Introduction
Among the many factors that potentially conspire against conservation of the world's biodiversity, including charismatic and popular large mammalian species, are infectious diseases, including those caused by metazoan parasites (Zhang et al., Reference Zhang, Daszak, Huang, Yang, Kilpatrick and Zhang2008). In general, we need an improved understanding of the parasites that infect these increasingly rare animals. At the same time, large host species can be viewed as arks that support a variety of unique symbiotic species, often including relatively host-specific parasites that may occur nowhere else in the world, and that are potentially susceptible to co-extinction events. Acknowledgment of the existence of this form of biodiversity and the need to preserve it adds even more incentive to characterizing and understanding the biology of the parasites of large, rare mammals.
This study focuses on two unrelated but prominent mammals of Chitwan National Park (CNP) in subtropical Nepal. The first is the Indian rhinoceros, also known as the greater one-horned rhinoceros or the Asian one-horned rhinoceros (Rhinoceros unicornis Linnaeus, 1758), which is listed by the International Union for the Conservation of Nature (IUCN) as ‘vulnerable’. The second is the Asian, or Indian, elephant Elephas maximus Linnaeus, 1758, listed as ‘endangered’ by IUCN. Whereas the biology and host–parasite relationships of vulnerable large mammals have been more intensively studied in other locations, such as African national parks (Southwell, Reference Southwell1921; Thapar, Reference Thapar1925; Fitzsimmons, Reference Fitzsimmons1962; Zumpt, Reference Zumpt1964; Penzhorn et al., Reference Penzhorn, Krecek, Horak, Verster, Walker, Boomker, Knapp and Quandt1994; Kinsella et al., Reference Kinsella, Deem, Blake and Freeman2004; Brant et al., Reference Brant, Pomajbíková, Modry, Petrželková, Todd and Loker2012), the study of the wild mammals of Nepal and their parasites is in its infancy. Our study focuses on schistosomes or blood flukes we have recovered from faecal samples of both elephants and rhinos in and around CNP.
The family Schistosomatidae is comprised of 14 recognized genera, five of which occur in mammals. Among the mammal-infecting genera is Bivitellobilharzia Dutt and Srivastava, 1955, with two described species, heretofore known only from elephants. Bivitellobilharzia loxodontae Vogel and Minnig, 1940 occurs in African forest elephants Loxodonta cyclotis Matschie, 1900 from the Democratic Republic of Congo (Vogel & Minning, Reference Vogel and Minning1940; Kinsella et al., Reference Kinsella, Deem, Blake and Freeman2004) and from the Central African Republic (Brant et al., Reference Brant, Pomajbíková, Modry, Petrželková, Todd and Loker2012). There have been no definitive reports of schistosomes from the African savanna elephant, Loxodonta africana Blumenbach, 1797. Bivitellobilharzia nairi (Mudaliar & Ramanujachary, Reference Mudaliar and Ramanujachary1945) Dutt and Srivastava, 1955 occurs in Asian elephants Elephas maximus Linnaeus, 1758 from India (Vogel & Minning, Reference Vogel and Minning1940; Mudaliar & Ramanujachary, Reference Mudaliar and Ramanujachary1945; Rao & Hiregauder, Reference Rao and Hiregauder1953; Dutt & Srivastava, Reference Dutt and Srivastava1961), Sri Lanka (Agatsuma et al., Reference Agatsuma, Rajapakse, Kuruwita, Iwagami and Rajapakse2004; Brant et al., Reference Brant, Morgan, Mkoji, Snyder, Rajapakse and Loker2006), Republic of the Union of Myanmar (Sundaram et al., Reference Sundaram, Padmanabha Iyer, Peter and Alwar1972) and Nepal (Devkota, Reference Devkota2008; Karki & Manandhar, Reference Karki and Manandhar2008). There are at least three recognized subspecies of Asian elephants: Elephas maximus maximus from Sri Lanka, the Indian elephant or Elephas maximus indicus from mainland Asia (including Nepal), and Elephas maximus sumatranus from the island of Sumatra. To our knowledge B. nairi has only been reported from the first two subspecies.
