Introduction
Tapeworms within the order Cyclophillidea display a large variation in the way they live their life (Mackiewicz, Reference Mackiewicz1988; Reid, Reference Reid and Calnek1991). Most species have one intermediate host, but some, such as Mesocestoides spp., can have two, while others, such as Hymenolepis nana and to some extent H. microstoma, can complete development and transmission without any intermediate host at all (Andreassen et al., Reference Andreassen, Ito, Ito, Nakao and Nakaya2004; Padgett & Boyce, Reference Padgett and Boyce2005). While many other groups of flatworms, such as digeneans or pseudophyllid tapeworms, have adapted to a taxonomically restricted group of intermediate hosts, larval forms of cyclophyllids may occur in both insects and vertebrates (Smirnova & Kontrimavichus, Reference Smirnova and Kontrimavichus1977; Conn, Reference Conn1985; Scholz et al., Reference Scholz, Bray, Kuchta and Řepová2004; Haukisalmi et al., Reference Haukisalmi, Konyaev, Lavikainen, Isomursu and Nakao2016).
The cestode Hymenolepis microps [syn: Weinlandia microps (Fuhrmann 1906) and H. tetraonis (Wolffhugel 1900)] is a common intestinal parasite of willow ptarmigan (Lagopus lagopus) and other tetraonid birds (Huus, Reference Huus1928; Wissler & Halvorsen, Reference Wissler and Halvorsen1977). Although this species has been suggested to have a significant effect on ptarmigan population dynamics (Holmstad et al., Reference Holmstad, Holstad, Karbøl, Revhaug, Schei, Vandvik and Skorping2004, Reference Holmstad, Hudson, Vandvik and Skorping2005a, Reference Holmstad, Hudson and Skorpingb), details on transmission mode and life cycle are absent. Since the majority of species in the genus Hymenolepis can use insects as intermediate hosts, it has been suggested that ptarmigans become infected by eating arthropods containing larval cestodes (Steen, Reference Steen1994). There is no evidence supporting monoxeny for this species. However, H. microps is maintained in populations of ptarmigan throughout the winter in high intensities without the usual seasonal decline that characterizes many other helminths (Schei et al., Reference Schei, Holmstad and Skorping2005). Thus, this cestode might have an unusually long life-span, and/or it may be transmitted year-round. In addition, H. microps is capable of keeping a relatively high prevalence even in low-density host populations (Holmstad et al., Reference Holmstad, Hudson and Skorping2005b), suggesting again the existence of an efficient transmission route. This suggests a continuous intake of the intermediate host(s) in the regular diet throughout the year. Such inclusion of invertebrates in the diet seems like a paradox, because willow ptarmigans are mainly herbivorous, consuming willow (Salix sp. – 80% of the diet) but also other bushy plants, such as Vaccinum spp., Alnus spp. and Betula spp. (Thomas, Reference Thomas1994). Insects are frequently included in the diet during the first 3 weeks after hatching (Spidsø, Reference Spidsø1980; Erikstad & Spidsø, Reference Erikstad and Spidsø1982). Hence, even if willow ptarmigans may ingest insects inadvertently while feeding on shrubs and bushes, accidental transmission is insufficient to explain the high prevalence of H. microps, especially in a subarctic environment, where insects are available only during a rather short time-window. The only invertebrates regularly ingested throughout the year are chewing lice belonging to the family Philopteridae (Phthiraptera: Ischnocera) also referred to as Mallophaga. Willow ptarmigans are parasitized by several species of lice and other ectoparasites (e.g. Diptera: Hyppoboscidae); among them, Goniodes lagopi and Lagopoecus affinis are reported with up to 90% prevalence in some host populations (Holmstad et al., Reference Holmstad, Jensen and Skorping2008). These two species show both horizontal and vertical transmission (Hillgarth, Reference Hillgarth1996; Darolova et al., Reference Darolova, Hoi, Kristofik and Hoi2001).
Chewing lice are mainly found on the head and neck region, on the breast and under the wings of the bird (Stock & Hunt, Reference Stock and Hunt1989). These lice feed on the feathers, and are not in contact with the bird skin. Lice and other ectoparasites are most efficiently controlled by grooming, plucking and preening of feathers (Clayton, Reference Clayton, Loye and Zuk1991).
