon, Randhawa, Luque and Mouillot2011; Marcogliese et al., Reference Marcogliese, Locke, Gélinas and Gendron2016). In both periods, length did not play a key role in parasitological descriptors, but we observed that in Period 1 the diversity and equitability remained constant across host lengths, meaning that the relative abundances of parasite species in each infra-community are constants relative to the length of the fish tested.
Many of these parasite communities experience temporal–structural or spatial–structural changes and this is related to seasonal variations in biotic and abiotic environmental factors and responds strongly to changes in host community composition. Over time these variations can reflect in parasite species’ composition and density (Anderson & Gordon, Reference Anderson and Gordon1982; Luque et al., Reference Luque, Mouillot and Poulin2004; Hechinger & Lafferty, Reference Hechinger and Lafferty2005; Gallegos-Navarro et al., Reference Gallegos-Navarro, Violante-González, Monks, García-Ibáñez, Rojas-Herrera, Pulido-Flores and Rosas-Acevedo2018). The species composition and structure of the parasite communities varied between periods, with species richness and diversity being highest in Period 1, although the endoparasites of P. lineatus exhibited similar distribution patterns in both periods, i.e., the low number of species, low diversity and numerical dominance of one parasite group.
Digenea species numerically dominated the parasite communities, accounting for 93% of the total parasites in Period 1 and 59% in Period 2, as well as S. margnorchis and S. nanni, were the representative species of Period 1, and C. ventrastomis and S. saccodontis were the representative species of Period 2. Transmission can occur when metacercariae encyst in aquatic vegetation or other surfaces (Al-Jahdali & Hassanine, Reference Al-Jahdali and Hassanine2012), and as the diet of P. lineatus is detritivorous and it feeds on the bottom, it facilitates infection by these parasites. Moreover, this is expected because, in tropical regions, Digenea is part of the most abundant and diverse group of fish parasitic helminths (Takemoto et al., Reference Takemoto, Amato and Luque1996; Luque & Poulin, Reference Luque and Poulin2008; Garcia-Prieto et al., Reference Garcia-Prieto, Mendoza-Garfias and Perez-Ponce de Leon2014). However, despite the dominance of the group for both periods, digenean parasites showed decreased infection levels in Period 2.
Acanthocephalans were the second most dominant group, mainly due to the high prevalence of N. curemai in both periods and the dominance of N. prochilodorum in Period 2. This dominance also can be explained by the feeding habits of the host, which includes a wide variety of invertebrates, presenting low food specificity, making it extremely susceptible to parasite acquisition through the trophic web (Fugi et al., Reference Fugi, Agostinho and Hahn2001; Lizama et al., Reference Lizama, Takemoto and Pavanelli2005). Other studies have already demonstrated the high prevalence of N. curemai in P. lineatus (Martins et al., Reference Martins, Moraes, Fujimoto, Onaka and Quintana2001; Leite et al., Reference Leite, Pelegrini, Agostinho, Azevedo and Abdallah2018; Duarte et al., Reference Duarte, Lehun, Leite, Consolin-Filho, Bellay and Takemoto2020; Lehun et al., Reference Lehun, Hasuike and Silva2020), which contributes to the high abundance in the present study, especially for Period 2.
The homogenization of the environment, caused by the construction of dams upstream of the reservoirs, directly affects the downstream river, with changes in the dynamics of fish assemblages, and may also cause a decrease in functional and taxonomic diversity, besides modifying the distribution and density of zoobenthos and zooplankton communities, which act as intermediate hosts for these parasites (Pinha et al., Reference Pinha, Aviz, Lopes Filho, Petsch, Marchese and Takeda2013; Simões et al., Reference Simões, Nunes, Dias, Lansac-Tôha, Velho and Bonecker2015; Petsch, Reference Petsch2016; Braghin et al., Reference Braghin, Almeida, Amaral, Canella, Gimenez and Bonecker2018; Lopes et al., Reference Lopes, Peláez, Dias, Oliveira, Rauber, Gomes and Agostinho2020). These areas may not have all the hosts needed for the complex life cycles of certain parasites, which can reduce the chance of these populations becoming established (Minchella, Reference Minchella1985; Marcogliese & Cone, Reference Marcogliese and Cone1997; Torchin & Mitchell, Reference Torchin and Mitchell2004; Hechinger & Lafferty, Reference Hechinger and Lafferty2005; Song & Proctor, Reference Song and Proctor2020). Thus, due to the high dependence on the hosts, the parasites are also affected by the physical, chemical and environmental changes that have been occurring in the lowland, although this effect is indirect (Karling et al., Reference Karling, Isaac, Affonso, Takemoto and Pavanelli2013).
As in all ecosystems and especially aquatic ecosystems (Winemiller et al., Reference Winemiller, McIntyre and Castello2016), environmental impacts have a strong negative effect on the life history of the host and, consequently, the parasite (Marcogliese & Cone, Reference Marcogliese and Cone1997; Tompkins et al., Reference Tompkins, Dobson, Arneberg, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2002). The life stage of each parasite will generally have its own direct and indirect responses to a stressor, as the impact becomes more evident as life cycle complexity increases (Lafferty & Kuris, Reference Lafferty and Kuris1999). Moreover, some studies in recent years have observed that the local extinction of parasite species, changes in prevalence, diversity, composition and dominance of parasitic infra-communities, an increase of monoxenous parasites (monogenetic, protozoa and crustaceans), and reduction of heteroxenous parasites (digenetic, nematodes and acanthocephalans) due to biotic and abiotic changes in the ecosystem (Karling et al., Reference Karling, Isaac, Affonso, Takemoto and Pavanelli2013; Yamada et al., Reference Yamada, Bongiovani, Yamada and Silva2017). We found that, over the years, the composition of the parasitic fauna of P. lineatus changed, and the dominant species was different in the two periods. This can be influenced by the environmental characteristics of the floodplain that suffers constantly from the impacts caused by dam construction.
Financial support
This work was financially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflicts of interest
None.