Adult males of Bivitellobilharzia are characterized by the presence of a tuberculated tegument, and by possession of up to 52 testes. Females are without tubercles and the ovary lies in the anterior fourth of the worm (Mudaliar & Ramanujachary, Reference Mudaliar and Ramanujachary1945). The eggs are asymmetrical and bear a terminal spine. We are otherwise largely ignorant of the biology of Bivitellobilharzia. We do not know the identity of the natural snail intermediate hosts and are poorly aware of the extent of their geographic distributions or patterns of host use. In molecular phylogenetic reconstructions, Bivitellobilharzia is basal to the prominent Schistosoma + Orientobilharzia Dutt and Srivastava, 1955 clade of schistosomes that occurs predominately in ruminants, primates and rodents (Brant et al., Reference Brant, Morgan, Mkoji, Snyder, Rajapakse and Loker2006, Reference Brant, Pomajbíková, Modry, Petrželková, Todd and Loker2012). Until recently, most of our current molecular knowledge of B. nairi has been from elephants in Sri Lanka (Agatsuma et al., Reference Agatsuma, Rajapakse, Kuruwita, Iwagami and Rajapakse2004; Brant et al., Reference Brant, Morgan, Mkoji, Snyder, Rajapakse and Loker2006) but very little is known about this species in other parts of its range. A recent study has provided the first molecular sequence data for B. loxodontae, confirming that the two species in the genus are distinct from one another and united within a monophyletic group, thus upholding Bivitellobilharzia as a distinct schistosome genus (Brant et al., Reference Brant, Pomajbíková, Modry, Petrželková, Todd and Loker2012).
Whereas elephants are well known for their role in hosting schistosomes, there has been but one prior report of schistosomes from any of the world's five extant species of rhinoceroses. Tiuria et al. (Reference Tiuria, Primawidyawan, Pangihutan, Warsito, Hariyadi, Handayani and Priosoeryarito2006) reported the eggs of Schistosoma spp. (199.4 × 111.8 μm) in a faecal sample from the Java rhino Rhinoceros sondaicus Desmarest, 1822 collected from Ujung Kulon National Park, Banten, Java, Indonesia. Further information to identify this schistosome species is lacking, and no images were included in the report. Other surveys of rhino parasites, whether from African or Asian rhinoceroses have not reported schistosome eggs (Zumpt, Reference Zumpt1964; Silberman & Fulton, Reference Silberman and Fulton1979; Palmieri et al., Reference Palmieri, Purmono and Ammann1980; Dutta et al., Reference Dutta, Bordoloi, Pathak and Choudhury1990; Chakraborty & Islam, Reference Chakraborty and Islam1993; Penzhorn et al., Reference Penzhorn, Krecek, Horak, Verster, Walker, Boomker, Knapp and Quandt1994; Chakraborty & Gogoi, Reference Chakraborty and Gogoi1995; Muryani et al., Reference Muryani, Tiuria, Andriansyah and Agil2008).
Given the vulnerable or endangered status of the world's rhinoceros and elephant species, this report represents an effort to characterize their schistosome parasite fauna using modern, non-invasive approaches before these animals are no longer available to study. Also, it is important to identify the parasite species harboured should they ever pose health problems for their hosts. Although there is some evidence that B. nairi infection can compromise survival of elephant calves (Anonymous, 1984), there has been little study of the impact schistosomes might have on the health of elephants or rhinoceroses, especially in the wild. This study also represents part of our ongoing effort to learn more about the poorly characterized schistosome genus Bivitellobilharzia, and the overall trematode fauna of Nepal.
Materials and methods
Collection and examination of faecal samples
Collections of fresh faecal samples from domestic or wild elephants, or from wild rhinoceroses, were made between 2007 and 2011, in and around CNP near Sauraha (27°30′0″N, 84°20′0″E) in south central Nepal. Fresh faecal samples were washed through a nested series of sieves (mesh sizes 450 μm, 150 μm, 75 μm and 32 μm) and eggs are retained on the 32 μm mesh screen. Sieved samples were either examined for unhatched eggs or were suspended in freshwater in 50 ml centrifuge tubes and exposed to sunlight to hatch the eggs. The upper layer of water in these tubes (1–2 ml) was collected and transferred to Petri dishes where miracidia were collected with the aid of a dissecting microscope. Fresh eggs were measured with the aid of a calibrated ocular micrometer at 400 × magnification using a compound microscope. Eggs or miracidia were preserved in RNAlater (Ambion The RNA Company, Life Technologies, Grand Island, New York, USA) or 96% ethanol. Each sample collected consisted of a small number (10–50) of individual miracidia or eggs, all recovered from the same faecal sample. All preserved samples were hand-carried with the permission (Ref. number 44, 25 July 2011) of the Nepal Health Research Council to the Department of Biology at the University of New Mexico, Albuquerque, New Mexico, USA for molecular analysis.
