Introduction
Nematode parasites of the genus Strongyloides includes over 50 different species that naturally infect mammals, birds, reptiles and amphibians (Viney & Kikuchi, Reference Viney and Kikuchi2017). Infection by Strongyloides begins when the infective third-stage larvae (iL3) penetrate the host skin and migrate through blood vessels to reach the lungs and then migrate to the digestive tract, where they become parasitic female adults that colonize the small intestine, producing eggs by parthenogenesis. The eggs or hatched larvae are excreted into the environment with the faeces, where they develop directly into iL3 forms via the homogonic route or develop into free-living adult stages via the heterogonic route, which reproduce sexually to produce eggs and then develop into infective larvae (Hino et al., Reference Hino, Tanaka, Takaishi, Fujii, Palomares-Rius, Hasegawa, Maruyama and Kikuchi2014).
Strongyloides ratti and Strongyloides venezuelensis are parasites from rats, but also are capable of infecting other rodents, such as mice and gerbils. Rodent hosts infected with S. ratti excrete both eggs and early-stage larvae, whereas hosts infected with S. venezuelensis excrete early-stage eggs with the faeces (Viney & Kikuchi, Reference Viney and Kikuchi2017). These two species are the most widely used to study Strongyloides infection and mucosal immunity in animal models. As both species can be maintained in the laboratory conditions, the different lifecycle stages may be obtained in considerable quantities and free of faecal debris. For example, larval stages can be obtained from faecal-charcoal cultures; parasitic females can be isolated from the rat's small intestine, whereas free-living adults can be obtained from faecal-agar cultures (Lok, Reference Lok2007; Hino et al., Reference Hino, Tanaka, Takaishi, Fujii, Palomares-Rius, Hasegawa, Maruyama and Kikuchi2014; Viney & Kikuchi, Reference Viney and Kikuchi2017). However, it is difficult to obtain eggs with a high purity from host faeces using parasitological concentration methods.
The recent genomic, transcriptomic and proteomic analyses of Strongyloides species have provided new insights into different aspects of the biology of this parasite (Soblik et al., Reference Soblik, Younis, Mitreva, Renard, Kirchner, Geisinger, Steen and Brattig2011; Hino et al., Reference Hino, Tanaka, Takaishi, Fujii, Palomares-Rius, Hasegawa, Maruyama and Kikuchi2014; Hunt et al., Reference Hunt, Tsai, Selkirk and Viney2017, Reference Hunt, Hino, Yoshida and Kikuchi2018; Maeda et al., Reference Maeda, Palomares-Rius, Hino, Afrin, Mondal, Nakatake, Maruyama and Kikuchi2019). The possibility of working with pure Strongyloides eggs opens up a wide range of future biological, biochemical and molecular studies.
In this study, we have developed a gradient centrifugal-flotation technique to isolate and purify S. venezuelensis eggs from rat faeces, which may be used in future biological, genomic, transcriptomic and proteomics studies.
Material and methods
Parasites and host animals
A Brazilian strain of S. venezuelensis was isolated from the wild rodent Bolomys lasiurus, in April 1986, in the Department of Parasitology of the Universidade Estadual de Campinas, Brazil, and was maintained in male Wistar rats (Rattus norvegicus) through experimental infections with iL3.
Experimental infection of animals
Three male rats (30 days of age) were inoculated subcutaneously in the abdominal region with 10,000 iL3, which were obtained from rat faecal-charcoal cultures. Briefly, infected rat faeces were moistened with tap water, mixed with animal bone charcoal (vol:vol) and incubated in wide glass jars at 28°C for two or three days; iL3 were recovered following the Baermann technique as described by Lok (Reference Lok2007). All procedures were conducted in accordance with the ethical guidelines adopted by the Ethics Committee for Animal Experimentation from Instituto de Medicina Tropical, Universidade de São Paulo, Brazil (Protocol 0356A).
Collection and preparation of samples
Faeces from the three infected rats were collected at days 6, 8, 12, 14, 16, 18 and 21 post-infection, and mixed to form a unique faeces sample; from this source, two samples of 1.0 g were taken and homogenized in 50 mL of distilled water, and filtered using four layers of gauze to remove the coarse material. The filtrates were centrifuged at 170 × g for 5 min and the supernatants were discarded. The pellets were resuspended in 50 mL of distilled water, centrifuged at 110 × g for 5 min and the supernatants discarded; this procedure was repeated one more time. One pellet was directly used for egg counting following the modified McMaster technique and the other one was used to purify eggs using our proposed method (as described below).