Willow ptarmigans produce two kinds of droppings: one moist and sticky, which is shed before feeding at dusk and dawn, and another kind, more fibrous and dry. Additionally, ptarmigan use residual heat from the second kind of droppings by roosting on top of them (Cramp & Simmons, Reference Cramp and Simmons1980; de Juana, Reference de Juana, del Hoyo, Elliott and Sargatol1994). Here, we suggest that chewing lice could be involved in the life cycle of H. microps, possibly as a consequence of this specific excrement heat-usage behaviour, which would allow cestode eggs shed in the faeces to contaminate the feathers of the ptarmigan. Chewing lice parasitizing the birds could thus ingest eggs while feeding on the feathers. Ischnocera lice are frequently ingested when ptarmigans groom feathers, as demonstrated by the presence of exoskeletons in the gut contents (P. Holmstad, pers. comm.). These considerations support the hypothesis that H. microps could use chewing lice as an intermediate host to reach and infect the final host efficiently. Hence, in order to test our hypothesis, we applied histological techniques and light microscopy to investigate the whole bodies of G. lagopi and L. affinis collected on willow ptarmigan for the presence of cysticercoids. In addition, we designed a polymerase chain reaction (PCR) assay targeting two different regions of the 18S rRNA of H. microps, to perform a molecular screening on DNA extracted from the two Ischnocera species collected on birds.
Materials and methods
A total of 61 willow ptarmigan were sampled from a single population in Kattfjord, Troms county, northern Norway during 2 weeks in September 2006. All birds were packed individually, in sealed plastic bags, and frozen at −20°C within 12 h after collection. Successively, birds were thawed at room temperature and examined for the presence of ectoparasites by brushing through the feathers over a sheet of white paper. The plastic bags were also checked for remaining ectoparasites. A total of 321 lice (see supplementary table S1 for details) were collected on 29 birds (47.5%) and all the lice collected from the same bird were stored in two single plastic vials for different usage. A large proportion of the collected lice were stored in a fixation solution for light microscopy analyses, while the remaining lice (n = 100) were stored in 96% ethanol for DNA extraction. Three birds (K64.01, K30.01 and K39.01) had a low number of ectoparasites (n < 6) and these lice were only used for DNA extraction and molecular testing. The guts of ptarmigans were examined for H. microps infection following published procedures (Holmstad & Skorping, Reference Holmstad and Skorping1998). All scoleces and attached proglottidis of H. microps collected on the same bird were stored in 96% ethanol in individual tubes (n = 30).
Collection and examination of samples
The lice were fixed by immersion in 1 ml solution (stock solution: 100 ml 10% formaldehyde, 10 ml 25% glutaraldehyde, 20 ml 0.2 m cacodylate buffer and 60 ml phosphate-buffered saline (PBS), pH = 7). Specimens (n = 4–9) were then dehydrated in ethanol and embedded in Technovit® 7100 (Heraeus Kulzer GmbH & Co., Hanau, Germany). Semi-thin sections (1 μm) were stained with toluidine blue and basic fuchsin–methylene blue before examination by light microscopy.
Molecular and phylogenetic analyses
The cestode H. microps (proglottids) and the two species of Mallophaga (G. lagopi and L. affinis), stored in 96% ethanol, were rinsed in distilled water and homogenized with a sterile pestle and later processed for DNA extraction using a commercial kit (DNeasy© Blood & Tissue kit, Qiagen, Hilden, Germany) following manufacturer's instructions.
In order to amplify the 18S rRNA of H. microps and the 18S rRNA of G. lagopi and L. affinis we used universal primers (18e-f: CAC CAG GTT GAT TCT GCC/1492r GGT TAC CTT GTT ACG ACT T) published previously by Weiss et al. (Reference Weiss, Zhu, Cali, Tanowitz and Wittner1994). PCR products were gel excised using Millipore Ultrafree®-DA and cloned using TOPO TA Cloning® kit and One Shot TOP10® chemically competent Escherichia coli (Thermo Fisher, Pittsburgh, Pennsylvania, USA) following the manufacturer's instructions. Positive clones were grown in Luria–Bertani (LB) medium (ampicillin 100 μg/ml) for 16 h in 4-ml volumes at 37°C. Plasmid DNA was extracted by means of QIAprep Spin Miniprep Kit (Qiagen). Finally, clones were sequenced from both strands using M13 primers. Sequences were checked for homology using BLASTn on the National Center for Biotechnology Information database. The 18S rRNA sequences of H. microps was deposited in GenBank under accession number KY403995. The sequences of H. microps, G. lagopi and L. affinis (partial) were aligned, and two hypervariable regions were defined by sequence comparison. Two sets of specific primers were designed for H. microps: HM1 F (CTC TGC GGC GTG CAT TAC AT), HM2 R (CGA GCC GAC TAG ACT CCA CA) and HM3 F (TAT GCG TAT CGT CAT GAT CA), HM4 R (AAG GTC TGA CTC GTT GAC AC). Each primer pair amplified a fragment of the ribosomal gene of length 226 and 249 bp, respectively. These primers were used to perform molecular screening on DNA extracted from Ischnocera lice collected on parasitized and non-parasitized birds. All precautions were taken to avoid contamination of lice with faeces or cestode eggs.