Molecular and phylogenetic analyses
DNA was extracted using the Qiagen DNeasy Blood and Tissue Kit (Valencia, California, USA). Schistosome eggs were digested overnight, whereas miracidia were digested for 2–3 h. The nuclear ribosomal gene 28S and the mitochondrial gene cytochrome oxidase I (cox1) were amplified by polymerase chain reaction. Methods and primers were as described in Brant et al. (Reference Brant, Pomajbíková, Modry, Petrželková, Todd and Loker2012). There are only two known species of Bivitellobilharzia and to confirm that our samples belonged to B. nairi and that they were different from B. loxodontae, we compared cox1 pairwise sequence differences. This allowed us to also relate our results with species differences among closely related species of Schistosoma.
The 28S and cox1 gene fragments were used in phylogenetic analyses using maximum parsimony (MP), maximum likelihood (ML) and minimum evolution (ME), carried out in PAUP* ver. 4.0b10 (Swofford, Reference Swofford2002). Bayesian inferences (BI) were made with the use of MrBayes (Huelsenbeck & Ronquist, Reference Huelsenbeck and Ronquist2001; Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003). jModel test was used to determine the best fit nucleotide substitution model for ML and ME analyses (Posada, Reference Posada2008). Optimal MP, ME and ML trees were reconstructed using heuristic searches: for the 28S dataset we ran 100 replicates for MP and ME and 10 replicates for ML; and for the cox1 dataset we ran 500 replicates for MP and ME and 100 replicates for ML. Nodal support was estimated by bootstrap: for the 28S dataset we ran 200 replicates with 10 addition sequence replicates for MP and ME and for the cox1 dataset we ran 500 replicates with 10 addition sequence replicates for MP and ME, and 200 replicates with 5 addition sequence replicates for ML. For the BI analysis of the 28S dataset, the parameters were unlinked: Nst = 6 rates = invgamma ngammacat = 4. For the BI analysis of the cox1 dataset, the parameters were unlinked; data were partitioned by gene, for codons one and two Nst = 2 and for codon three Nst = 6 rates = gamma, ngammacat = 4. For both 28S and cox1 datasets, four chains were run simultaneously for 5,000,000 generations, trees were sampled every 100 cycles, the first 5000 trees with pre-asymptotic likelihood scores were discarded as burn-in, and the retained trees were used to generate a 50% majority-rule consensus trees and posterior probabilities.
Results
From 2007 to 2011 we collected 22 fresh domestic elephant faecal samples and two fresh wild elephant faecal samples. The latter samples were determined to be from a wild elephant because they were recovered deep within CNP and had a plant composition typical of that consumed by wild elephants. Additionally, fresh faecal material was taken from each of 14 different rhinoceros faecal middens, all located in and around CNP. Rhinos use these middens to mark their territories. The locality information from our collections is summarized in table 1. Among them, two elephant samples (one domestic and one wild), and seven rhinoceros samples were found positive for schistosome eggs/miracidia.
Table 1 Host, status with respect to schistosome infection, date of collection and location (including co-ordinates) of the positive faecal samples from the Chitwan National Park (CNP) and surrounding sites; one sample was collected from each site unless otherwise stated.
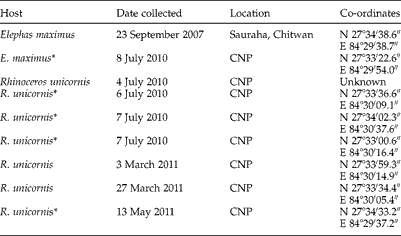
* Samples sequenced successfully.
