Introduction
Trichinella spiralis is the chief causative agent of trichinellosis (Murrell & Pozio, Reference Murrell and Pozio2011). Humans become infected by eating raw or undercooked meat containing viable infective T. spiralis larvae (Pozio, Reference Pozio2007). Adult females of this parasitic species reside in the small intestinal epithelium of the host, leading to immune-mediated inflammation and hypersensitivity reactions that induce evident intestinal pathology (Khan, Reference Khan2008). Moreover, T. spiralis has an exceptional relation with skeletal muscle owing to its distinctive ability to undergo intracellular growth in muscle cells. Once infected, muscle cells undergo extensive morphological and biochemical changes that allow them to develop into a protective refuge (a nurse cell) for the parasite (Wu et al., Reference Wu, Sofronic-Milosavljevic, Nagano and Takahashi2008). The long-term infection of muscles with Trichinella results in a very powerful interaction with the host immune system, leading to marked inflammation of the affected muscles (Bruschi & Chiumiento, Reference Bruschi and Chiumiento2011).
Many factors other than direct injury induced by the parasite itself contribute to tissue damage in trichinellosis. One of the main causes of this damage is the oxidative stress state that accompanies Trichinella infection, as revealed by the increased production of various stress markers, such as GSTO-1, haem oxygenase I (Bruschi et al., Reference Bruschi, Saviozzi, Piaggi, Malvaldi and Casini2003), superoxide dismutase and malondialdehyde (MDA) (Mido et al., Reference Mido, Fath, Farid, Nonaka, Oku and Horii2012). Furthermore, inflammatory cells that produce excessive amounts of reactive oxygen species (ROS), nitrogen species and other free radicals upon activation are recruited (Chiumiento & Bruschi, Reference Chiumiento and Bruschi2009). Moreover, there is increased production of lipoperoxidized proteins in nurse cells (Bruschi et al., Reference Bruschi, Saviozzi, Piaggi, Malvaldi and Casini2003). Therefore, anti-oxidants and anti-inflammatory drugs are expected to help protect hosts against these injurious factors (Shimoni et al., Reference Shimoni, Klein, Weiner, Assous and Froom2007; Kazemzadeh et al., Reference Kazemzadeh, Mohammad and Mohammad2014). However, universally prescribed nonsteroidal or steroidal anti-inflammatory drugs possess many adverse effects that limit their usage (Barnes, Reference Barnes2014; Badri et al., Reference Badri, Miladi, Nazari, Greige-Gerges, Fessi and Elaissari2016; Oray et al., Reference Oray, Abu Samra, Ebrahimiadib, Meese and Foster2016). Accordingly, there is a pressing need to examine new, safe and efficient compounds that have anti-inflammatory and anti-oxidant properties (Kunnumakkara et al., Reference Kunnumakkara, Sailo, Banik, Harsha, Prasad, Gupta, Bharti and Aggarwal2018).
Resveratrol (RSV) is a polyphenolic stilbene abundant in the rinds of red fruits such as berries and grapes and in nuts (Szkudelska & Szkudelski, Reference Szkudelska and Szkudelski2010). Plants produce it as a phytoalexin, and it makes them more resistant to stressful conditions and microbial and fungal infections (Chedea et al., Reference Chedea, Vicas, Sticozzi, Pessina, Frosini, Maioli and Valacchi2017). It has a wide safety margin and is commonly used as an over-the-counter nutraceutical (Vidavalur et al., Reference Vidavalur, Otani, Singal and Maulik2006). Accumulating data indicate its beneficial effects in many diseases, such as diabetes (Su et al., Reference Su, Hung and Chen2006), muscular dystrophy (Hori et al., Reference Hori, Kuno, Hosoda, Tanno, Miura, Shimamoto and Horio2011) and atherosclerosis (Wu & Hsieh, Reference Wu and Hsieh2011). Additionally, it possesses neuroprotective effects (Sun et al., Reference Sun, Xiao, Cai, Chen, Wei, Liu, Lv and Zou2010), cardioprotective effects (Bradamante et al., Reference Bradamante, Barenghi and Villa2004; Tanno et al., Reference Tanno, Kuno, Yano, Miura, Hisahara, Ishikawa, Shimamoto and Horio2010) and anti-inflammatory effects (Donnelly et al., Reference Donnelly, Newton, Kennedy, Fenwick, Leung, Ito, Russell and Barnes2004). Furthermore, several studies have demonstrated that RSV has antimicrobial activities (Docherty et al., Reference Docherty, Fu, Stiffler, Limperos, Pokabla and DeLucia1999; Chan, Reference Chan2002; Wang et al., Reference Wang, Lai, Hsueh, Chiou, Lin and Liaw2006).
