Introduction
Invasive species (i.e. a non-native organism) can potentially cause serious problems in many ecological habitats worldwide (Gurevitch & Padilla, Reference Gurevitch and Padilla2004; Pejchar & Mooney, Reference Pejchar and Mooney2009), including losses in biodiversity, changes in ecosystem functions and impacts on economic enterprises (Lovell et al., Reference Lovell, Stone and Fernandez2006). Parasites may play a key role in mediating the impacts of biological invasion (Lymbery et al., Reference Lymbery, Morine, Kanani, Beatty and Morgan2014). Alien parasites are usually introduced in new habitats with an alien host species (Lymbery et al., Reference Lymbery, Morine, Kanani, Beatty and Morgan2014), and may lead to the emergence of new diseases in native hosts (Taraschewski, Reference Taraschewski2006). In the literature, the most commonly reported co-introduced parasites into new habitats are parasitic worms (helminths) (Lymbery et al., Reference Lymbery, Morine, Kanani, Beatty and Morgan2014).
Ligula intestinalis (Linnaeus 1758) is a globally distributed diphyllobothriidean cestode (Kennedy, Reference Kennedy1974) and has been reported from a wide range of cyprinid fish species from Europe (Vanacker et al., Reference Vanacker, Masson and Beisel2012), North America, Asia and Australia (Stefka et al., Reference Stefka, Hypsa and Scholz2009). Recently it has also been found in Africa, both in Lake Victoria and in Lake Nyasa (Cowx et al., Reference Cowx, Rollins and Tumwebaze2008; Msafiri et al., Reference Msafiri, Kwendwa, Nestory and Alistidia2014). In Lake Nyasa the parasite infects a small pelagic cyprinid Engraulicypris sardella (Günther, 1868) (Msafiri et al., Reference Msafiri, Kwendwa, Nestory and Alistidia2014; Rusuwa et al., Reference Rusuwa, Ngochera and Maruyama2014). Engraulicypris sardella, locally called usipa, is a small (common size 100 mm length), annual, pelagic, cyprinid fish endemic to Lake Nyasa (Rufli & Van Lissa, Reference Rufli and Van Lissa1982; Thompson, Reference Thompson1996). Having a high growth rate, they reach a maximum length of 130 mm in less than a year (Eccles, Reference Eccles1992). Young E. sardella feed exclusively on phytoplankton and switch to zooplankton when they attain adulthood (Degnbol, Reference Degnbol1982; Allison et al., Reference Allison, Irvine, Thompson and Ngatunga1996). They are widespread within the lake and are found in both nearshore areas and offshore pelagic water, down to depths of approximately 200 m (Maguza-Tembo et al., Reference Maguza-Tembo, Palsson and Msiska2009).
Ligula intestinalis has a complex life cycle involving two aquatic intermediate hosts and a piscivorous bird as the definitive host (Dubinina, Reference Dubinina1980). Copepods and various cyprinid fish species serve as first and second intermediate hosts, respectively. Ligula plerocercoids (the parasite larva found in the second intermediate host) cause severe pathogenic effects on the fish intermediate host, suppressing gonad development and perhaps causing complete castration in roach and chub (Kennedy et al., Reference Kennedy, Shears and Shears2001). This could potentially lead to population collapses of infected host species and changes in the abundance and composition of the fish community within lakes (Wilson, Reference Wilson1971; Wyatt & Kennedy, Reference Wyatt and Kennedy1988).
Infections of L. intestinalis in E. sardella in Lake Nyasa were first observed by Mwambungu et al. (Reference Mwambungu, Ngatunga, Kihedu and Mlay1996) and Kihedu et al. (Reference Kihedu, Mlay, Mwambungu and Ngatunga2001) during long-line research surveys. In Lake Nyasa, only E. sardella has been found to be infected, but it is known that this parasite can switch between different cyprinid hosts (Vanacker et al., Reference Vanacker, Masson and Beisel2012). The fishery of E. sardella in Tanzania contributes significantly to the livelihood of local people, by providing them with cheap animal protein. Severe infections with L. intestinalis could potentially lead this endemic species to extinction, as has been reported for other fish species (Wyatt & Kennedy, Reference Wyatt and Kennedy1988).