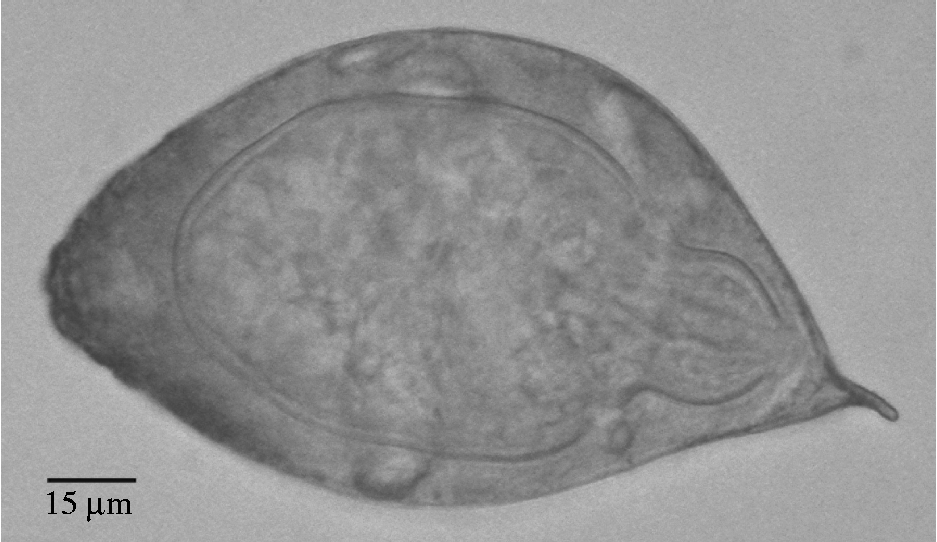
All eggs resembled those of B. nairi. Those from R. unicornis samples measured 132–156 μm (average 144 μm) in length (excluding spine) by 81.6–86.4 μm (average 83.4 μm) wide with a spine length of 9.6–12 μm (average 11.4 μm) (fig. 1). Dimensions of the schistosome eggs from elephants were not obtained, but these eggs were similar in size and shape to eggs obtained from rhinos. In table 2, the dimensions of schistosome eggs obtained from our rhino samples are compared to egg measurements from the literature for schistosomes from elephant and rhinoceros faecal samples.

Fig. 1 Schistosome egg obtained from faecal samples of R. unicornis from Chitwan National Park.
Table 2 Schistosome eggs obtained from rhino samples compared to egg measurements from the literature for schistosomes from elephant and rhinoceros faecal samples from other localities (all measurements are in μm, average values in parentheses).
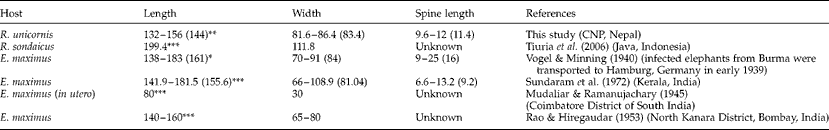
Terminal spine: *included in measurement; **not included in measurement; ***not known if terminal spine was included in measurement.
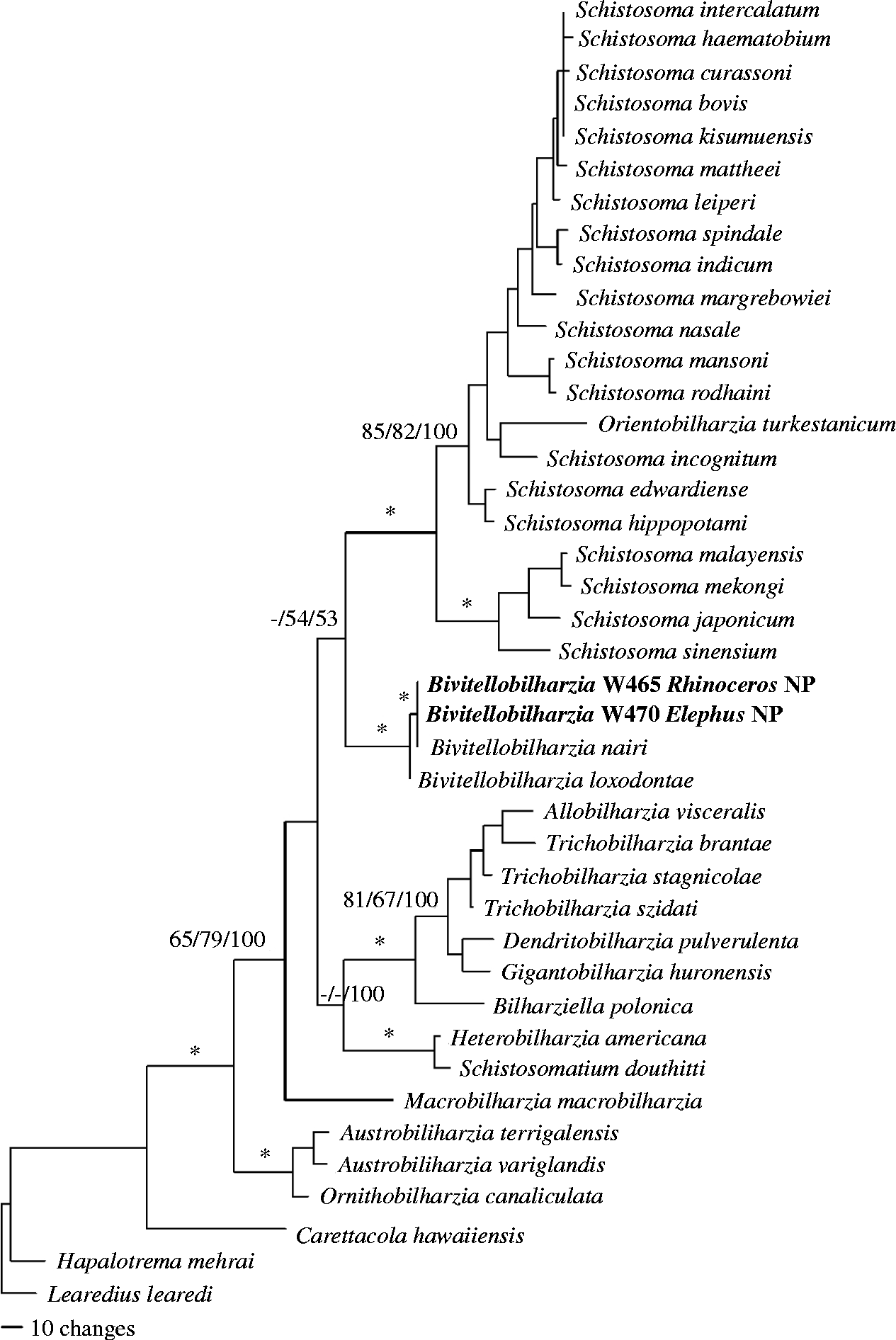
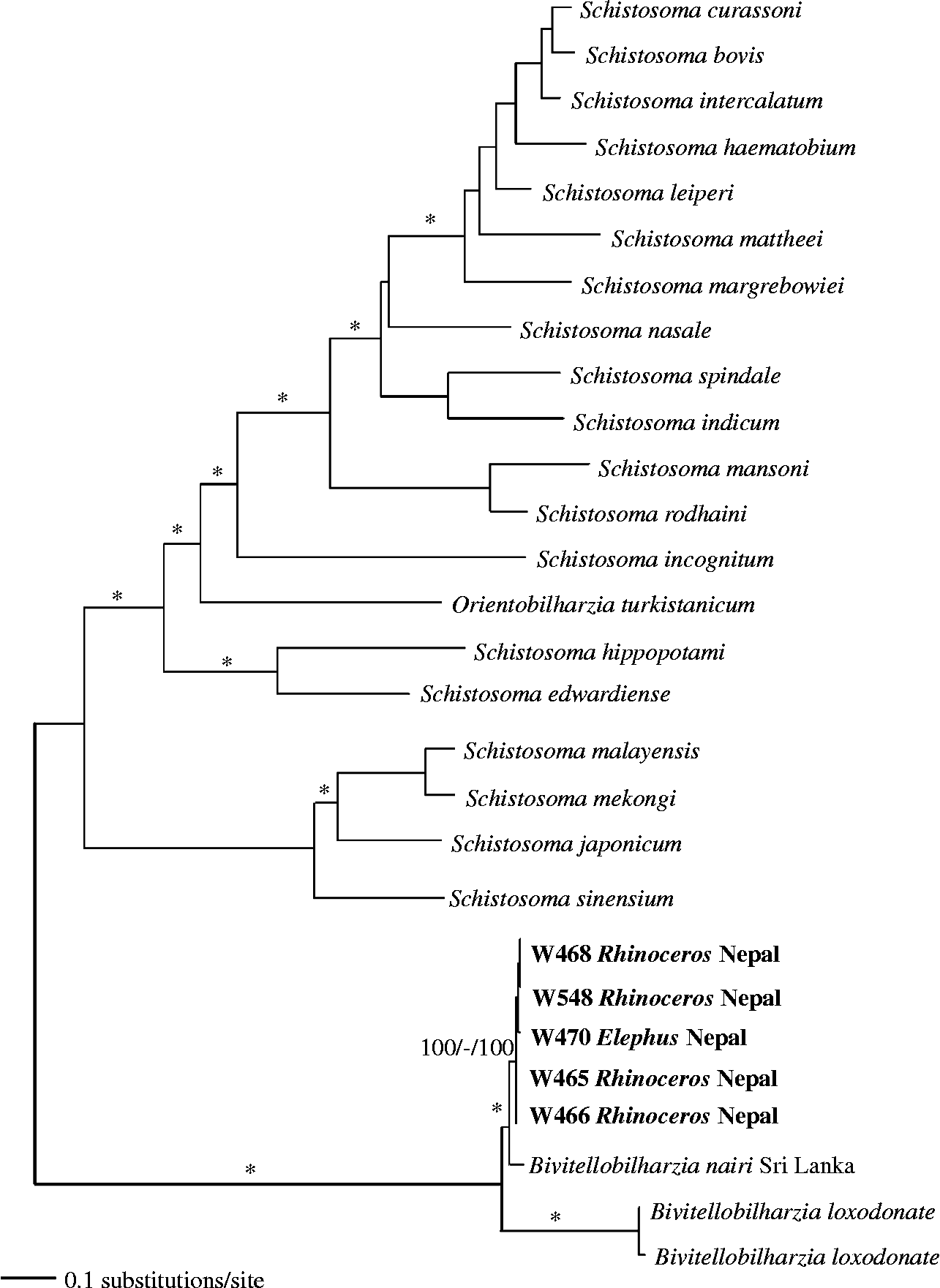
