Introduction
Environmental changes and ecological disturbances, either through natural or human-induced phenomena, can have major impacts on ecosystem functioning and stability (Morley, Reference Morley2007). Ecosystem alterations affect free-living and parasite species equally and can have large effects on parasite and disease dynamics (Lafferty & Kuris, Reference Lafferty and Kuris1999, Reference Lafferty, Kuris, Thomas, Guégan and Renaud2005; Patz et al., Reference Patz, Graczyk, Geller and Vittor2000; Morley & Lewis, Reference Morley and Lewis2006; Vidal-Martinez et al., Reference Vidal-Martinez, Pech, Sures, Purucker and Poulin2010). There is indeed evidence that disturbances in ecosystems can affect parasite populations and subsequently that of their hosts, or vice versa (Holmes, Reference Holmes1996; Blanar et al., Reference Blanar, Munkittrick, Houlahan, MacLatchy and Marcogliese2009). Increasing human impacts on ecosystems through land use may thus modify the distribution and abundance of parasites, and interactions among hosts and parasites (Johnson & Chase, Reference Johnson and Chase2004). However, parasites can be affected in widely different and unpredictable ways (Vidal-Martinez & Wunderlich, Reference Vidal-Martínez and Wunderlich2016). In some cases, ecosystem alterations can drive disease emergence (Dobson & Foufopoulos, Reference Dobson and Foufopoulos2001). For example, eutrophication of freshwater ecosystems has been linked with a drastic increase in trematode infection and limb deformities in amphibians (Johnson & Chase, Reference Johnson and Chase2004). Indirect, positive effects of nutrient enrichment on the abundance of Planorbella spp. snails, first intermediate hosts of the parasite, induced an increase in the standing crop of parasite larvae (Johnson et al., Reference Johnson, Chase, Dosch, Hartson, Gross, Larson, Sutherland and Carpenter2007). In contrast, environmental stressors that depress host population density can reduce parasite abundance, or even the ability of a parasite to persist at all, because a decline in host density also reduces contact rates between host and parasite (Lafferty & Holt, Reference Lafferty and Holt2003; Sures, Reference Sures2004). For instance, freshwater acidification, through reduction of intermediate host snail abundance, can significantly decrease the abundance and diversity of digenean parasites (Marcogliese & Cone, Reference Marcogliese and Cone1996). Generally, alterations of the ecological context in which hosts and parasites interact can have dramatic and unanticipated consequences for populations and community dynamics (Lafferty & Kuris, Reference Lafferty and Kuris1999; Ostfeld & Holt, Reference Ostfeld and Holt2004).
Responses of parasites to perturbation can be positive, negative or absent, often depending on how host densities are affected by particular ecological disturbances (Lafferty, Reference Lafferty1997; Vidal-Martinez et al., Reference Vidal-Martinez, Pech, Sures, Purucker and Poulin2010). Parasites with complex life cycles are likely to be particularly sensitive to changes in the environment as they rely on several host species to complete a generation. Aquatic parasites with complex life cycles relying on trophic transmission (i.e. infected prey eaten by a predator definitive host) are highly vulnerable to changes in the environment if intermediate host prey abundance and predator definitive host abundance or diet are affected (Lafferty, Reference Lafferty2008). Generally, the abundance and diversity of invertebrates are key components determining the parasite community in aquatic ecosystems. Parasite abundance and diversity in fish hosts are also highly dependent on individual fish diet (Knudsen et al., Reference Knudsen, Amundsen, Nilsen, Kristoffersen and Klemetsen2008). As a result, changes in parasite communities in fish have been reported repeatedly after alterations of the invertebrate prey community (Khan & Hooper, Reference Khan and Hooper2007; Morley, Reference Morley2007). Anthropogenic pressure on freshwater ecosystems may thus have common, but often ignored, effects on parasite dynamics. Additionally, geographically localized alterations of lotic freshwater ecosystems can have downstream effects on many aspects of ecosystem structure and dynamics, including parasites (Morley, Reference Morley2007). Again, effects on parasites are often highly unpredictable, ranging from increasing prevalence to complete extinction of specific parasites downstream of the disturbance (Mackie et al., Reference Mackie, Morton and Ferguson1983; Thompson & Nehring, Reference Thompson and Nehring2000). Riparian land use (e.g. forestry) and management can seriously affect lotic freshwater ecosystems, both locally and downstream of affected areas (Broadmeadow & Nisbet, Reference Broadmeadow and Nisbet2004; Richardson & Béraud, Reference Richardson and Béraud2014). Impacts can be large and diverse, even when the stream physical environment is not greatly affected (Lagrue et al., Reference Lagrue, Kominoski, Danger, Baudoin, Lamothe, Lambrigot and Lecerf2011; Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012; De Nadaï-Monoury et al., Reference De Nadaï-Monoury, Gilbert and Lecerf2014; Evangelista et al., Reference Evangelista, Boiche, Lecerf and Cucherousset2014). However, effects of riparian forest alterations on parasites can vary greatly among parasite species, even within the same host–parasite community, and are thus extremely difficult to predict (McKenzie, Reference McKenzie2007).
The nematode Cystidicoloides ephemeridarum is a common and widely distributed parasite of salmonid fish in the Holartic (Moravec, Reference Moravec1994). It uses larvae of the mayfly Ephemera danica as its main intermediate host (Moravec & Frantova, Reference Moravec and Frantova2003). Mayfly larvae become infected if they accidentally ingest C. ephemeridarum eggs when they feed on fine organic matter contained in the sediments in which they live; eggs hatch within the gut of the insect host. Parasite larvae migrate through the intestinal wall, coil and encyst in the host tissue. The cycle is completed when the mayfly is consumed by a fish definitive host in which parasites mature, reproduce and lay eggs. Eggs are passed with the faeces and deposit with fine organic matter in streambed sediments. Cystidicoloides ephemeridarum may thus be affected indirectly by environmental impacts reducing or increasing the abundance of its two different hosts. Alternatively, the parasite may not be affected by environmental changes if these do not impact the parasite's hosts. For example, small-scale forest harvesting did not influence brown trout, Salmo trutta, densities in headwater streams also containing E. danica mayfly and the parasite (Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012); C. ephemeridarum definitive host availability was thus not altered. As a result, this specific environmental impact may have no effect on the parasite's dynamics. However, riparian tree removal is also known to affect stream habitats severely, including channel morphology, substrate composition, sedimentation rates and sediment size, water temperature and chemistry (Sweeney et al., Reference Sweeney, Bott, Jackson, Kaplan, Newbold, Standley, Hession and Horwitz2004; Zhang et al., Reference Zhang, Richardson and Pinto2009). Environmental impacts on stream habitats could thus directly impact both mayfly host and parasite through alteration of sedimentation rates, for example. Changes in densities of the intermediate host (E. danica) of C. ephemeridarum may also subsequently affect that of the parasite. Variations in parasite abundance following ecosystem alterations may further affect both host and parasite in a feedback loop: a decrease in parasite densities may have positive effects on host populations and vice versa.