RSV is a potent anti-oxidant. It decreases intracellular ROS production through different mechanisms, including direct scavenging and activating enzymes, such as mitochondrial superoxide dismutase, that provide anti-oxidant defence (Leonard et al., Reference Leonard, Xia, Jiang, Stinefelt, Klandorf, Harris and Shi2003; Robb et al., Reference Robb, Page, Wiens and Stuart2008; Tanno et al., Reference Tanno, Kuno, Yano, Miura, Hisahara, Ishikawa, Shimamoto and Horio2010). Additionally, it acts as a modulator of several vital enzymes that play a role in cell survival, such as cyclooxygenases, protein kinase C, lipoxygenase, protein tyrosine kinase, ribonucleotide reductase, inducible nitric oxide synthase and P450 (Soliman et al., Reference Soliman, Ismail, Badr and Nasr2017). Therefore, we aimed to explore the potential effects of RSV administration on the therapeutic outcome of the intestinal and muscular phases of experimental trichinellosis.
Materials and methods
Animals and parasites
Male Swiss albino mice aged 6–8 weeks and weighing 25–30 g were purchased from the Theodore Bilharz Research Institute (Giza, Egypt). They were maintained in accordance with institutional and national guidelines. The mice were acclimatized for seven days prior to being used in the experiments. The mice were infected with T. spiralis L1 larvae orally according to the method described by Dunn & Wright (Reference Dunn and Wright1985). The Trichinella species used in this study was genotyped as T. spiralis (Trichinella - Istituto Superiore di Sanita code: ISS6158) by the European Union Reference Laboratory for Parasites, Superior Institute of Health, Rome, Italy. Trichinella spiralis larvae were orally inoculated at a dose of 200 larvae per infected mouse.
Experimental design
Animals were divided into four groups: the uninfected mouse group (20 mice; negative control); the infected mouse group, which did not receive any medication (40 mice; positive control); the early treatment group (20 mice), which received RSV for two weeks starting from the first day post-infection (p.i.); and the late treatment group (20 mice), which was treated with RSV for two weeks starting from the 28th day p.i. Additionally, seven mice received only the solvent carboxymethyl cellulose in distilled water.
On the 15th day p.i., ten mice from each of the control groups and from the early treatment group were sacrificed, and the small intestines were collected; one piece from each sample (1 cm from the jejunum) was used for histopathological examination, and another part was used for biochemical analysis. Another ten mice from the positive control group and from the early treatment group were sacrificed, and the small intestines were used to count adult worms.
On the 43rd day p.i., ten mice from the positive control group and from the late treatment group were sacrificed, and total larvae were counted in the muscles. Furthermore, ten mice from each of the control groups and from the late treatment group were sacrificed, and similar skeletal muscle samples were taken and used for the histopathological study, immunohistochemical study and biochemical analysis.
Drugs
Resveratrol powder was purchased from Sigma-Aldrich Chemie (Steinheim, Germany) and was administered orally as a freshly prepared suspension in 0.5% carboxymethyl cellulose in distilled water at a dose of 20 mg/kg once daily (Soliman et al., Reference Soliman, Ismail, Badr and Nasr2017).
Parasitological assays
Isolation and counting of adult worms
Mice were euthanized, and the small intestines were separated. After the intestines were washed with physiological saline, they were divided into 1-cm portions and kept in 10 ml physiological saline for 2 h at 37°C. The saline was collected using a pipette, and the intestines were washed three times with physiological saline. All of the fluid was collected and centrifuged at 2000 rpm for 3 min. The supernatant was removed, the sediment was reconstituted in 3–5 drops of physiological saline and the number of adults was counted by examining the reconstituted sediment drop by drop at a magnification of 20× (Wakelin & Lloyd, Reference Wakelin and Lloyd1976).