DNA sequence data were deposited in GenBank, under accession numbers JQ975005 and JQ975006 for the 28S (1310 bp) data set and JQ975007–JQ975011 for the cox1 (1071 bp) data sets. The results of the analyses used to reconstruct the phylogenetic relationships of our samples to known samples were congruent other than variation in nodal support (figs 2 and 3). Bivitellobilharzia grouped as a sister clade to Schistosoma, and our Nepalese rhinoceros and elephant samples grouped with the known sample of B. nairi from Sri Lanka, to the exclusion of B. loxodontae from the Central African Republic. The cox1 tree (fig. 3) included four samples from R. unicornis and one sample from wild E. maximus; these samples were not significantly different from each other, indicating that it is unlikely there is a separate schistosome species in each of the two host species. All samples grouped with, but were genetically different from (cox1 2.7–3.2%), the sample of B. nairi from Sri Lanka.

Fig. 2 Bayesian inference tree based on 28S sequences. Samples in bold are those collected for this study. Node support is indicated by MP, ME bootstrap values and Bayesian posterior probabilities (PP), respectively. The asterisks indicate MP and ME bootstrap values of >90 and PP of >97. The dashes indicate no significant node support.

Fig. 3 Maximum likelihood tree based on partial cox1 sequences. Samples in bold are those collected for this study. Node support is indicated by MP and ME bootstrap values and Bayesian PP, respectively. The asterisks indicate bootstrap values of >90 and a PP of >97. The dash indicates no significant node support.