In the study reported here we looked at the effects of riparian forest management on the prevalence and abundance of the parasitic nematode C. ephemeridarum and the abundance of its insect intermediate host E. danica in forested headwater streams. We assessed whether tree removal along short (<500 m) stream reaches affected mayfly host and/or parasite population dynamics locally (i.e. at the level of the disturbance) but also upstream and downstream of the disturbance, to document potential repercussions on unaltered stream sections through aerial dispersal of adult mayfly or downstream drift of E. danica larvae. We predicted that forest management may markedly alter the densities of mayfly larvae and thus parasite abundance in the ecosystems. We also discuss potential cascading effects of changes in mayfly larvae and/or parasite abundances on infection dynamics in fish definitive hosts. However, potential changes in infection levels and parasite effects on fish hosts could not be assessed directly as we did not obtain permits to sample and dissect trout (S. trutta) in our study streams.
Materials and methods
Study sites
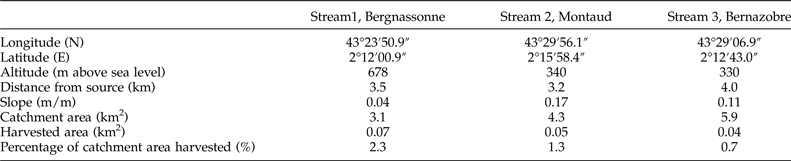
This study was carried out in the Montagne Noire in south-western France, a 1450 km2 highland region covered by mixed broadleaf forest dominated by beech (Fagus sylvatica) and oak (Quercus robur) and drained by a high density of low-order, permanent streams (Lagrue et al., Reference Lagrue, Kominoski, Danger, Baudoin, Lamothe, Lambrigot and Lecerf2011). Surface waters are characterized by circumneutral pH, low conductivity and concentrations of dissolved reactive phosphorus, and high nitrate concentrations owing to atmospheric deposition (Lecerf et al., Reference Lecerf, Dobson, Dang and Chauvet2005). We selected three adjacent and structurally analogous second-order streams. The Bergnassonne, Montaud and Bernazobre streams are referred to as streams 1, 2 and 3 (see table 1 for details). These streams are naturally heavily shaded (>90% riparian shading cover) but also comprised a ‘harvested’ reach affected by recent (2–4 years old) clear-cutting of riparian areas. Vegetation along harvested reaches was composed of saplings, bramble and soft-stem plants (Lecerf et al., Reference Lecerf, Evangelista, Cucherousset and Boiché2016). Harvested patches accounted for less than 5% of the total catchment area (table 1). Forestry operations were carried out to avoid streambed destruction by harvesting machines and to limit fine sediment contamination of the surface water (Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012).
Table 1. Physical features of the three streams selected at the level of the harvested reaches.
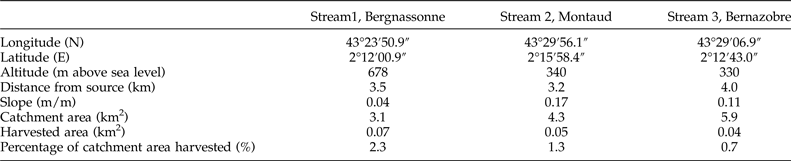
In each of the three streams, a section of length 50 m was selected in the middle of the harvested reach; these sections are hereafter referred to as harvested sections (H). We also selected a non-harvested upstream section of length 50 m and a 50-m non-harvested downstream section, both flowing under mature forest, in each stream. Upstream (Up) and downstream (Dn) sections were at least 50 m away from the edge of the harvested reach of the stream to avoid transitional effects between harvested and non-harvested reaches.
Riparian canopy
Stream exposure to sunlight and riparian canopy cover were assessed for each of the nine selected stream sections (3 sections × 3 streams) by determining daytime photosynthetically active radiation (PAR; μmol/s/m2) and canopy openness to sky (%) at the fully foliated canopy state (Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012). PAR was measured above the water surface, in the middle of each section, using a Li250A Photometer (LI-COR, Lincoln, Nebraska, USA). Canopy openness (%) was quantified from three hemispherical digital images per stream section taken from the middle of the stream with a Pentax (Tokyo, Japan) *ist D camera equipped with a SIGMA (Tokyo, Japan) 4.5 mm F2.8 EX DC circular fisheye lens. Gap Light Analyzer v2 software (http://www.ecostudies.org/gla/) was used to assess percentage gap area, assuming an equisolid angle projection, according to the lens manufacturer's instructions.
Geomorphology, organic matter content, temperature and chemistry
Streambed characteristics at each selected stream section were assessed from ten equally spaced transects. On each transect, we measured wet channel width and determined the proportion of streambed made of depositional sediments (%), the preferential habitat of E. danica larvae (Macan, Reference Macan1979; Tokeshi, Reference Tokeshi1985). Organic matter contents of depositional sediments were also determined using a loss-on-ignition method, to assess the habitat quality of E. danica in terms of resource availability (De Nadaï-Monoury et al., Reference De Nadaï-Monoury, Gilbert and Lecerf2014). For this purpose, we collected five sediment samples (20 g each) at equally distant points along each stream section on one occasion. Water temperature, oxygen levels and conductivity were measured in situ on each of the three sampling dates using multi-parameter probes.