Egg counting procedure
The eggs contained in the pellet (obtained from 1.0 g of rat faeces) were counted following a modified version from the McMaster technique described by Zajac & Conboy (Reference Zajac and Conboy2012). Briefly, the pellet was resuspended in 14 mL of Sheather's flotation solution, but without formalin (454 g of sucrose + 360 mL of distilled water; specific gravity of 1.27) and both sides of the McMaster plastic chamber slide were filled with 1 mL of the mixture and then allowed to sit for 5 min before examining. All eggs within both chambers were counted and then multiplied by the factor of 50 to obtain the number of eggs per gram of faeces.
Sucrose gradient procedure
The other pellet was resuspended in 14 mL of distilled water and then carefully layered over a discontinuous sucrose density gradient, which was made with 15 mL of Sheather's flotation solution without formalin followed by 15 mL of 50% Sheather's flotation solution (diluted vol:vol with distilled water) in a 50 mL polypropylene conical tube. The gradient was centrifuged at 240 × g for 7 min and the eggs (concentrated in the water–sucrose interface) were carefully recovered (approximately 5 mL) using a plastic Pasteur pipette and immediately transferred to a new 50 mL polypropylene conical tube; this recovered volume was diluted tenfold with distilled water and centrifuged at 110 × g for 3 min. The pellet was resuspended in 40 mL of distilled water and centrifuged at 110 × g for 3 min, and this step was then repeated one more time. The pellet containing pure eggs was finally dissolved in 1 mL of distilled water containing 0.2% trypan blue in order to determine the percentage of viability (de Victorica & Galván, Reference de Victorica and Galván2003) and counted following the modified version of the McMaster technique. Additionally, 0.1 ml of the final suspension was deposited on the surface of nutrient agar plates and incubated at 28°C for 24 h to permit the embryonic and hatching process of the larvae, in order to confirm the viability of the Strongyloides eggs. The percentage of hatched larvae was determined by microscopic observation.
Results and discussion
Using the modified version of the McMaster technique, we have observed that infected rats release considerable quantities of eggs from day 6 to day 10 post-infection, and this excretion begins to decrease by day 12 post-infection until the end of the experiment (day 21). The mean egg excretion per gram of faeces in the different days post-infection is summarized in the table 1.
Table 1. Evaluation of the sucrose gradient procedure for purifying Strongyloides venezuelensis eggs.
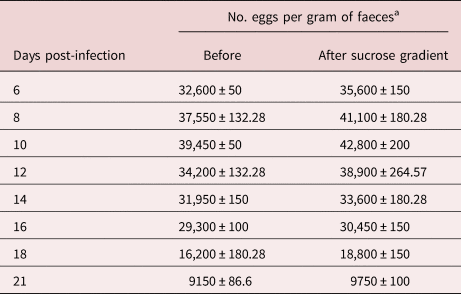
a Determined by the modified McMaster technique. The results are presented as the mean ± standard deviation from three experiments in different periods.
Initial experiments for purifying Strongyloides eggs using the Sheather's solution as a unique flotation medium were not satisfactory because small faecal debris were retained and concentrated together with the eggs in the water–sucrose interface (data not shown). Although several subsequent washing cycles were carried out to eliminate the faecal debris, a poor-quality material was always obtained.
In order to solve this inconvenience, the next step was to improve the flotation system by testing sucrose solutions with specific gravities of less than 1.27 but greater than the specific gravity of Strongyloides egg. Thus, two different layers derived from the Sheather's sucrose solution were finally used to purify the Strongyloides eggs from the faecal debris. Using this discontinuous sucrose density gradient, all the Strongyloides eggs were retained and concentrated in the first interface, while the faecal debris were retained and concentrated in both the second interface and the bottom of the tube (fig. 1). The dye exclusion test using 0.2% trypan blue showed that none of the Strongyloides eggs were broken during the purification process, and the agar culture showed that all of these eggs became embryonated and evolved into larvae, meaning that all of them were 100% alive (data not shown).