In small lakes, ponds and reservoirs, L. intestinalis typically shows epidemic cycles lasting a few years, where infection levels increase rapidly followed by population crashes of the intermediate host (Kennedy et al., Reference Kennedy, Shears and Shears2001). In larger lakes, the situation may be different, with local extinctions in some areas and increasing parasite abundance at other locations within the lake. Within Lake Nyasa we would therefore expect more spatial variation in prevalence than in the typical small lakes where this parasite has been most frequently studied. Moreover, in larger lakes, fish species diversity is usually higher (Barbour & Brown, Reference Barbour and Brown1974), providing the parasite with alternative hosts and potentially facilitating host switching when fish populations crash. This might cause the parasite to become persistent within the lake. Furthermore, small lakes usually have relatively shallow depths and are therefore not usually stratified (Vanacker et al., Reference Vanacker, Masson and Beisel2012). Larger lakes usually stratify (Ngupula et al., Reference Ngupula, Ezekiel, Kimirei, Mboni and Kashindye2012) and this provides marked biotic and abiotic changes with water depth, allowing us to study how this parasite affects the depth preference of the intermediate host.
As a first step towards understanding the dynamics of this parasite in a huge lake like Lake Nyasa, here we present data on the spatial and temporal variation in prevalence and intensity. Since ecological conditions affecting parasite transmission rates are likely to vary at different locations and at different times of the year, we expected to find marked effects of site and season on the infection level of the parasite. Moreover, since it has been shown that the final host tends to catch a disproportionately high share of infected fish (Brown et al., Reference Brown, Loot, Grenfell and Guégan2001; Loot et al., Reference Loot, Brosse, Lek and Guegan2001a), we explored how prevalence varied with water depth.
Materials and methods
The study was conducted in Lake Nyasa (also known as Lake Malawi or Lake Niassa), the southernmost of the large African rift valley lakes (fig. 1). Lake Nyasa is shared by three states: Malawi, Mozambique and Tanzania. The lake is located at an altitude of 472 m above sea level, it has a mean surface area of 29,000 km2 and a maximum depth of 785 m (Bootsma & Hecky, Reference Bootsma and Hecky1993). The mean surface temperature of the lake is between 24 and 28°C (Vollmer et al., Reference Vollmer, Bootsma, Hecky, Patterson, Halfman, Edmond, Eccles and Weiss2005) and the annual rainfall ranges between 1000 and 2800 mm (LNBWB, 2013). The Lake Nyasa basin is characterized by two marked seasons: a dry season (May–September) and a double-maxima rainy season, with a long rainy season in January–April and a short rainy season during October–December.

Fig. 1. Map of Lake Nyasa, showing all sampling stations (Matema, Lupingu and Mbamba bay). Reproduced with permission from Msafiri et al. (Reference Msafiri, Kwendwa, Nestory and Alistidia2014).
The present study was conducted at three different fishing stations – Matema, Lupingu and Mbamba Bay – situated in the south-western part of the United Republic of Tanzania. At each fishing station, two sampling sites were established, one site close to the lake shore (littoral zone) and the second site located offshore (pelagic zone).