Sampling and dissections of Ephemera danica larvae
Mayfly larvae were sampled on three occasions, one month apart, in February, March and April 2010, before larvae started emerging as adult mayfly (Tokeshi, Reference Tokeshi1985). Larvae were sampled using a standard Surber sampler net with a 0.1 m2 horizontal metal frame (0.33 × 0.3 m) fitted with a nylon net (mesh 250 μm) (Surber, Reference Surber1937; Hauer & Lamberti, Reference Hauer and Lamberti2011). Five samples of depositional sediments haphazardly distributed across each section were obtained on each occasion (3 streams × 3 sections × 3 dates × 5 samples). Samples were taken by embedding the Surber's metal frame into the sediment. Substrate and E. danica larvae enclosed within the frame were manually scooped up into the net to a depth of 10 cm, or until hard substrate was reached, so that all E. danica larvae contained within the metal frame were captured in the net. Animals and substrate contained in the net were transferred on to a sieve (mesh size 500 μm) so that fine sediment could be rinsed off. Samples containing fewer than 30 larvae were then stored individually in jars filled with 10% formaldehyde for later sorting, counts, measurements and dissections. When samples contained more than 30 E. danica individuals, larvae were counted to determine density (number of E. danica larvae/m2 of depositional sediment area) and a subsample was haphazardly selected and preserved for later measurements and dissections. Mean densities of mayfly larvae in each stream section were estimated by correcting densities recorded in depositional sediments (as described above) by the proportion of streambed area made of depositional sediments (%). In the laboratory, preserved E. danica larvae were rinsed in tap water and measured (body length excluding caudal filaments). Mayfly individuals were then dissected to determine the prevalence of the nematode parasite C. ephemeridarum (proportion of mayfly hosts infected; Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997) and the numbers of parasite larvae were counted to determine abundance (mean number of parasites per host; Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997). Parasite density (number of C. ephemeridarum individuals/m2 of streambed) was estimated as the product of parasite abundance and E. danica host density.
Statistical analyses
Potential differences among streams and stream sections in canopy openness, streambed wet channel width, depositional sediment surface area and organic matter contents were tested using General Linear Models (GLM) with stream (1, 2 and 3) and stream section (Up, H and Dn) as categorical predictors. Canopy openness (%) and depositional sediment area (%) were arcsine transformed, while wet channel width and organic matter content (OM) were log transformed before analyses.
Ephemera danica size and densities (log transformed) were compared among streams and stream sections using a GLM to test for potential effects of riparian tree removal on mayfly populations; stream (1, 2 and 3) and stream section (Up, H and Dn) were used as categorical predictors. Potential differences in parasite prevalence among streams and stream sections were compared using Fisher's exact tests; the proportions of infected mayfly larvae among stream sections were compared in a pair-wise manner. Potential differences among streams and stream sections in C. ephemeridarum abundance (log transformed) in mayfly larvae were tested using a GLM with the same categorical predictors as above. Mayfly larval size was also added as a continuous predictor to control for potential effects of host size on parasite abundance. Finally, a GLM was used to test for differences in C. ephemeridarum densities (number of parasites/m2 of streambed) among streams and stream sections. Sampling occasions/dates were treated as replicates, and sampling date was also included as a categorical predictor in the three above GLMs. All models were run using STATISTICA Software 6.0 (StatSoft Inc., Paris, France).
Results
Environmental variables
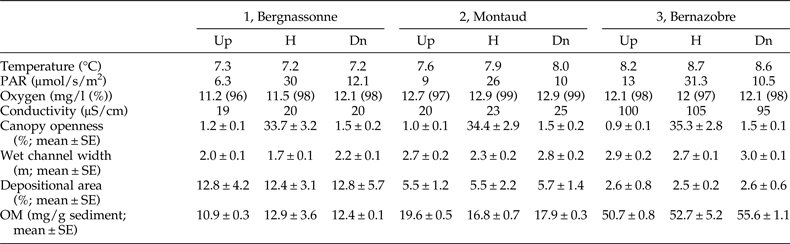
Although there were small differences among streams in water temperature, PAR and water conductivity, these variables were highly similar across sections within streams (table 2). Dissolved oxygen levels were very high and almost identical among all stream sections (table 2).
Table 2. Characteristics of the nine stream sections (Up, upstream; H, harvested; Dn, downstream) sampled, including temperature, photosynthetically active radiation (PAR), dissolved oxygen levels, conductivity, riparian canopy openness, stream wet channel width, proportion of river bed surface made of depositional sediments, and organic matter (OM) contents of depositional sediments.
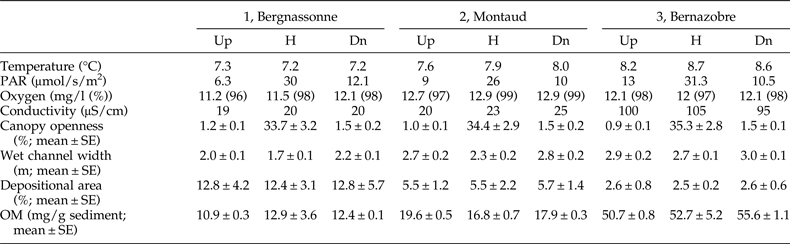
As expected, canopy openness was significantly higher in harvested sections (GLM, F 2,18 = 1423.3, P < 0.0001) but did not differ among streams (GLM, F 2,18 = 0.326, P = 0.726; table 2). Wet channel width varied significantly among streams (GLM, F 2,81 = 36.24, P < 0.0001) and among stream sections within each stream (GLM, F 2,81 = 8.54, P = 0.0004); stream channel was consistently narrower in the harvested section (H) than in both upstream (Up) and downstream (Dn) sections in all three streams (table 2). The proportion of streambed made of depositional sediments was significantly different among streams (GLM, F 2,81 = 13.82, P < 0.0001) but did not differ among stream sections (GLM, F 2,81 = 0.18, P = 0.835; table 2). Finally, organic matter contents (OM, mg/g sediments) varied significantly among streams (GLM, F 2,36 = 248.5, P < 0.0001) but not among stream sections (GLM, F 2,36 = 0.57, P = 0.571; table 2).