Based on our cox1 data, B. nairi and B. loxodontae were 12–12.5% different, a value similar to those of closely related species pairs: for example, Schistosoma indicum–S. spindale 14.4%, S. mansoni–S. rodhaini 11.7% (this is based on the sequence data we used to generate the phylogenetic tree). The average pairwise difference among the B. nairi we recovered and species of Schistosoma was 21–25%. The similarity in cox1 mtDNA sequences between schistosomes from the two large mammal species (0.0–0.5% divergence) suggests they share the same schistosome in CNP.
Discussion
The recovery of B. nairi from both domestic elephants that live adjacent to CNP and from wild elephants that live within the park represents the most northerly records for this species, which is otherwise known from India, Sri Lanka and the Republic Union of Myanmar (Vogel & Minning, Reference Vogel and Minning1940; Mudaliar & Ramanujachary, Reference Mudaliar and Ramanujachary1945; Rao & Hiregauder, Reference Rao and Hiregauder1953; Dutt & Srivastava, Reference Dutt and Srivastava1961; Sundaram et al., Reference Sundaram, Padmanabha Iyer, Peter and Alwar1972; Agatsuma et al., Reference Agatsuma, Rajapakse, Kuruwita, Iwagami and Rajapakse2004). Domestic elephants have a relatively low prevalence of B. nairi, perhaps because they spend relatively little time in natural snail habitats compared to wild elephants or rhinoceroses.