The 18S rRNA sequence of H. microps was aligned with available cyclophyllidean sequences downloaded from GenBank. Gblocks v 0.91b (Castresana, Reference Castresana2000) was used on the ribosomal alignment to eliminate the unalignable or ambiguous regions. Gblocks settings included less strict flanking positions, gap positions within blocks and small final blocks, which resulted in a fragment of 1780 bp (80% of the original 2233 bp). The best evolutionary model, selected using jModelTest 2.1.7 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012), was GTR + G+Γ according to the Bayesian information criterion (BIC).
Analyses were run in MrBayes v3.2 (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003) using two runs of four chains, each running over 20,000,000 generations, sampling every 10,000 generations, and using 50% burn-in for the complete dataset. The convergence of the two runs was confirmed (standard deviation of split frequencies < 0.01).
Results
The 61 examined L. lagopus showed high variability in the number of ectoparasites and also in the number of endoparasites in the gut (supplementary table S1). Two species of cestodes were found in the intestine, H. microps and Paroniella urogalli, with a prevalence of 49% and 10%, respectively. Hymenolepis microps was found in 30 birds with variable degrees of infestation (n scolices = 1–496). A total of 321 Ischnocera lice (154 G. lagopi and 167 L. affinis) were collected from 29 birds, with a mean intensity of 11.06 lice per bird.