Sampling was carried out monthly from January to December in 2015 and a total of 6987 E. sardella were collected in situ from artisanal fishermen operating light-attraction fishery during the nights of a dark moon-phase. All fish were caught using an open-water sein net with 10 mm mesh size, and were obtained at a depth of about 100 m for both the lake habitats (i.e. littoral and pelagic zones). Coordinates of the sampled area were recorded using a hand-held global position system (Garmin GPS 72H; Garmin Ltd, Olathe, Kansas, USA). The length and weight of both E. sardella and L. intestinalis were measured to the nearest millimetre and 0.01 g. The fish were later dissected for parasite determination, and the cestodes were identified according to the protocol of Dobben (Reference Dobben1952) as L. intestinalis. Determinations of the parasite prevalence and intensity in E. sardella were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
To test whether the parasite prevalence varied with water depth, we conducted a sampling survey at Matema fishing station, fishing 225 E. sardella during the dark moon-phase nights in deeper water, up to 200 m depth. Within the sampling site, four vertical sampling depths were used: one close to the surface (i.e. 50 m depth) and the others at 100, 150 and 200 m depths, respectively. However, at the 200 m depth, E. sardella was not found in the catch and therefore we decided to exclude this location from the analysis. Data on the host E. sardella, prevalence and measurements of L. intestinalis were collected using the methodology described above.
Statistical analysis
Statistical computing and graphics were carried out using R, version 3.2.3 (http://r-project.org). Analyses of the prevalence of L. intestinalis in E. sardella were performed using a Generalized Linear Model (GLM). In the model, infection was fitted as a binary response variable, with parasite occurrence used as 0 for absence and 1 for presence. Lake habitat (levels: littoral and pelagic) and sampling stations (levels: Matema, Lupingu, Mbamba Bay) were added as categorical predictors in the model. The model also included an interaction between the two predictors. Analysis of parasite prevalence at different times of the year (2015) was carried out using a Generalized Additive Model (GAM). In the