Density and size of Ephemera danica
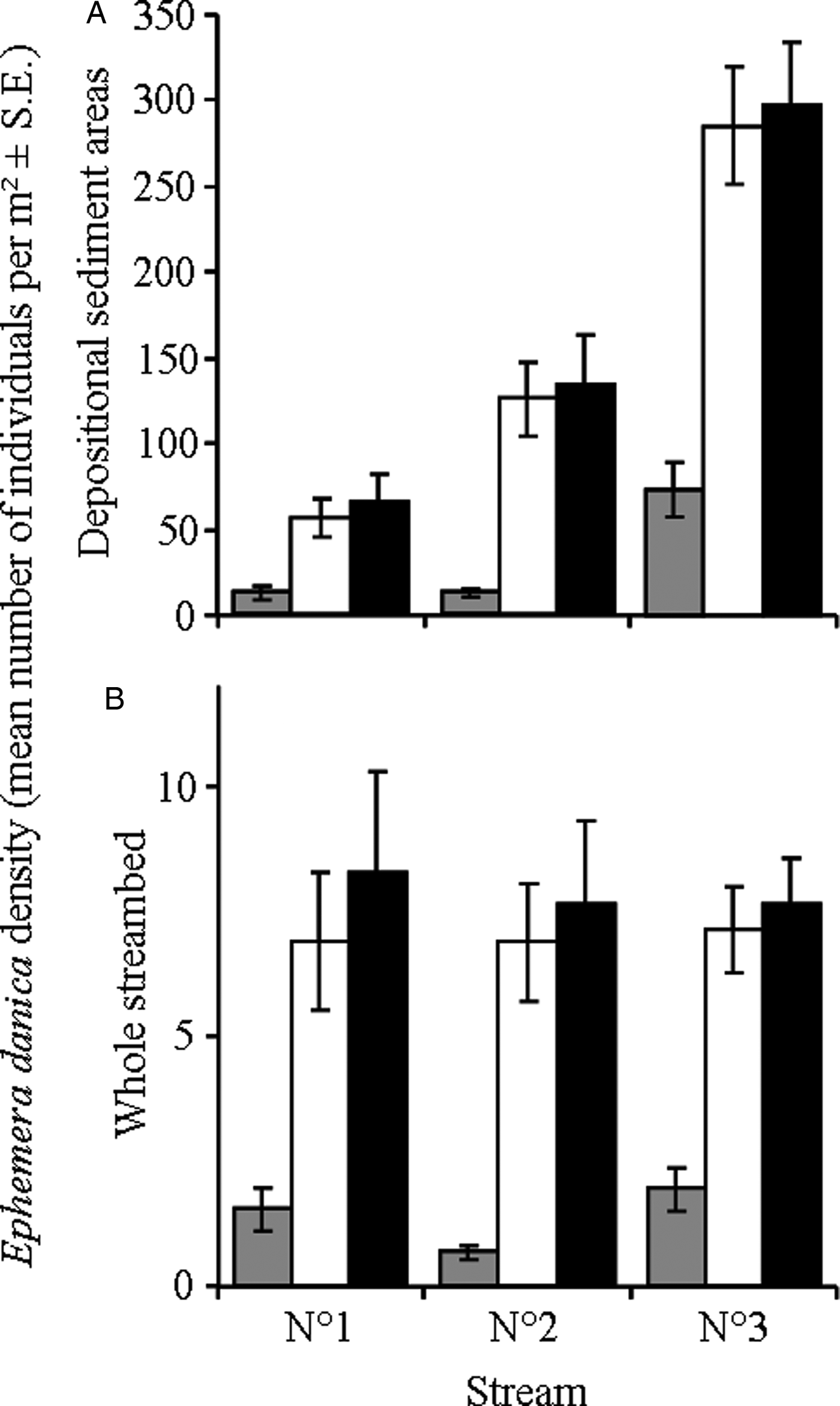
Densities of E. danica (number of individuals/m2) in depositional sediments varied significantly among streams (GLM, F 2,108 = 64.6, P < 0.0001), stream sections (GLM, F 2,108 = 79.4, P < 0.0001) and dates (GLM, F 2,3108 = 14.1, P < 0.0001). The density of mayfly larvae was significantly lower in samples collected in February than in both March and April (Tukey HSD, P = 0.0002 and 0.0001, respectively); there was no difference between samples collected in March and April (Tukey HSD, P = 0.804). Densities were lower in stream 1 than in both the other streams (Tukey HSD, P = 0.0018 and 0.0001 for streams 2 and 3, respectively); the density was also higher in stream 3 than in stream 2 (Tukey HSD, P = 0.0001; fig. 1A). Finally, densities of mayfly larvae were significantly lower in upstream sections than in both harvested and downstream sections (Tukey HSD, both P = 0.0001; fig. 1A); there was no difference between harvested and downstream sections (Tukey HSD, P = 0.975; fig. 1A).