From a total of 221 examined lice, 12 cysticercoid-like structures were found in 2 G. lagopi and 10 L. affinis sampled from ptarmigan which were also infected with H. microps. Each of two specimens of L. affinis harboured two cysticercoid-like structures. These structures were clearly not associated with the normal insect anatomy (fig. 1). Besides, the cysticercoids were only reported in Ischnocera lice collected from birds parasitized by H. microps.

Fig. 1. Cysticercoids (arrowed) occurring in the abdomen of three different specimens of chewing lice; scale bar = 50 μm.
Two samples out of a total of four, consisting of DNA extracted from a pool of 25 lice, were positive in the PCR screening. From each positive sample two different fragments of 18S rRNA were amplified; the gene fragments showed 100% identity with the 18S rRNA of H. microps sequenced in this study.
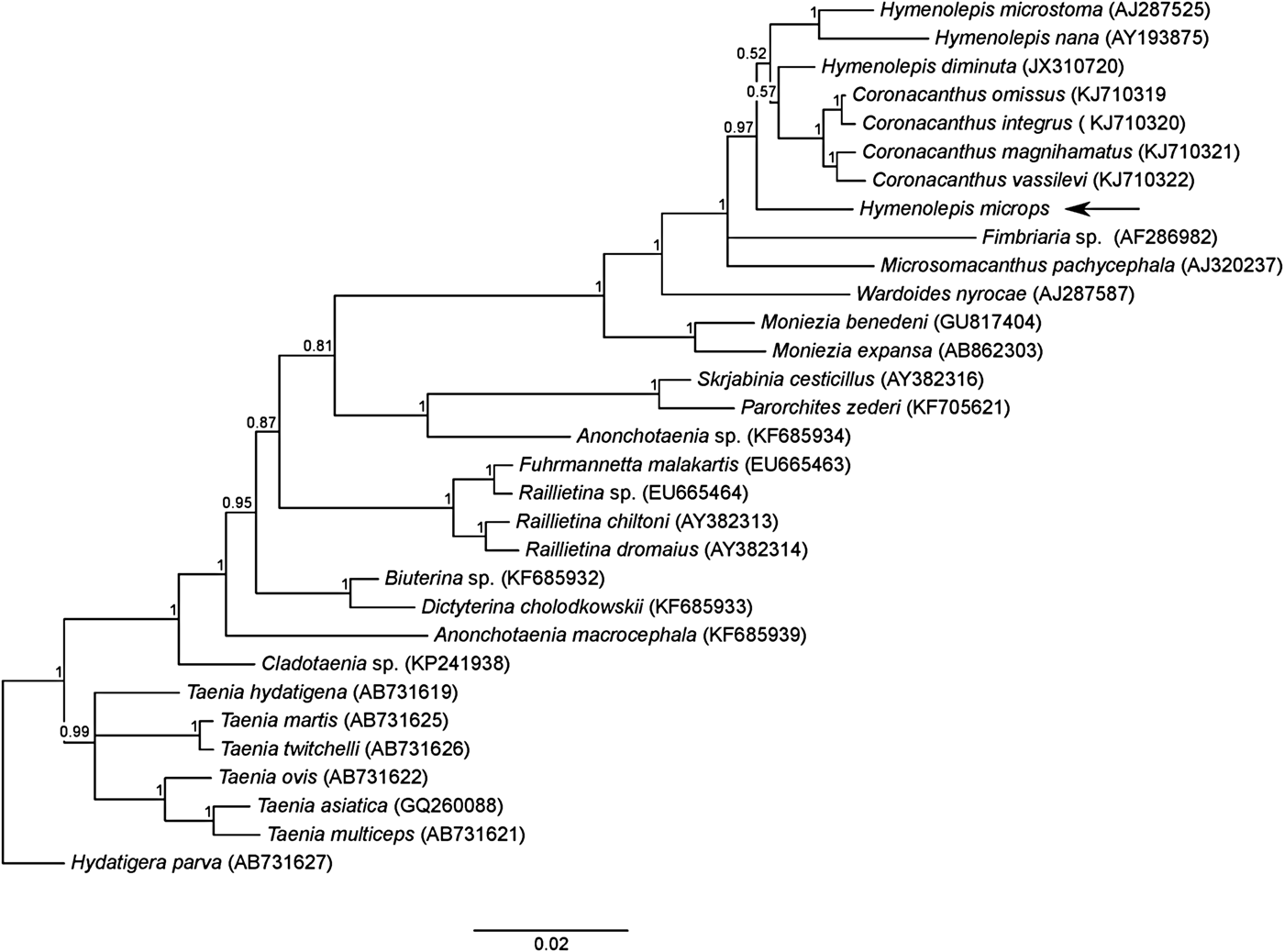
Bayesian analysis showed a well-resolved tree topology, where H. microps grouped together with the other Hymenolepis species (fig. 2). However, the genus Hymenolepis was paraphyletic (pp = 0.97), with the genus Coronacanthus nested inside. A group including the genera Hymenolepis, Coronacanthus, Fimbriaria and Microsomacanthus had maximum node support (pp = 1). The phylogenetic tree was rooted on Hydatigera parva; the genus was recently resurrected to accommodate several species more distantly related to Taenia spp. (Nakao et al., Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013).