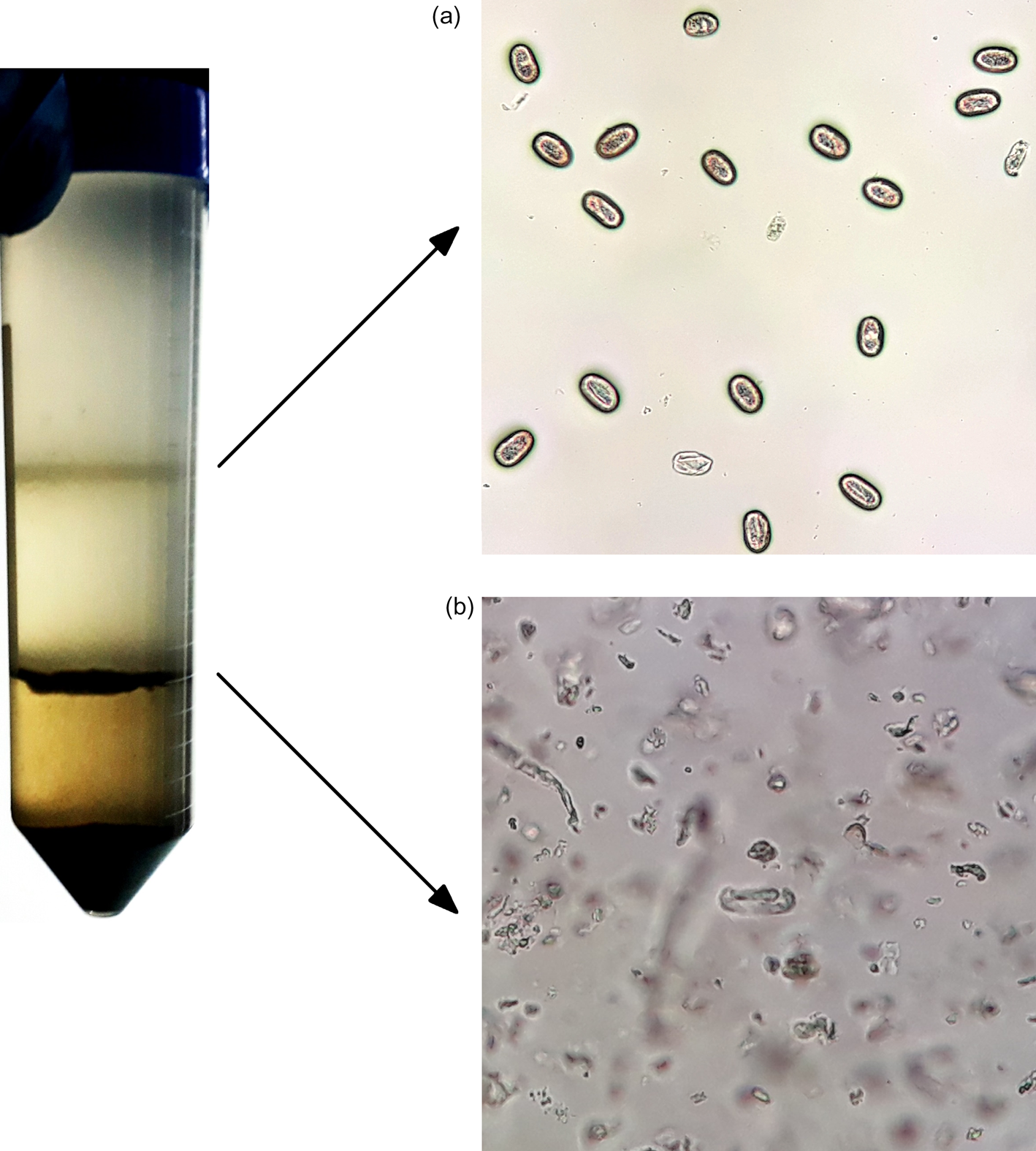
Fig. 1. Sucrose gradient procedure carried out in a 50 mL polypropylene conical tube, showing the first interface containing pure Strongyloides venezuelensis eggs (a) and the second interface containing faecal debris (b).