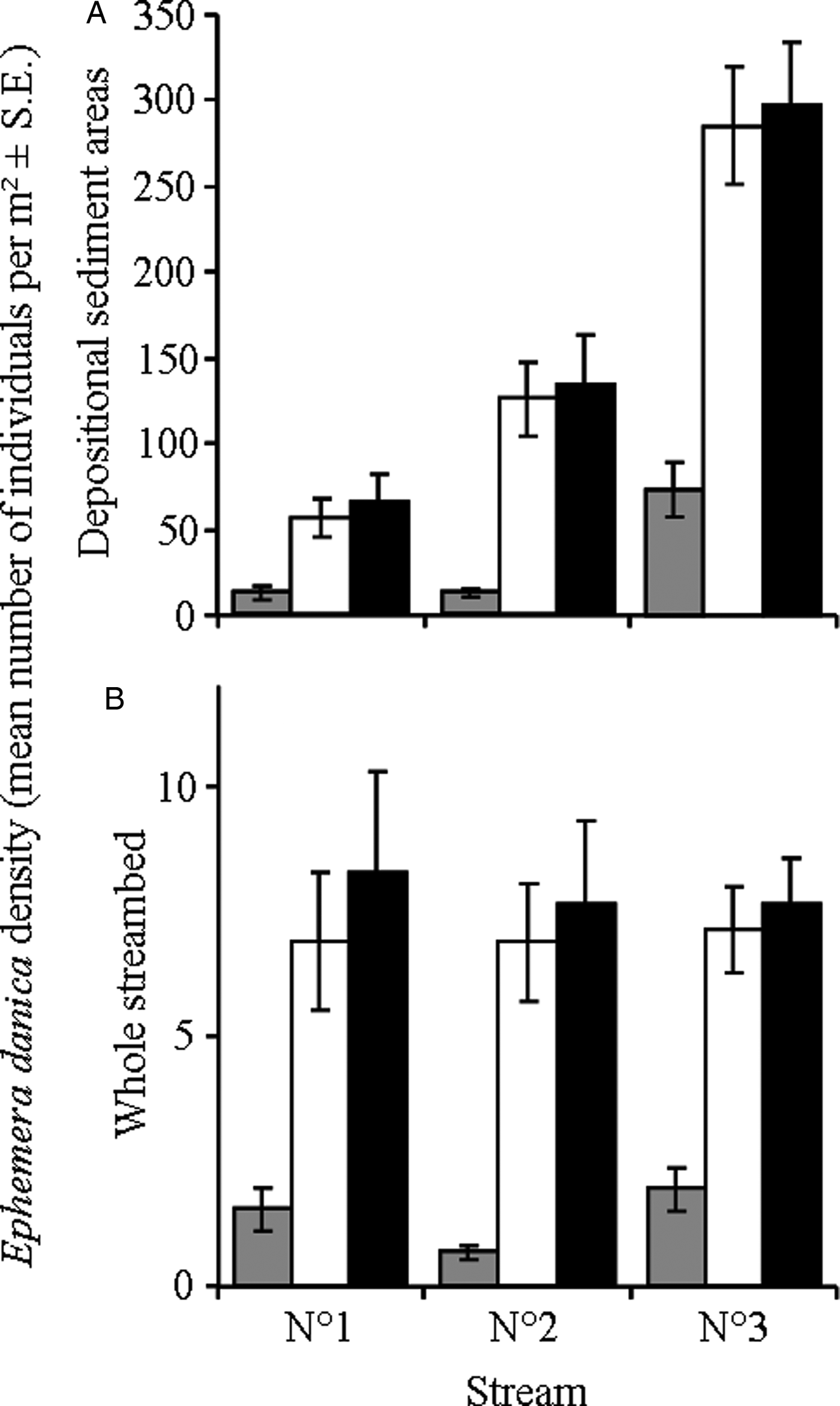
Fig. 1. Density of larvae (mean number of individuals/m2 ± SE) of the mayfly Ephemera danica in each stream section (upstream (grey bars), harvested (white bars) and downstream (black bars)) of the three study streams in (A) depositional sediment areas only and (B) the whole streambed.
However, E. danica larvae densities were recorded from depositional sediment areas and the proportion of streambed made of depositional sediments was also significantly different among streams (see above). When mayfly densities were corrected and expressed in terms of number of individuals/m2 of streambed, there was no difference among streams (GLM, F 2,108 = 0.75, P = 0.475; fig. 1B). Differences among sampling dates (GLM, F 2,108 = 11.5, P < 0.0001) and stream sections remained the same (GLM, F 2,108 = 48.5, P < 0.0001; fig. 1B).
Mayfly larval size did not vary among streams (GLM, F 2,702 = 0.04, P = 0.962; body length in mm (± SE): 18.9 ± 0.4, 18.1 ± 0.2 and 18.2 ± 0.2 in streams 1, 2 and 3, respectively) or among sections (GLM, F 2,702 = 0.26, P = 0.773; 18.6 ± 0.3, 18.5 ± 0.2 and 17.9 ± 0.2 in upstream, harvested and downstream sections, respectively). However, E. danica larval size increased significantly between sampling dates (GLM, F 2,702 = 28.9, P < 0.0001; 17.1 ± 0.2, 18.0 ± 0.2 and 19.6 ± 0.2 in February, March and April, respectively). Sampling date and host size were thus included in the following model testing for the effects of stream and section on parasite abundance.
Prevalence, abundance and density of Cystidicoloides ephemeridarum
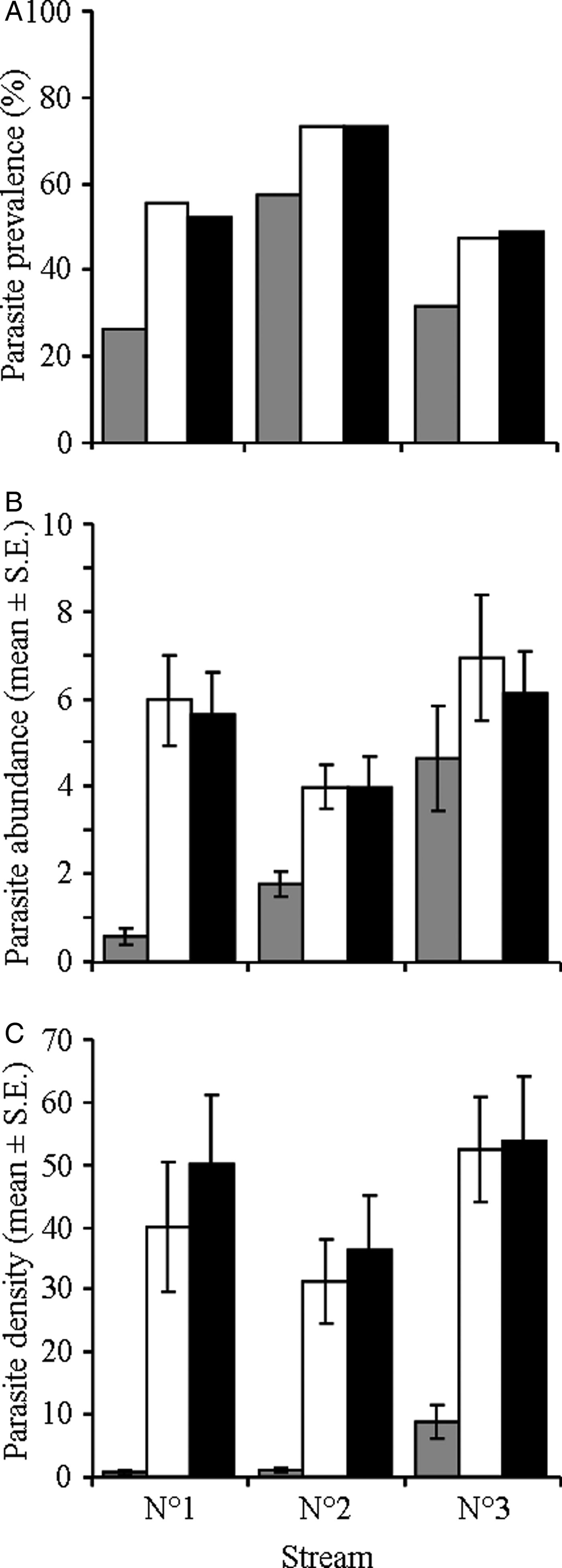