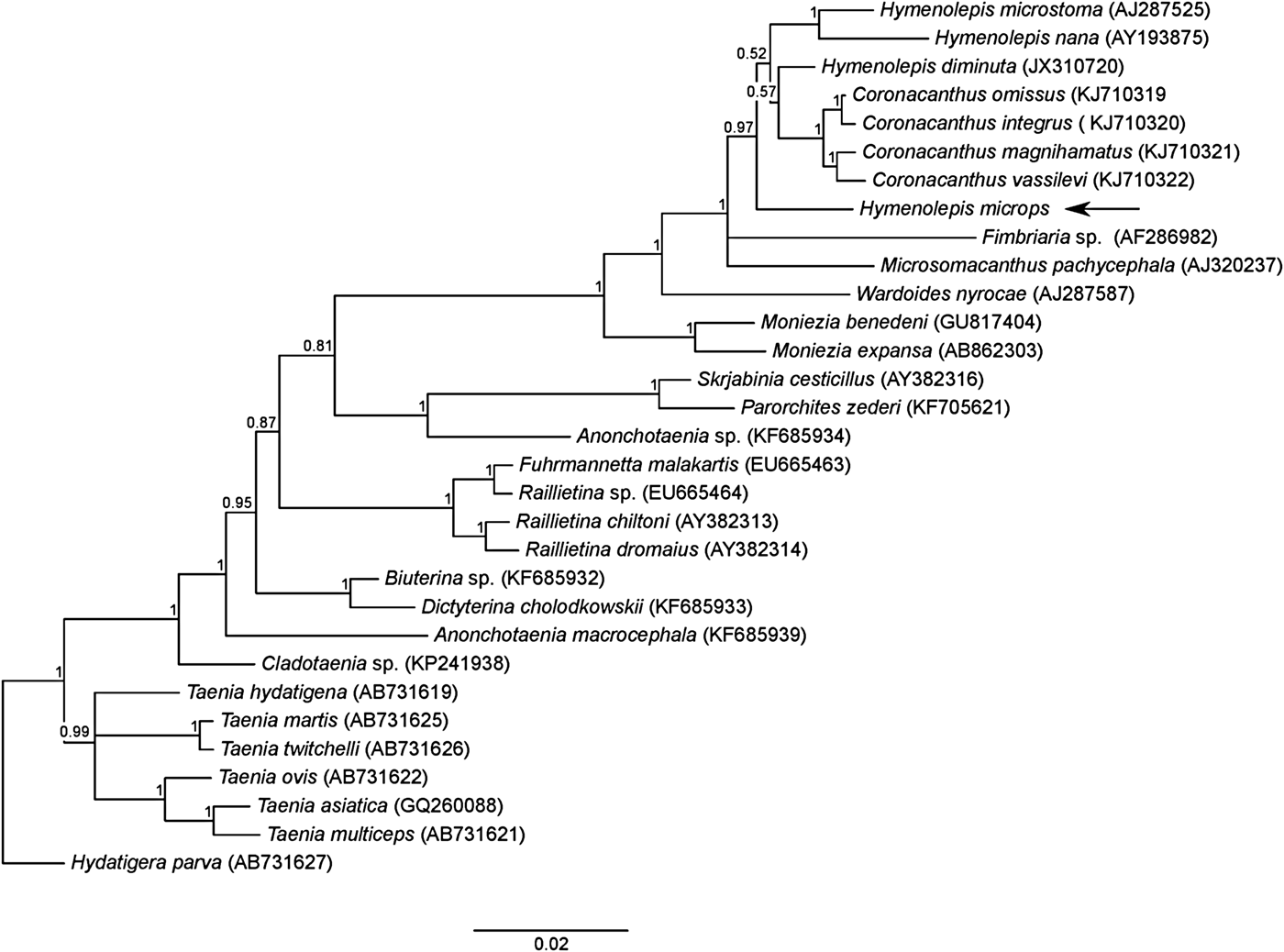
Fig. 2. Phylogenetic tree showing relationships among cestodes belonging to the order Cyclophyllidea, inferred by Bayesian analysis based on an alignment of 31 sequences and 1780 positions of the 18S rRNA gene. Posterior probabilities are reported above the nodes. The arrow indicates the position of H. microps.
Discussion
The only definitive method of examining the life cycle of a parasite is by experimental infections. However, performing transmission experiments on wild animals is a daunting task and involves both practical and legal problems. Microscopy and molecular techniques will therefore, in many cases, represent the only available tools to test hypotheses on parasite life cycles.
The cysticercoid-like structures encountered in the abdomen of G. lagopi and L. affinis did not resemble any parts of the normal anatomy of the insect. They were found only in lice collected on birds parasitized by H. microps and the morphology showed characteristics highly comparable with other cycticercoids described previously in beetles (Voge & Heyneman, Reference Voge and Heyneman1957; Valkounova, Reference Valkounova1983). Indeed, the structure found in the abdomen measured 150–200 μm in diameter, in accordance with the range (130–600 μm) measured for cysticercoids of H. microstoma described previously in beetles (Voge, Reference Voge1964).
Besides, the molecular detection of H. microps DNA in G. lagopi and L. affinis constitutes a further proof in the correct identification of these structures. Therefore, two independent lines of evidence seem to confirm the presence of cysticercoids of the cestode H. microps in the Ischnocera lice.