Total larval burden in muscles
Mice were euthanized on the 43rd day p.i. Muscle larval counts in whole carcasses were determined according to the method described by Dunn & Wright (Reference Dunn and Wright1985). Briefly, each mouse was dissected and digested in 1% pepsin-hydrochloride in 200 ml distilled water. Following incubation of the mixture at 37°C for one hour with continuous stirring by means of an electric stirrer, encysted larvae were collected via the sedimentation technique and then washed several times in distilled water. The number of larvae was counted microscopically using a McMaster counting chamber (Lauda-Konigshofen, Germany).
Biochemical assays
Homogenates and protein concentration
Intestinal and muscle specimens were dissected and washed with ice-cold saline, cut into multiple small pieces, weighed, homogenized with 50 mm phosphate buffer (pH 7.4) and centrifuged at 12,000×g for 20 min at 4°C. Then, the resultant supernatant (free of insoluble materials) was frozen at −80°C until analysis. Tissue protein content was determined by the method described by Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951).
Assessment of oxidant/anti-oxidant status in intestinal and muscle tissue homogenates
Biochemical assays for xanthine oxidase (XO) activity and total anti-oxidant capacity (TAC) were analysed as previously described by Litwack et al. (Reference Litwack, Bothwell, Williams and Elvehjem1952) and Koracevic et al. (Reference Koracevic, Koracevic, Djordjevic, Andrejevic and Cosic2001), respectively. Additionally, MDA and reduced glutathione (GSH) levels were assayed using commercial kits supplied by Biodiagnostic (Giza, Egypt).
Enzyme-linked immunosorbent assays (ELISAs)
Interleukin 4 (IL-4) and pentraxin 3 (PTX3) levels in intestinal and muscle tissue homogenates were measured using ELISA kits (RayBiotech Inc., Peachtree Corners, Georgia, USA, and Chongqing Biospes Co., Chongqing, China, respectively). All ELISA techniques were performed according to the manufacturer's protocol and read on a microplate reader (Stat Fax®2100, Fisher Bioblock Scientific, Strasbourg, France) at 450 nm with a correction wavelength set at 570 nm.
Histopathological study
Tissue samples from the studied groups were fixed in 10% formalin for 24 h, washed in water for 12 h, dehydrated in ascending grades of alcohol, cleared in xylene and embedded in paraffin blocks that were sectioned at a thickness of 5 μm by a microtome and then stained with haematoxylin and eosin. Subjective semiquantitative histopathological scoring was used to assess the histopathological characteristics of the small intestine and muscle sections (Othman et al., Reference Othman, Abou Rayia, Ashour, Saied, Zineldeen and El-Ebiary2016). Tissue section examination and scoring were carried out in a blinded manner.
For small intestinal specimens, histopathological evaluation of the following parameters was performed: the extent of inflammatory cell infiltrates within the core of the intestinal villi and submucosa (+1 = mild reaction; +2 = moderate reaction; and +3 = intense reaction); pathological changes in the intestinal architecture, including epithelial changes and hyperplasia of goblet cells (+1 = mild; +2 = moderate; and +3 = severe); and the mucosal architecture and the degree of villous atrophy (+1 = mild; +2 = moderate; and +3 = severe). For skeletal muscle specimens, the extent of the inflammatory reaction surrounding the capsule was evaluated and scored (+1 = mild reaction; +2 = moderate reaction; and +3 = intense reaction).
To assess the aforementioned histopathological parameters, five histological sections/mouse and ten low-power fields (100×) from each of the examined histological sections were examined, and then the average score was calculated.
Study of vascular endothelial growth factor (VEGF) in skeletal muscles
Muscle sections were deparaffinized and treated with 3% hydrogen peroxide in methanol to block endogenous peroxidase activity. Antigen retrieval was performed. Then, a monoclonal antibody against VEGF (clone: EP1176 y, ready-to-use, GENOVA Diagnostics Company, Asheville, North Carolina, USA) was applied to the sections and incubated for 30 min at room temperature. The antigen–antibody complex was visualized using the biotin–streptavidin–peroxidase method. The colour was developed with diaminobenzidine solution, and the sections were lightly counterstained with haematoxylin. The slides were then dehydrated and mounted.