Overall parasite prevalence (i.e. proportion of infected hosts) varied among streams. Prevalence was significantly higher in stream 2 than in the other two streams (Fisher's exact tests, χ2 = 23.8 and 43.3, both P < 0.0001; 46.3%, 69.9% and 42.3% in streams 1, 2 and 3, respectively); there was no difference between streams 1 and 3 (Fisher's exact test, χ2 = 0.41, P = 0.232). Parasite prevalence did not vary among dates in any of the three streams (Fisher's exact tests, all P > 0.05). However, the prevalence of C. ephemeridarum varied significantly among stream sections (fig. 2A). In all three streams, parasite prevalence was lower in the upstream section than in both harvested and downstream sections (Fisher's exact tests, all P < 0.05; fig. 2A). Finally, parasite prevalences were similar between the harvested and downstream sections in all three streams (Fisher's exact tests, all P > 0.05; fig. 2A).
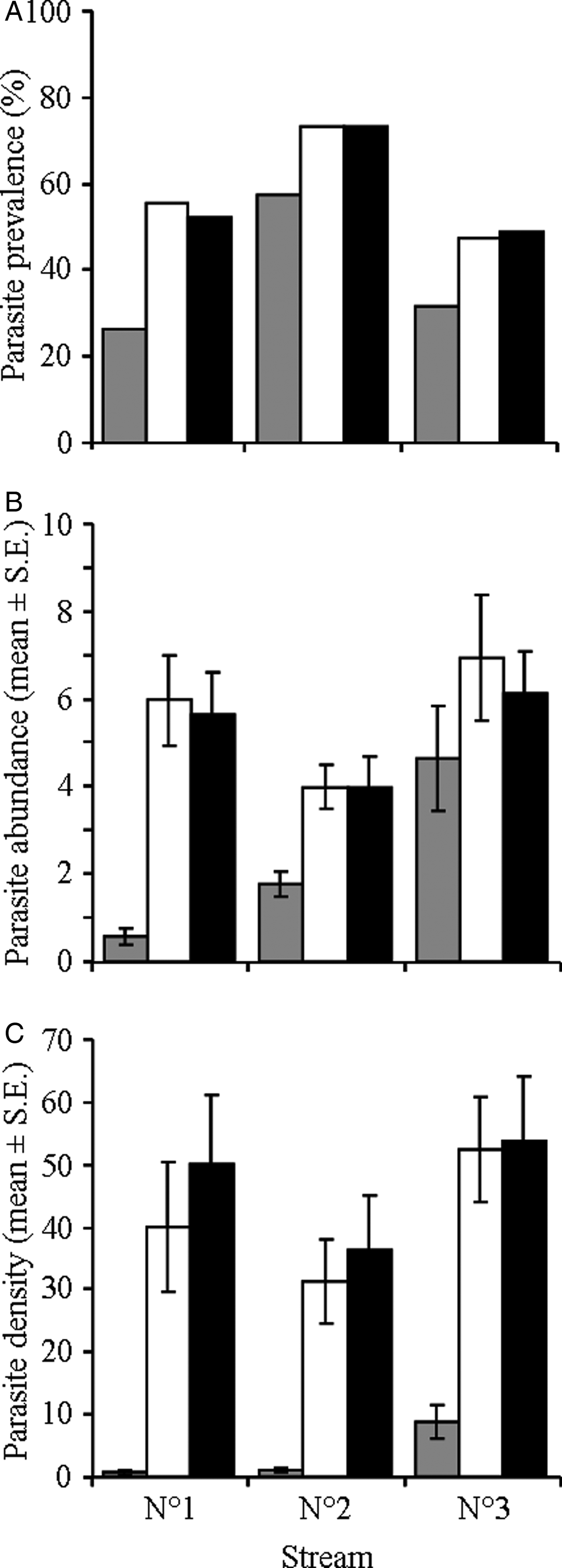
Fig. 2. Infection levels of the nematode parasite Cystidicoloides ephemeridarum in its mayfly intermediate host in each stream section (upstream (grey bars), harvested (white bars) and downstream (black bars)) of the three study streams. (A) Prevalence (proportion of infected mayfly larvae); (B) abundance (mean number (± SE) of parasites per individual host); and (C) density (mean number (± SE) of parasites/m2 of streambed). Parasite density was calculated as the product of mayfly host density (per m2 of streambed) and parasite abundance.
Parasite abundance (i.e. mean number of parasites per individual host) did not differ among streams (GLM, F 2,701 = 0.27, P = 0.734), or sampling dates (GLM, F 2,701 = 2.04, P = 0.131; fig. 2B) but increased significantly with host size (GLM, F 1,701 = 130.9, P < 0.0001; r = 0.355). More importantly, C. ephemeridarum abundance was significantly influenced by stream section (GLM, F 1,701 = 16.4, P < 0.0001). Parasite abundance was significantly lower in upstream than in both harvested and downstream sections (Tukey HSD, both P < 0.0001; fig. 2B). There was no difference between harvested and downstream sections (Tukey HSD, P = 0.953; fig. 2B).