Many arthropod species from different taxa have been described as intermediate hosts for cyclophyllidean cestodes, including crustaceans and mites, but also insects in the orders Collembola, Orthoptera, Lepidoptera and Coleoptera (Wahl, Reference Wahl1967; Smirnova & Kontrimavichus, Reference Smirnova and Kontrimavichus1977; Heicher & Gallati, Reference Heicher and Gallati1978; Valkounova, Reference Valkounova1983; Shostak, Reference Shostak2014). Lice and fleas in the order Phthiraptera are also vectors of various mammalian and avian endoparasites (Marshall, Reference Marshall1967; Marshall, Reference Marshall1981; Bartlett, Reference Bartlett1993).
Further support for our hypothesis of Ischnocera as intermediate hosts comes from behavioural and ecological considerations. First, both young and adult ptarmigan ingest lice. Chewing lice are kept under control by the host through preening, using the bill-tip and the foot (Clayton, Reference Clayton, Loye and Zuk1991; Møller & Rózsa, Reference Møller and Rózsa2005). Second, ischnocerans as intermediate hosts would explain the high prevalence of H. microps at all seasons of the year. Lice are present in cestode-infested birds during both summer and winter. Schei et al. (Reference Schei, Holmstad and Skorping2005) observed that infection levels of H. microps tended to be high and stable during the winter season. The usual seasonal decline observed in other helminth species was not registered when comparing samples collected in autumn and winter. Most free-living insects are absent during the winter in northern Norway and those that overwinter tend to be inactive and concealed beneath the snow. A transmission system based on accidental ingestion of free-living insects would certainly not explain the high levels of H. microps infestations recovered in adult L. lagopus.
A complicating factor that seems to contradict the idea of high transmission throughout the year is that H. microps tends to show a marked morphological change in the transition from autumn to winter, known as destrobilation. Basically, the cestode consists of large and numerous gravid proglottids in summer and autumn, but it is reduced to the scolex and a few non-gravid proglottids in the winter (Huus, Reference Huus1928; Schei et al., Reference Schei, Holmstad and Skorping2005). In samples of willow ptarmigan collected during winter, the destrobilated form of H. microps is found most frequently, but some gravid proglottids have also been reported (Schei et al., Reference Schei, Holmstad and Skorping2005; Holmstad, pers. comm.). The destrobilated form has been found in other species of cestodes as well (Dick & Burt, Reference Dick and Burt1970; Delahay, Reference Delahay1999). Destrobilation seems to be linked to periods of low transmission, when the cestode decreases investment in egg production.
Although this observation would suggest that transmission during winter is quite low, it is still not incompatible with the hypothesis of ischnocerans as the main transmission route. Chewing lice will only be able to infect other individuals during periods of close contact. This takes place during the mating period in spring, and in late summer/early autumn when parents (both males and females) shelter the chicks from cold weather. During other times of the year these birds do not have any close contact, which means that if the only means of transmission is via chewing lice, the birds would mostly just infect themselves.
The possibility that H. microps might also have a direct (re-)infestation cycle combined with the inclusion of facultative intermediate hosts cannot be ruled out. We found only one or two cysticercoids within a single louse, casting doubt on the possibility that the number of cysticercoids might reach similar intensities (n = 4–129) as reported from other insect species (Case & Ackert, Reference Case and Ackert1940).
Further studies will be necessary to elucidate other aspects of the life cycle of H. microps. Although difficult to realize, controlled infection experiments could be performed to confirm that cestodes can be transmitted from infested to non-infested birds after ingestion of Ischnocera lice and under conditions that would exclude monoxeny. Moreover, the bird plumage might be investigated for the presence of cestode eggs, to evaluate possible mechanisms favouring egg intake by lice.
Our observation of cestode DNA in lice samples is not sufficient evidence to conclude that chewing lice serve as intermediate hosts for H. microps, since cestode DNA might have come from contamination or from eggs that are not developing into infective cysticercoids. However, given that we observed both cysticercoid-like structures in lice under light microscopy and identified cestode DNA from batch samples of lice, the most plausible conclusion is that H. microps eggs are able to infect and develop in Ischnoceran hosts. Nevertheless, this study cannot exclude the possibility that other arthropods might be suitable hosts for this cestode in other bird species.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X17000141
Acknowledgements
The authors are grateful to Harald Kryvi, Teresa Cieplinska and Maurizio Dioli for technical assistance and advice during the preparation of the histological slides of the Ischnocera lice for optical microscopy.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.