model, parasite prevalence was the response variable, and day of the year was included as the predictor in the model. Moreover, rainfall data were added to the figure of the model, with the aim of showing variations of parasite prevalence in relation to precipitation. The relationship between prevalence of L. intestinalis and host depth was explored using a binary logistic regression model. In the model, infection was fitted as a binary response variable, with parasite occurrence used as 0 for absence and 1 for presence, and lake depths were added as categorical predictors in the model.
Results
Overall prevalence from all stations throughout the study period was 14.98 ± 2.43% (mean ± SE). Number of cestodes per infected fish ranged from 1 to 4, and overall mean intensity was 1.13 ± 0.01 (mean ± SE). The majority of infected fish carried one parasite (87.84%), with 11.07% having two parasites, 1% three parasites and 0.1% four parasites. Engraulicypris sardella sampled from the littoral zone had a significantly higher prevalence of infection than those sampled in the pelagic zone (GLM: F (1,6985) = 5480.5, P < 0.001) (fig. 2).
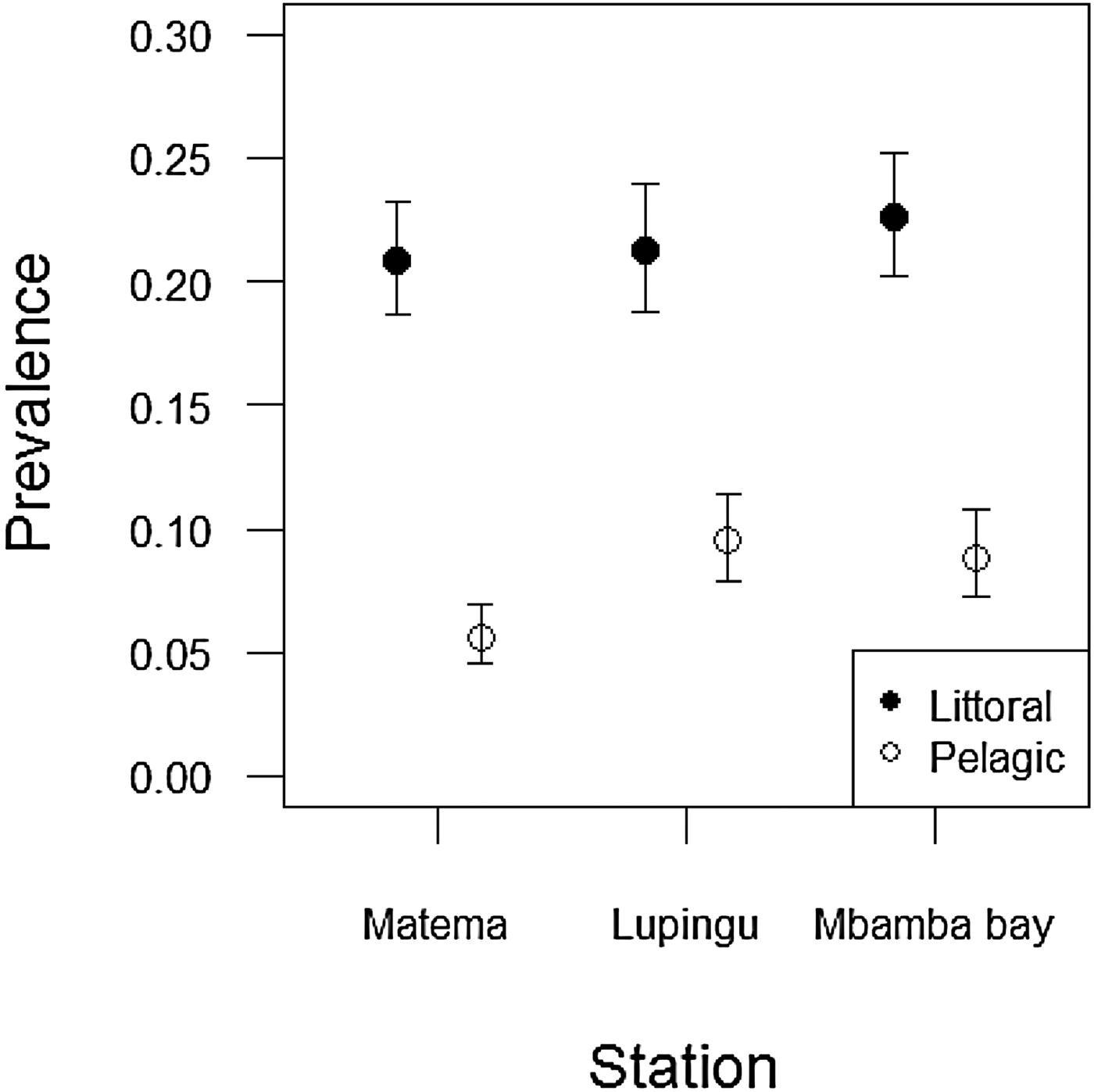
Fig. 2. Prevalence (±SE) of L. intestinalis in E. sardella depending on lake habitat (littoral zone vs pelagic zone) and station (Matema, Lupingu and Mbamba Bay) in Lake Nyasa, 2015.
Additionally, we found a significant interaction between the lake habitats and sampling stations, i.e. the change in prevalence when going from littoral zone to pelagic zone was not the same for all sampling stations (GLM: F (2,6981) = 5463.0, P < 0.01). Treatment contrasts revealed that the change was largest in Matema compared to the two other stations. However, this effect was small compared to the overall difference between the littoral and pelagic habitat.
Parasites were found throughout the year; but the prevalence was higher during the rainy season (19.68 ± 3.99%) from October to April compared to the dry season (8.83 ± 1.46%) between May and September, and the variations in parasite infestation between months of rainy seasons and dry seasons were statistically significant (GAM: edf = 4.959, Ref. df = 6.045, F = 2.744, P < 0.05) (fig. 3). The average rainfall intensity per month for rainy and dry seasons was 123 mm and 2 mm, respectively.