Analysis of VEGF staining
Cells positive for VEGF immunostaining showed brownish cytoplasmic staining. Immunohistochemical scores (IHSs) were determined by combining the percentage of positively stained cells (quantity score) with the staining intensity score. The scores ranged from 0 to 4 as follows: 0 = no immunostaining; 1 = 1–10% of the cells were positive; 2 = 11–50% of the cells were positive; 3 = 51–80% of the cells were positive; and 4 = ≥81% of the cells were positive. The staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). IHSs were obtained by multiplying the quantity score (0–4) by the staining intensity score (0–3) and ranged from 0 to 12. A score of 9–12 was considered strong immunoreactivity (+3), a score of 5–8 was considered moderate immunoreactivity (+2), a score of 1–4 was considered weak reactivity (+1) and a score of 0 was considered negative (Gou et al., Reference Gou, Chen, Zhu, Jiang, Yang, Cao and Hou2011). To evaluate VEGF expression, five histological sections/mouse and ten high-power fields (400×) from each examined section were examined, and then the average score was calculated.
Statistical analysis
The data are presented as the mean ± standard deviation. Analysis of variance was used to compare more than two groups, and the probability of a significant difference between the means of two groups was determined by a post-hoc test (for biochemical indices) and Monte Carlo exact test for chi square (for histopathological and IHSs). Differences were considered significant when P < 0.05. Statistical analyses were performed according to conventional procedures using Statistical Package for the Social Sciences (SPSS Inc., Chicago, Illinois, USA) software for Windows version 20.
Results
Number of adult worms in the small intestine and larvae in the muscles
The mean number of adult worms in the small intestines of the early treatment group (23.40 ± 2.41) was significantly lower than that in the positive control group (39.80 ± 3.70) (P = 0.001). Likewise, our results revealed a significant reduction in the number of total larvae in the late treatment group (7548.20 ± 840.74) compared to the positive control group (12,635.40 ± 803.61) (P = 0.001).
Biochemical findings
Assay of oxidant/anti-oxidant markers
The levels of XO and MDA in small intestinal homogenates and muscle homogenates were significantly increased in the positive control group compared to the negative control group (P = 0.001). However, the levels of these markers were significantly decreased in the RSV-treated groups compared to the positive control group (P = 0.001). On the other hand, significant decreases in TAC and GSH levels in small intestinal homogenates and muscle homogenates were observed in the positive control group compared to the negative control group (P = 0.001). The TAC and GSH levels were significantly enhanced in RSV-treated mice compared to positive control mice (tables 1 and 2).
Table 1. Redox parameters in small intestine homogenates (n = 10).

P1: negative control group vs positive control group.
P2: negative control group vs early treatment group.
P3: positive control group vs early treatment group.
n = number of studied mice in each group.
* P < 0.05 (significant). SD, standard deviation.
Table 2. Redox parameters in skeletal muscle homogenates (n = 10).

P1: negative control group vs positive control group.
P2: negative control group vs late treatment group.
P3: positive control group vs late treatment group.
n = number of studied mice in each group.
* P < 0.05 (significant). SD, standard deviation.
IL-4 and PTX3 in small intestinal homogenates and muscle homogenates
The levels of IL-4 and PTX3 were compared between all groups, and they were significantly increased in the positive control group compared with the negative control group. Following the administration of RSV to mice, IL-4 and PTX3 levels were significantly reduced compared to those in the positive control group (tables 3 and 4).
Table 3. Interleukin 4 levels in the different groups (n = 10).

P1: negative control group vs positive control group.
P2: negative control group vs early treatment group.
P3: positive control group vs early treatment group.
P4: negative control group vs late treatment group.
P5: positive control group vs late treatment group.
n = number of studied mice in each group.
* P < 0.05 (significant). SD, standard deviation.
Table 4. Pentraxin 3 levels in the different groups (n = 10).

P1: negative control group vs positive control group.
P2: negative control group vs early treatment group.
P3: positive control group vs early treatment group.