We were surprised to find readily hatching schistosome eggs derived from Asian rhino dung samples collected from characteristic rhino dung middens in CNP. This is the first report of a schistosome infection in R. unicornis. Molecular signatures obtained from rhino-derived eggs or miracidia indicated they were very similar with respect to both cox1 and 28S sequences to B. nairi eggs or miracidia obtained from CNP elephant dung samples. The degree of cox1 sequence divergence (genetic distance) between specimens of B. nairi recovered from elephants and rhinos in CNP (0.0–0.5%) is low in comparison to the 1.4% degree of variability observed within a locality for S. mansoni, another mammalian schistosome parasite (Stothard et al., Reference Stothard, Webster, Weber, Nyakaana, Webster, Kazibwe, Kabatereine and Rollinson2009). Nothing about the sequence data we collected suggests that rhinos and elephants are supporting separate species or variants of B. nairi in CNP, although further study would be helpful to confirm this point.
In contrast, the divergence for cox1 between B. nairi collected from Sri Lanka and Nepal (2.7–3.2%) is appreciable. Although this probably merely reflects geographic variation in B. nairi between the two localities, which are separated by over 2300 km, the possibility that more discrete differences occur between worms from the two localities should not be ignored, especially given that the distribution of elephants in southern Asia is not continuous, and the Sri Lankan specimens are from an island population.
A second reason to keep an open mind regarding the possibility of additional diversity in Asian Bivitellobilharzia stems from the one prior report of schistosome eggs from Rhinoceros sondaicus from Java (Tiuria et al., Reference Tiuria, Primawidyawan, Pangihutan, Warsito, Hariyadi, Handayani and Priosoeryarito2006). This is because the egg sizes reported are larger (table 2) than those reported here for B. nairi from R. unicornis. Although no additional data are available (and may never be, given the rarity of Javan rhinos in the wild), given the disparity in egg sizes, further study is needed to determine if this schistosome is likely to be B. nairi as well. To our knowledge, elephants are not present in the part of Java in which the infected Javan rhino, or rhinos, were found.
In general, based on what we know now, our results are in agreement with a recent study of B. loxodontae from African forest elephants (Brant et al., Reference Brant, Pomajbíková, Modry, Petrželková, Todd and Loker2012): the Nepalese elephant/rhino schistosome supports the concept of two separate species within Bivitellobilharzia, and that this genus represents a distinct lineage within the Schistosomatidae. Whereas Bivitellobilharzia has been traditionally considered a genus of ‘elephant schistosomes’, our report of B. nairi from Indian rhinos, and the earlier report of a putative Bivitellobilharzia species from Javan rhinos (Tiuria et al., Reference Tiuria, Primawidyawan, Pangihutan, Warsito, Hariyadi, Handayani and Priosoeryarito2006), indicate Bivitellobilharzia can occur in animals other than elephants.
It is intriguing that Indian elephants and Asian rhinos both host the same schistosome in CNP, yet are not close relatives. A recent phylogenetic study of mammals suggests rhinos (Rhinoceratidae) and elephants (Elephantidae) last shared a common ancestor at about 100 million years ago (Meredith et al., Reference Meredith, Janečka, Gatesy, Ryder, Fisher, Teeling, Goodbla, Eizirik, Simão, Stadler, Rabosky, Honeycutt, Flynn, Ingram, Steiner, Williams, Robinson, Burk-Herrick, Westerman, Ayoub, Springer and Murphy2011). Both species spend a considerable amount of time in water, a factor expected to predispose them to schistosome infection. However, both species also have very thick skin, which raises the question as to how elephants and rhinos become infected in the first place.