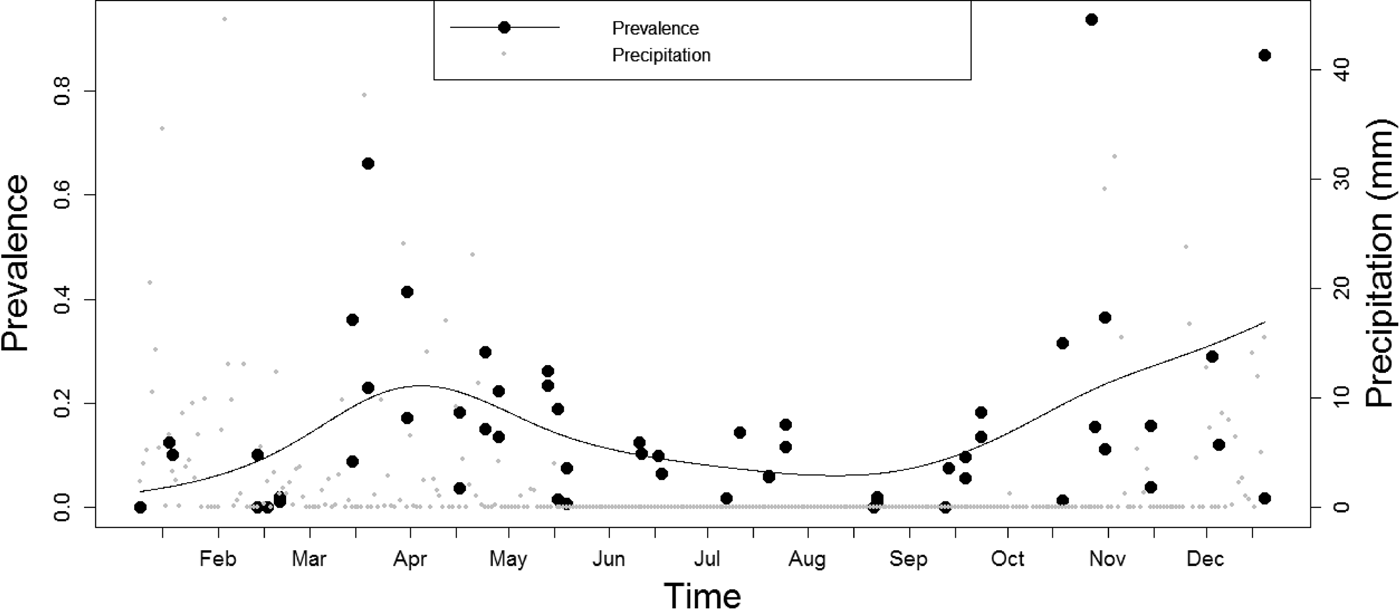
Fig. 3. Variations of parasite prevalence (GAM model represented by black line and raw data by solid black circles) and daily rainfall (grey solid circles) in Lake Nyasa from January to December, 2015.
Parasite prevalence varied significantly with the depth at which fish hosts were located, with the highest prevalence in the upper 50 m and declining with depth (GLM: F (1,223) = 242.67, P < 0.05) (table 1). Below 150 m we found no infected E. sardella. The mean length of fish also tended to decline with water depth. Moreover, male E. sardella were more frequently infected than females and were also more common in the surface water than at deeper water levels.
Table 1. Distributions of fish sample size, mean fish size, fish sex, prevalence and mean intensity at each sampling depth in Lake Nyasa.

Discussion
Spatial and temporal variations of L. intestinalis in different cyprinid fish species are well documented (Sweeting, Reference Sweeting1976, Reference Sweeting1977; Bean & Winfield, Reference Bean and Winfield1992; Loot et al., Reference Loot, Brosse, Lek and Guegan2001a; Dejen et al., Reference Dejen, Vijverberg and Sibbing2006; Britton et al., Reference Britton, Jackson and Harper2009; Zhokhov & Pugacheva, Reference Zhokhov and Pugacheva2012). However, to our knowledge there are few studies of this parasite from large and deep lakes (Marshall & Cowx, Reference Marshall, Cowx and Cowx2003; Msafiri et al., Reference Msafiri, Kwendwa, Nestory and Alistidia2014; Rusuwa et al., Reference Rusuwa, Ngochera and Maruyama2014). Marshall & Cowx (Reference Marshall, Cowx and Cowx2003) studied the effects of L. intestinalis on Rastrineobola argentea (closely related to E. sardella) in Lake Victoria and found no discernible trend in parasite prevalence between different parts of the investigated lake (but without presenting any data). Studies on L. intestinalis in E. sardella are even fewer. Recently, Msafiri et al. (Reference Msafiri, Kwendwa, Nestory and Alistidia2014) and Rusuwa et al. (Reference Rusuwa, Ngochera and Maruyama2014) reported the occurrence of this parasite in E. sardella in Lake Nyasa and found high parasite prevalences of over 30 and 50%, respectively, in the investigated parts of the lake. However, their findings were only obtained from a small part of the lake and did not cover all seasons.