P4: negative control group vs late treatment group.
P5: positive control group vs late treatment group.
n = number of studied mice in each group.
* P < 0.05 (significant). SD, standard deviation.
Histopathological results
Small intestine findings
Histopathological examination of sections of the small intestine samples from the positive control group showed signs of inflammation in the mucosa and submucosa and the core of the villi. The inflammatory infiltrates principally consisted of eosinophils and neutrophils. Furthermore, there was ulceration and sloughing of the intestinal mucosal epithelium, goblet cell hyperplasia and a decrease in the length of the villi (fig. 1a). Compared to sections from the infected control group, sections from the early treatment group exhibited improvements in histopathological changes and a reduction in inflammatory cell infiltrates (fig. 1b).The histopathological findings in small intestinal sections of all studied groups are shown in Table 5.
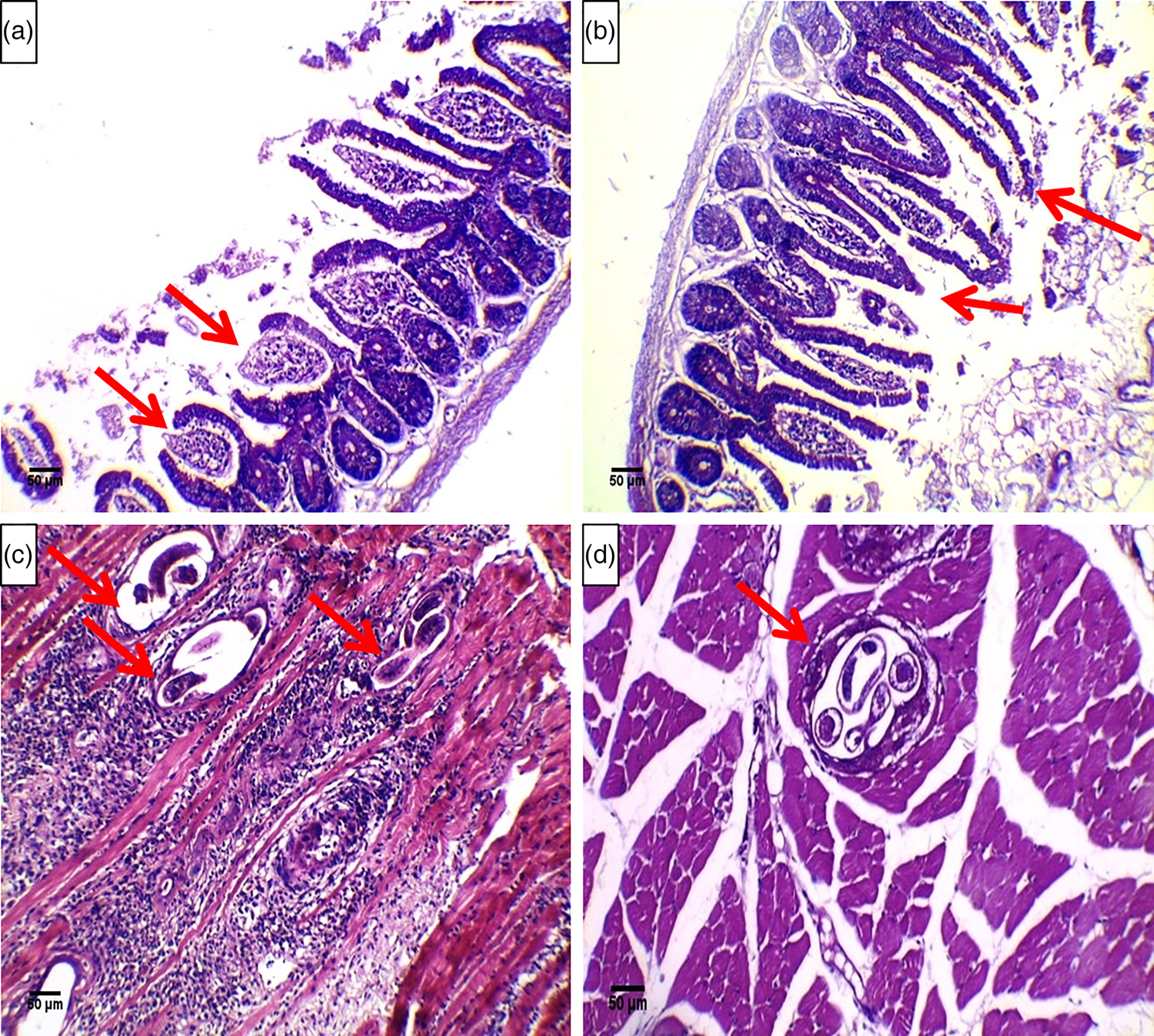
Fig. 1. Histopathological analysis of small intestine sections from (a) the positive control group showing dense inflammatory cellular infiltrates mainly in the core of the villi and extending into the submucosa, hyperplasia of the crypts of Lieberkuhn, a decrease in the villous height to crypt depth ratio and ulceration of the mucosa (arrows) (Haematoxylin and eosin [H&E], 200×) and (b) the early treatment group showing improvements in changes in the small intestines with an evident decrease in inflammatory cell infiltrates (arrows) (H&E, 200×). Histopathological analysis of skeletal muscle sections from (c) the positive control group showing a large number of larvae deposited within the muscle fibres (arrows) surrounded by an intense inflammatory reaction (H&E, 200×) and (d) the late treatment group showing fewer larvae in the muscle (arrows) and a decreased number of inflammatory cell infiltrates (H&E, 200×).
Table 5. Histopathological findings in small intestine sections from the studied groups (n = 10).

n = number of studied mice in each group.
* P < 0.05 (significant).
Skeletal muscle findings
Histopathological examination of skeletal muscle sections from the positive control group revealed that multiple sections contained encysted T. spiralis larvae surrounded by nurse cells and a collagen capsule. Moreover, a massive inflammatory cell infiltrate composed mainly of histiocytes, lymphocytes, plasma cells and eosinophils surrounded the encysted larvae and diffusely infiltrated the affected muscle fibres (fig. 1c). Upon examination of sections from the late treatment group, we found a significant decrease (P = 0.001) in the extent of inflammatory cell infiltration and a decrease in the number of muscle larvae compared to those in sections from the positive control group (fig. 1d).The extent of the inflammatory cellular infiltarion in the skeletal muscle sections of the studied groups is shown in Table 6.