Parasite density differed significantly among sampling dates (GLM, F 2,108 = 31.0, P < 0.0001) and stream sections (GLM, F 2,108 = 190.9, P < 0.0001). Similarly to host density, C. ephemeridarum density was significantly lower in samples collected in February than in both March and April (Tukey HSD, both P = 0.0001); there was no difference between samples collected in March and April (Tukey HSD, P = 0.698). Parasite densities were also significantly lower in upstream sections than in both harvested and downstream sections (Tukey HSD, both P = 0.0001; fig. 2C); there was no difference between harvested and downstream sections (Tukey HSD, P = 0.999; fig. 2C). In contrast to host densities, parasite densities also varied among streams (GLM, F 2,108 = 18.3, P < 0.0001). Parasite densities were higher in stream 3 than in both the other streams (Tukey HSD, both P = 0.0001); there was no difference between streams 1 and 2 (Tukey HSD, P = 0.813; fig. 2C).
Discussion
Parasites are now recognized as being important components of ecosystem communities and dynamics (Combes, Reference Combes1996; Mouritsen & Poulin, Reference Mouritsen and Poulin2002; Hudson et al., Reference Hudson, Dobson and Lafferty2006; Wood et al., Reference Wood, Byers, Cottingham, Altman, Donahue and Blakeslee2007). They can also respond strongly to environmental disturbances and further increase extinction risk in host species already affected by environmental stressors (Sures, Reference Sures2001; Smith et al., Reference Smith, Sax and Lafferty2006). Understanding how human disturbances affect both hosts and parasites is thus vital when trying to assess overall impacts on ecosystem community and dynamics. Previous studies showed that small-scale forest management and riparian tree removal had no detectable impacts on trout abundance, the definitive host of the nematode parasite C. ephemeridarum (Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012; Evangelista et al., Reference Evangelista, Boiche, Lecerf and Cucherousset2014). Here, we found that impacts on the physical habitat (depositional zones) of mayfly larvae were minimal, as also noted by De Nadaï-Monoury et al. (Reference De Nadaï-Monoury, Gilbert and Lecerf2014) whose study covered a larger number of streams. Although stream channel width was lower in harvested than non-harvested stream reaches, differences were small (20–50 cm) and habitat availability for the mayfly intermediate host was not affected (table 2). Despite broadly similar geomorphological features among the study reaches along the streams, both the density of mayfly intermediate hosts (E. danica) and parasite abundance were substantially higher in harvested stream reaches. Ecosystem disturbance also seemed to increase host densities and parasite infection levels in downstream reaches, where riparian forest was preserved. Natural upstream–downstream increases in infection levels of fish parasites have been documented before over large distances (Blasco-Costa et al., Reference Blasco-Costa, Koehler, Martin and Poulin2013). However, the spatial scale, metres rather than kilometres, and magnitude of the changes observed here are most likely consequences of the ecosystem disturbance. Riparian forest harvesting had thus indirect positive effects on both E. danica (intermediate host) and C. ephemeridarum (parasite).
The distribution of aquatic insect larvae influences that of their adult stages, but many adults, including mayfly, migrate upstream for reproduction (Hubbard, Reference Hubbard and Campell1991; Winterbourn et al., Reference Winterbourn, Chadderton, Entrekin, Tank and Harding2007). Upstream migration of adult aquatic insects most often follows the stream corridor but can be strongly influenced by habitat characteristics in general and riparian vegetation in particular (Brittain, Reference Brittain1982; Delettre & Morvan, Reference Delettre and Morvan2000; Petersen et al., Reference Petersen, Masters, Hildrew and Ormerod2004). Here we found markedly and consistently lower densities of E. danica larvae in stream reaches immediately upstream of logged patches, indicating that forest harvesting has potentially strong effects on mayfly dispersal and recruitment (fig. 1). Evidence suggests that the presence of mature forest canopy can prevent mass emergence and swarming of adults, thus reducing egg and larval densities (Winterbourn et al., Reference Winterbourn, Chadderton, Entrekin, Tank and Harding2007). In contrast, open stream reaches can experience huge concentrations of reproductive adult mayflies and, subsequently, locally high larval densities. Here, the dense, hedgerow-type vegetation present at the boundary between harvested and upstream reaches may form a barrier to the upstream migration of adult E. danica (Stamps et al., Reference Stamps, Buechner and Krishnan1987; Delettre & Morvan, Reference Delettre and Morvan2000; Málnás et al., Reference Málnás, Polyák, Prill, Hegedüs, Kriska, Dévai, Horváth and Lengyel2011). Although dense vegetation at the boundary between harvested and upstream reaches is probably permeable to some degree, the abrupt transition from open, harvested stream reaches to dense vegetation may create an optical barrier to the relatively large adult E. danica mayfly (Málnás et al., Reference Málnás, Polyák, Prill, Hegedüs, Kriska, Dévai, Horváth and Lengyel2011). Accumulation of adult mayflies against this barrier may thus create large concentrations of reproductive adults followed by the much localized deposition of large numbers of eggs, and thus high larval densities, in impacted stream reaches.
In addition to flight, aquatic insects disperse downstream through drift, a process which probably largely accounted for increasing E. danica larval densities in downstream, unaltered reaches (Gyselman, Reference Gyselman, Flannagan and Marshall1980; Brittain, Reference Brittain1982). The striking and consistent difference in mayfly densities observed between upstream and harvested reaches could thus be the result of natural downstream drift of larvae, not totally compensated for by upstream flight of reproductive adults due to the barrier formed by the forest edge (fig. 1; Hubbard, Reference Hubbard and Campell1991; Málnás et al., Reference Málnás, Polyák, Prill, Hegedüs, Kriska, Dévai, Horváth and Lengyel2011). However, this hypothesis would need to be tested experimentally. Whether riparian forest removal has negative upstream, positive local and downstream effects, or all of the above, on the densities of E. danica larvae remains unclear. Generally, by creating patchiness in the mayfly habitat, discontinuities in riparian forest seem to induce high heterogeneity in the distribution of E. danica larvae.