In the present study, we observed a surprisingly small spatial variation in prevalence of L. intestinalis in E. sardella between different fishing stations, despite the fact that the southernmost station (Mbamba Bay) is more than 180 km from the most northerly station (Matema). This suggests that the parasite is widely distributed throughout the lake. Based on our data, there is no indication of local extinctions at some localities and population increases at others.
We found an average prevalence of about 15% and few individual hosts had more than one parasite. This is an infection level well below that found in many other studies where high parasite abundance has been followed by severe population declines in the fish intermediate host (Sweeting, Reference Sweeting1977; Kennedy et al., Reference Kennedy, Shears and Shears2001). However, this prevalence estimate is based on samples taken at about 100 m depths throughout the year and at all localities. When we sampled fish throughout the water column (during the end of the rainy season), from near the surface and down to 150 m, we observed an average prevalence of about 24%, confirming the concern noted by Kennedy et al. (Reference Kennedy, Shears and Shears2001) that obtaining representative samples of Ligula infection levels is rather difficult.
Both the observed infection level, as well as the rather uniform distribution of the parasite within similar habitats, suggests that the host–parasite dynamics may be different in a huge lake like Nyasa, as compared to smaller lakes. One interpretation of our findings is that E. sardella has a significantly higher risk of becoming infected in the littoral zone than in the pelagic, since we observed consistent differences in prevalence between these habitats. An alternative mechanism that could cause such a pattern would be if infected fish tend to aggregate in the littoral zone after they have become infected. Our data do not allow us to distinguish between these two hypotheses.
Another factor that could affect the host–parasite dynamics is suggested by our observations on the vertical distribution of infected hosts, where we found a consistent decrease in prevalence with increasing depths. Some other studies (for instance Bean & Winfield, Reference Bean and Winfield1992) have reported the same trend, but others found no difference in the proportion of infected fish at various depths (Museth, Reference Museth2001), suggesting that parasite distribution across depths depends on the fish host species.
In a huge lake like Nyasa, and with a host species that goes to depths below 150 m, we have a system that can demonstrate this pattern with a much higher resolution than in most other lakes. Our results could indicate that the risk of becoming infected appears to vary, not only between the littoral and the pelagic, but also along a vertical dimension. This could be an important factor preventing dramatic declines of the intermediate host population (and the parasite), which have been observed in smaller lakes (Sweeting, Reference Sweeting1976, Reference Sweeting1977; Bean & Winfield, Reference Bean and Winfield1992; Kennedy et al., Reference Kennedy, Shears and Shears2001), because there could be sites within the lake where transmission is significantly reduced.
However, from the present study, we cannot conclude that our observation on the distribution of infected E. sardella at different depths is a reflection of different transmission rates. Potentially, there could be no difference in transmission over different depths and what we observe is the result of different preferences of E. sardella once they have become infected. This could either be caused by some physiological side-effect of being infected – for example, a need for a higher saturation of oxygen (Lester, Reference Lester1971) or increased energy requirement (Milinski, Reference Milinski, Barnad and Behnke1990) – or it could reflect active manipulation by the parasite to increase its fitness (Poulin, Reference Poulin2010; Moore, Reference Moore2013). In any case, having an intermediate host that prefers to stay in the upper water levels must be adaptive to the parasite, since it depends on reaching its final host (fish-eating birds) through trophic transmission.