Table 6. The extent of the inflammatory cell infiltrates in skeletal muscle sections from the studied groups (n = 10).

n = number of studied mice in each group.
* P < 0.05 (significant).
Immunohistochemical findings
In the negative control group, no VEGF reaction was detected in muscle sections, whereas a strong (+3) reaction was detected in the positive control group. This positive immunostaining was predominantly obvious in the cytoplasm of nurse cells and the cytoplasm of the surrounding inflammatory cells (fig. 2a). A significant reduction in VEGF expression was observed in the late treatment group compared to the positive control group (P < 0.05) (fig. 2b). VEGF expression in the experimental groups is summarized in table 7.

Fig. 2. Immunohistochemical staining of VEGF in skeletal muscle sections from (a) the positive control group showing (+3) VEGF expression within the cytoplasm of the nurse cells and the surrounding inflammatory cells (immunoperoxidase, ×200) and (b) the late treatment group showing weak (+1) cytoplasmic VEGF expression within the nurse cells (immunoperoxidase, 200×).
Table 7. Comparison of VEGF expression in skeletal muscle sections (n = 10).

n = number of studied mice in each group.
* P < 0.05 (significant).
Discussion
Diverse plant-derived polyphenols have been used extensively in various studies in an attempt to find innovative drugs that have inhibitory effects against various pathogens (Lai & Roy, Reference Lai and Roy2004; Taguri et al., Reference Taguri, Tanaka and Kouno2004). One of these compounds is RSV. Many studies have investigated its effectiveness and have shown that RSV has considerable antibacterial, antifungal and antiviral properties (Docherty et al., Reference Docherty, Fu, Stiffler, Limperos, Pokabla and DeLucia1999; Chan, Reference Chan2002; Jung et al., Reference Jung, Hwang, Sung, Kang, Kang, Seu and Lee2005). In addition, the inhibitory effects of RSV against protozoans that infect humans, such as Leishmania major and Encephalitozoon cuniculi, have been demonstrated (Leiro et al., Reference Leiro, Cano, Ubeira, Orallo and Sanmartín2004; Kedzierski et al., Reference Kedzierski, Curtis, Kaminska, Jodynis-Liebert and Murias2007). Nevertheless, few studies have examined its anthelmintic activities (Soliman et al., Reference Soliman, Ismail, Badr and Nasr2017).