Synergistic positive effects on parasite dynamics and host densities induced an overall 6- to 66-fold increase in parasite densities in impacted reaches compared to upstream, unaltered stream sections (fig. 2). Furthermore, this marked increase was also observed downstream of the disturbance, in stream reaches not directly impacted by forest harvesting, suggesting that even small ecological disturbances can have ripple effects beyond areas directly impacted. Such downstream repercussions of localized disturbances are common in lotic systems (Morley, Reference Morley2007). Increased intermediate host densities are expected to have positive effects on parasite dynamics by increasing the overall standing crop of the parasite (Johnson & Chase, Reference Johnson and Chase2004; Johnson et al., Reference Johnson, Chase, Dosch, Hartson, Gross, Larson, Sutherland and Carpenter2007). Here, prevalence and abundance of C. ephemeridarum are likely to be amplified further by increased transmission rates to fish definitive hosts (Arneberg et al., Reference Arneberg, Skorping, Grenfell and Read1998). Densities of mayfly larvae increased significantly in harvested and downstream reaches (present study), while trout definitive host densities were little affected (Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012; Evangelista et al., Reference Evangelista, Boiche, Lecerf and Cucherousset2014). Encounter rates between intermediate (mayfly) and definitive hosts (trout), along with transmission opportunities for the parasite, are thus likely to increase simply because of higher predation rates on the intermediate host (Holt & Roy, Reference Holt and Roy2007). Furthermore, trout are selective feeders, often overexploiting the most abundant prey (Ringler, Reference Ringler1979; Rincón & Lobón-Cerviá, Reference Rincón and Lobón-Cerviá1999). As a result, trout may preferentially feed on the abundant mayfly larvae in impacted stream reaches, further increasing infection dynamics of C. ephemeridarum and potentially explaining the documented increase in both prevalence and abundance of the parasite in E. danica larvae. Alternatively, or concomitantly, highly infected mayfly larvae may be more vulnerable to predation by trout. Higher predation rates on infected hosts can be due to simple pathological side-effects of the infection, or to parasite-driven changes in host behaviour increasing the predation risk of infected hosts (Poulin, Reference Poulin1995; Lagrue et al., Reference Lagrue, Kaldonski, Perrot-Minnot, Motreuil and Bollache2007; Cézilly et al., Reference Cézilly, Thomas, Médoc and Perrot-Minnot2010). For example, C. ephemeridarum may render E. danica larvae more prone to trout predation through higher drift rates (Williams et al., Reference Williams, Townsend and Poulin2001). However, this cannot be determined here and would require further studies.
Seasonal dynamics of E. danica and C. ephemeridarum have been well studied. Sampling of E. danica in the present study corresponded to the high prevalence and abundance period preceding the season of high predation on emerging mayfly larvae and massive infections in fish hosts (Frantova & Moravec, Reference Frantova and Moravec2003). Given the patterns of host and parasite densities shown in our study, fish occupying the harvested and downstream reaches of these streams must be exposed to much higher parasite levels, because of both an increase in the availability of mayfly larvae and higher parasite abundances. Although parasite transmission to trout definitive hosts can be affected by fish feeding preference and alternative prey availability, transmission rates are highly dependent upon abundance of the intermediate host and availability of parasite larvae (Aho & Kennedy, Reference Aho and Kennedy1984, Reference Aho and Kennedy1987). It is uncertain how far downstream of the impacted reach these effects can be detected, but they may have repercussions over long distances and well away from the disturbance itself. Intestinal helminth parasites can have strong negative effects on their fish host, often inducing a reduction in body condition and increased mortality in cases of severe infection (Mladineo et al., Reference Mladineo, Zrnčić and Oraić2009). Although C. ephemeridarum has relatively low pathological impacts, an increase in parasite standing crop following disturbances can translate into infection intensities orders of magnitude higher than in undisturbed ecosystems (Johnson & Chase, Reference Johnson and Chase2004; Johnson et al., Reference Johnson, Chase, Dosch, Hartson, Gross, Larson, Sutherland and Carpenter2007). The synergistic, positive effects of riparian forest removal on both intermediate host prey and parasite levels documented here could thus induce a massive increase in infection levels in trout definitive hosts, although other seasonal factors, such as water temperature, may modulate infection levels in fish (Aho & Kennedy, Reference Aho and Kennedy1984, Reference Aho and Kennedy1987). Riparian forest management may thus reduce fish body condition or survival indirectly, even though it did not directly influence trout densities (Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012; Evangelista et al., Reference Evangelista, Boiche, Lecerf and Cucherousset2014). However, infection levels and parasite effects on fish could not be assessed here as we did not obtain permits to sample and dissect fish.
Understanding how environmental disturbances influence host–parasite interactions, parasite dynamics and potential disease emergence is key to predicting how perturbations may affect wildlife populations and further impact already vulnerable host species (Smith et al., Reference Smith, Sax and Lafferty2006; Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012). Overall, we show that both host and parasite were positively affected by environmental disturbance in the studied ecosystem. There was also a synergistic effect whereby increases in host abundance and parasite levels induced a higher than expected increase in parasite densities (fig. 2). As a result, fish definitive hosts in these ecosystems are likely to be carrying much higher parasite burdens than normal. Furthermore, local, small-scale forest harvesting had similarly strong downstream effects on host and parasite dynamics. Although these results cannot be generalized to all parasites, it is likely that riparian vegetation management will have strong indirect effects on freshwater parasites and/or hosts, even without directly impacting the aquatic ecosystem. Small-scale forest harvesting is often considered to be a good alternative to large-scale clear-cut logging, but it may have large, unforeseen impacts on freshwater ecosystem structure and functioning, as shown here and in a previous study (Lecerf et al., Reference Lecerf, Baudoin, Besson, Lamothe and Lagrue2012). Finally, because physical differences between harvested and unaltered sections were minimal, the striking changes in host and parasite populations reported here support the view that some parasites can be useful as bioindicators of subtle environmental changes (Khan & Hooper, Reference Khan and Hooper2007).
Acknowledgements
We are grateful to E. Cano and S. Lamothe for field and laboratory assistance. We thank Professor R. Poulin for very constructive comments on a previous version of the manuscript. We also thank an anonymous reviewer and the Deputy Editor for very constructive comments on a previous version of the manuscript.
Financial support
This study was supported by funding from ONEMA (French National Agency for Water and Aquatic Environments).
Conflict of interest
None.