The results described in table 1 show that our method allows recovering Strongyloides eggs free of faecal debris on the different days from the experimentation time, with an amount apparently greater than 100% of the value obtained when the samples were counted directly by the modified McMaster technique before the purification process. A possible explanation for this phenomenon is that faecal debris could be hindering the spontaneous flotation process of the eggs in the McMaster technique, thus obtaining low values during the egg count. Perhaps more float time would be necessary before examining the plastic chamber slide. In any case, the McMaster technique should only be taken as an approximation of the true number of eggs per gram of faecal sample.
Parasitological concentration methods are used to separate parasite eggs or cysts from faecal debris and are classified into sedimentation or flotation techniques, based on the use of solutions with lower or higher specific gravity than the parasitic organisms, respectively. The main advantage of the flotation techniques is to produce a cleaner material than the sedimentation techniques. However, the chemicals used to prepare the flotation solution may deform or break the egg or cyst walls, making their identification and purification difficult (Ballweber et al., Reference Ballweber, Beugnet, Marchiondo and Payne2014).
Several authors have developed different methods to isolate and recover helminth eggs. For example, Wassall & Denham (Reference Wassall and Denham1969) and Beer (Reference Beer1972) described methods for purifying helminth eggs using semi-mechanical procedures for sieving, concentrating and collecting eggs from faeces. Likewise, McClure et al. (Reference McClure, Kruk and Misaghi1973) and Schaad & Walker (Reference Schaad and Walker1975) described methods for purifying eggs from the plant-parasitic nematode Meloidogyne sp. by using centrifugal-flotation techniques.
With regards to Strongyloides eggs, Lok (Reference Lok2007) reported unsuccessful results at adapting the standard technique used for purifying Caenorhabditis elegans eggs to yield viable Strongyloides stercoralis eggs in quantity. With the aim of obtaining antigenic extracts from S. venezuelensis eggs, Goulart de Carvalho et al. (Reference Goulart de Carvalho, Neto de Sousa, Gonçalves, da Cunha-Junior and Costa-Cruz2015) described a semi-mechanical procedure to separate eggs by passing an aqueous mixture of rat faeces through different sieves and several washing cycles by centrifugation. Unfortunately, the authors did not describe the performance of this procedure and whether the final product was free from faecal debris.
In this study, a rapid and simple method to separate Strongyloides eggs from rat faeces was developed using basic laboratory materials and equipment, obtaining a product with a high grade of purity in less than one hour, which is very convenient due to the rapid larval development inside the egg (Viney & Kikuchi, Reference Viney and Kikuchi2017).
So far, there is no information on the specific gravity of Strongyloides sp. eggs, but taking into consideration the specific gravity of Ancylostoma caninum eggs, which is approximately 1.056 (David & Lindquist, Reference David and Lindquist1982), a Sheather's solution diluted by half was sufficient to obtain a specific gravity of approximately 1.12, which is enough to make the eggs float, and for them to be isolated and purified.
Several authors have used centrifugal-flotation techniques for either the recovery (Dryden et al., Reference Dryden, Payne, Ridley and Smith2005), count (Cringoli et al., Reference Cringoli, Rinaldi, Maurelli, Morgoglione, Musella and Utzinger2011) or the determination of the specific gravidity (David & Lindquist, Reference David and Lindquist1982; Norris et al., Reference Norris, Steuer, Gravatte, Slusarewicz, Bellaw, Scare and Nielsen2018) of some helminth eggs. Recently, Choi et al. (Reference Choi, Park and Park2019) have described a method for purifying Trichuris suis eggs by using a combination of sieving, concentrating and centrifugal flotation, obtaining good results. However, their method is complex and difficult to reproduce in a laboratory with basic materials and equipment. Our proposed method eliminates the possibility of using complex apparatus, different chemical substances or time-consuming procedures.
Finally, this study constitutes the first report in the scientific literature on purifying Strongyloides eggs using a discontinuous sucrose density gradient, and future studies must be carried out to assess the efficiency of this method to isolate and purify other nematode eggs from animal or human faecal samples.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. W.H.R. is receiving a scholarship from Edital MCT/CNPq-Brazil, process number 142056/2018-9. F. M. de P. is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (grant number 2016/06185-0).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.