In the present study, the effect of RSV on experimental trichinellosis was investigated. A significant reduction in the parasite burden in intestinal and muscle tissues was observed in treated mice compared to positive control mice. These results are consistent with the findings of in vitro study by Ozkoc et al. (Reference Ozkoc, Tuncay, Delibas and Akisu2009), which showed that RSV has a significant direct lethal effect on newborn larvae of T. spiralis and adult T. spiralis. However, Ozkoc et al. (Reference Ozkoc, Tuncay, Delibas and Akisu2009) showed that a significant lethal effect on muscle larvae was detected only upon exposure to very high concentrations of RSV (440 and 880 μM) for more than 48 h. Researchers have used the ability of RSV to inhibit polyamine metabolism, block calcium channels, induce antimitotic effects by terminating tubulin polymerization and interfere with oxygen consumption by the parasite to explain its antiparasitic effects (Leiro et al., Reference Leiro, Cano, Ubeira, Orallo and Sanmartín2004; Kedzierski et al., Reference Kedzierski, Curtis, Kaminska, Jodynis-Liebert and Murias2007; Lamas et al., Reference Lamas, Morais, Arranz, Sanmartín, Orallo and Leiro2009). Additionally, some RSV analogues showed nematocidal effects through the same mechanisms, including the inhibition of tubulin polymerization and oxygen consumption by the parasite (Stadler et al., Reference Stadler, Dagne and Anke1994; Schneider et al., Reference Schneider, Chabert, Stutzmann, Coelho, Fougerousse, Gossé, Launay, Brouillard and Raul2003; Chabert et al., Reference Chabert, Fougerousse and Brouillard2006). Furthermore, we cannot exclude the possibility that the observed effectiveness of RSV in decreasing muscle larval counts may be attributed to its direct effect on adult worms, which results in a reduction in the number of larvae that reach the muscles.
During the course of T. spiralis infection, a large amount of free radicals and ROS are produced both by the parasite itself and by the host during innate and acquired defence reactions against infection (Bruschi et al., Reference Bruschi, Saviozzi, Piaggi, Malvaldi and Casini2003; Othman et al., Reference Othman, Abou Rayia, Ashour, Saied, Zineldeen and El-Ebiary2016). Anti-oxidants have an important role in metabolism and help protect the host from oxidant-mediated harmful effects (Chiumiento & Bruschi, Reference Chiumiento and Bruschi2009). Our analysis of oxidative stress biomarkers showed that they were significantly upregulated in the small intestinal and skeletal muscle tissues of the positive control group compared to the negative control group. These results are in agreement with those of several previous studies (Wojtkowiak-Giera et al., Reference Wojtkowiak-Giera, Wandurska-Nowak, Michalak, Derda and Łopaciuch2012; Blum et al., Reference Blum, Mohanan, Fabre, Yafawi and Appleton2013; Kazemzadeh et al., Reference Kazemzadeh, Mohammad and Mohammad2014). Oxidative stress was decreased in the RSV-treated groups, as revealed by the increase in TAC and the GSH level and the decrease in XO and MDA levels. These findings are consistent with those of several studies (Jiang et al., Reference Jiang, Peng, Luo, Li and Lin2008; Palsamy & Subramanian, Reference Palsamy and Subramanian2008; Das et al., Reference Das, Mukherjee, Gupta, Rao and Vasudevan2010; Toklu et al., Reference Toklu, Sehirli, Ers-ahin, Sü leymanoğ lu, Yiğiner, Emekli-Alturfan, Yarat, Yeğen and Sener2010; Moridi et al., Reference Moridi, Karimi and Sheikh2015; Hamza & El-Shenawy, Reference Hamza and El-Shenawy2017; Soliman et al., Reference Soliman, Ismail, Badr and Nasr2017; Turkmen et al., Reference Turkmen, Birdane, Demirel, Kabu and Ince2019) that reported that RSV possesses powerful anti-oxidant properties that help ameliorate oxidative stress.