We also found that a considerable proportion of the intermediate host population was infected with L. intestinalis at depths between 100 and 150 m. It is highly unlikely that any bird would be able to catch these individuals, and it may seem that tapeworms present in this part of the intermediate host population are not able to be transmitted to the final host. An alternative hypothesis is that this part of the Ligula population consists of immature individuals, not yet ready to be transmitted to birds, and that immature parasites are actively manipulating the host to avoid being predated upon, as has been shown recently for other trophically transmitted parasites (Mikheev et al., Reference Mikheev, Pasternak, Taskinen and Valtonen2010; Dianne et al., Reference Dianne, Perrot-Minnot, Bauer, Gaillard, Léger and Rigaud2011; Gopko et al., Reference Gopko, Mikheev and Taskinen2015; Hafer & Milinski, Reference Hafer and Milinski2015, Reference Hafer and Milinski2016). We are currently exploring this hypothesis further.
We also observed a marked variation in prevalence throughout the year, where the highest infection levels coincided with the rainy seasons in March–May and in November–December. White-breasted cormorants (Phalacrocorax carbo) (one of the definitive hosts of L. intestinalis and therefore an important organism for the completion of the parasite's life cycle) are among the most abundant piscivorous birds of the lake (Linn & Campbell, Reference Linn and Campbell1992) and these exhibit, to some extent, seasonal migration, with the lowest abundance in the period March through May, i.e. at the same period when we observed a very high Ligula prevalence in the intermediate host. From the eggs of the tapeworm being dispersed in the lake by birds until it turns up as plerocercoid larvae in fish, there is a considerable time delay, because the eggs have to hatch into free-swimming larvae, and then the parasitic stage in copepods needs to develop into a fully developed procercoid stage before being infective to fish (Loot et al., Reference Loot, Francisco, Santoul, Lek and Guégan2001b). Even after having infected the second intermediate host, the parasite takes a considerable time to grow and mature (Loot et al., Reference Loot, Francisco, Santoul, Lek and Guégan2001b), and early stages can be difficult to discover. We should therefore not expect to see any direct correlation between the seasonal abundance of piscivorous birds and the observed prevalence of L. intestinalis in fish.
Interestingly, prevalence appeared to decline very rapidly after the peak levels. In the second intermediate host, L. intestinalis is a long-lived species, tending to live as long as the fish. On the other hand, in the piscivorous bird, the parasite survives for only a few days (Kennedy et al., Reference Kennedy, Shears and Shears2001). The marked decline in prevalence in the fish host can therefore not be explained by the disappearance of parasites from already infected hosts. Parts of this pattern are probably explained by a dilution effect, where young and uninfected individuals are recruited into the population during a period of low transmission. However, an additional mechanism that would contribute to the fast decline in prevalence could be that infected fish are removed from the population much faster than the uninfected ones. Our observations on the vertical distribution of the proportion of infected fish support this last hypothesis.
Acknowledgements
We are grateful to G. Salaka and W. Mgui of Mbamba Bay fisheries station, G. Godi and D. Leziray of Lupingu and Matema wards councils, respectively, and E. Magesa of Tanzania Fisheries Research Institute Kyela Centre for their assistance during field work research in Lake Nyasa. We thank A.G.V. Salvanes for her expert comments on handling fish. Moreover, we thank F. Midtøy for technical assistance during field sampling, and fishermen who assisted us during fishing operations. Finally, we thank K.H. Jensen and R. Club at the Department of Biology, University of Bergen, for their expert advice on statistical computing and graphics.
Financial support
This study was funded by the University of Bergen, Norway.
Conflict of interest
None.
Ethical standards
This research received ethical approval from the Tanzanian Fisheries Research Institute (application ID: TAFIRI/HQ/PF 637/100).