The answer to this question would become clearer if we knew the identity of the natural intermediate host for B. nairi. Vogel & Minning (Reference Vogel and Minning1940) recovered B. nairi cercariae from experimentally exposed lab-reared snails of Planorbidae: Biomphalaria pfeifferi, originally from Africa, and Planorbis sp. from Germany. Despite considerable searching among thousands of freshwater snails recovered both within and outside CNP, we have failed to find any snails shedding B. nairi cercariae. Experimental infections of some of the more prominent freshwater snail species from the area with miracidia derived from either rhinos or elephants have also failed to result in infections. Keeping experimentally exposed snails alive in the heat of the Terai region is a persistent challenge. Although it would be unprecedented for schistosomes and for most digenetic trematodes except sanguinicolids, perhaps the intermediate host for B. nairi is not a snail, or perhaps not an aquatic snail. It is also conceivable that infected intermediate hosts are actually ingested along with vegetation eaten by these large herbivores. That this is even a possibility is supported by the finding of mollusc shell contents in R. unicornis faecal samples from CNP (Laurie, Reference Laurie1978). In such a case, cercariae might never be shed from the intermediate host as is typical for other schistosomes. Dissections of the majority of snails collected have not, however, yielded cryptic infections. Our preferred hypothesis, suggested by Vogel & Minning's (Reference Vogel and Minning1940) work, is that the life cycle is a conventional one, but that snail infections simply happen to be extremely rare. The longevity of rhinos and elephants would favour persistence of B. nairi in nature in two ways: these large animals could slowly accrue worms and acquire bisexual infections, and once infected, would be available to produce eggs to infect intermediate hosts – even at a potentially low level – over a span of decades.
The report of B. nairi from one, and possibly two, rhino species from Asia and from the Indian elephant raises the issue as to whether this is originally a rhino parasite that has colonized elephants, or vice versa. The presence of the only known congener, B. loxodontae in African forest elephants, suggests that Bivitellobilharzia worms are first and foremost elephant parasites. To our knowledge there are no reports of schistosomes in African rhinos, which in general are less aquatic than their Asian counterparts. Given the long and complex history of both elephants and rhinos, and that the extant species we see today are but a small proportion of the many species that once existed, it may be very difficult to fully answer this question. Also, it is difficult to resolve whether Bivitellobilharzia originated in Africa or Asia.
The presence of a shared schistosome between Indian elephants and Asian rhinos also raises a question as to whether each species is able to maintain B. nairi in nature, or whether transmission really depends primarily on the eggs derived from one species or the other. Certainly the presence of B. nairi in elephants from locations, like Sri Lanka, where rhinos are absent suggests that elephants are able to propagate the infection themselves. Whether rhinos can do the same in the few localities where they currently exist without elephants remains to be determined. Once again, their longevity may be a factor that favours their ability to maintain B. nairi on their own. It is also conceivable that additional mammalian species transmit B. nairi in CNP. We examined 10 faecal samples from chital deer (Axis axis) and they were negative (unpublished observations). Gaur (Bos guaros) are also present, but rare, and we have not had the opportunity to examine faecal specimens from this species. Domestic buffaloes (Bubalus bubalis) and cattle (Bos primigenius indicus) are generally absent inside the park but we have had a chance to examine 100 faecal samples of domestic buffalo in areas adjacent to CNP. All were negative for B. nairi infection.
Finally, the presence of a shared potential pathogen between elephants and rhinos is noteworthy from a management point of view. Schistosomes are capable of causing considerable pathology, so it is important to keep in mind that both host species in Nepal could be affected by B. nairi. Translocations or population increases of one host species may result in unexpected transfer of B. nairi to the other species. Perhaps the two host species share other parasites as well, possibly even including the pathogenic liver fluke Fasciola jacksoni or amphistome flukes, both known from elephants. We must also remain alert to the possibility that B. nairi may also infect other valuable hoof stock in CNP.
Acknowledgements
The University of New Mexico supported this study, through a National Institutes of Health grant to E.S.L. (RO1 A144913) and a National Science Foundation grant to S.V.B. (DEB 1021427). Technical assistance at UNM Molecular Biology Facility was supported by NIH grant 1P20RR18754 from the Institute Development Award program of the National Center for Research Resources. We are grateful to the officials of the Department of National Parks and Wildlife Conservation, Chitwan National Park and the Nepal Health Research Council (Permit No. 44) for their cooperation with this research. We are also indebted to Miss Susma Dhakal, Mr Sulav Dhakal, Mr Mohan Pandey, Mr Bikas Sapkota and Mr Ramesh Ghimire for providing field support to collect the samples.