Infection with T. spiralis triggers a very powerful Th2 response, which controls a diversity of responses that are specific to this helminthic infection, including mucosal mastocytosis, goblet cell hyperplasia and intestinal eosinophilic infiltration. There is a broad consensus that Th2 cytokines, including IL-4, IL-5, IL-9 and IL-3, can create a hostile environment for intestinal helminth parasites by producing intense intestinal pathology that may result in the expulsion of these parasites (Urban et al., Reference Urban, Maliszewski, Madden, Katona and Finkelman1995; Finkleman et al., Reference Finkleman, Shea-Donohue, Goldhill, Sullivan, Morris, Madden, Gause and Urban1997; Scales et al., Reference Scales, Ierna and Lawrence2007). IL-4 plays an important role in the induction of these pathological changes through the Stat6 signalling pathway (Arizmendi et al., Reference Arizmendi, Yepez-Mulia and Cedillo-Rivera2001; Khan et al., Reference Khan, Blennerhasset, Ma, Matthaei and Collins2001). On the other hand, Lawrence et al. (Reference Lawrence, Paterson, Higgins, MacDonald, Kennedy and Garside1998) showed that in IL-4-deficient mice, the expulsion of T. spiralis adult worms can still occur in the absence of severe enteropathy. Furthermore, in the same study, the researchers concluded that intestinal pathological changes are not the main factor that contributes to the expulsion of the parasite. In the present work, there was a significant upregulation in the expression of IL-4 in the small intestinal and muscle homogenates of untreated infected mice compared to negative control mice. Similarly, previous studies by Sofronic-Milosavljevic et al. (Reference Sofronic-Milosavljevic, Radovic, Ilic, Majstorovic, Cvetkovic and Gruden-Movsesijan2013) and Ding et al. (Reference Ding, Bai, Wang, Shi, Cai, Luo, Liu and Liu2017) reported that IL-4 expression is enhanced in T. spiralis-infected animals compared to uninfected animals. According to our results, there was a significant reduction in the expression of IL-4 in the small intestinal and muscle homogenates of RSV-treated infected mice compared to untreated infected mice. These results are in agreement with those of Lee et al. (Reference Lee, Kim, Kwon, Oh, Lee and Ahn2009), who reported that RSV significantly reduces IL-4 in a mouse model of OVA-induced allergic asthma. We think that a decrease in IL-4 expression may be beneficial in the course of trichinellosis by leading to a decrease in intestinal mastocytosis, which play a major role in the damage and atrophy of the villi during T. spiralis infection. Mast cells produce leukotrienes and 5-hydroxytryptamine, which provoke epithelial injury at the tips of villi, a mechanism that may induce villus atrophy (Serna et al., Reference Serna, Porras and Vergara2006).
The levels of both short and long pentraxins in the blood or tissues rapidly rise under inflammatory conditions and are generally correlated with the severity of the condition that provokes their expression (Norata et al., Reference Norata, Garlanda and Catapano2010). The local production of PTX3 (a member of the long pentraxin family) by macrophages, dendritic cells and endothelial cells rapidly occurs upon inflammatory stimulation (Garlanda et al., Reference Garlanda, Bottazzi, Bastone and Mantovani2005), and neutrophils liberate PTX3 from cytoplasmic granules at the location of tissue damage or in response to microbial agents (Jaillon et al., Reference Jaillon, Peri and Delneste2007). As PTX3 production occurs locally at sites of inflammation, it is believed to be a good self-regulating marker of disease severity (Fazzini et al., Reference Fazzini, Peri and Doni2001). In the present study, PTX3 levels were elevated in the small intestinal and muscle tissues of the positive control group compared to the negative control group. These findings correspond with the results of Savchenko et al. (Reference Savchenko, Inoue and Ohashi2011), who reported that PTX3 concentrations increase quickly in response to pro-inflammatory signals. However, PTX3 levels are decreased in the treated groups, indicating improvements in tissue injury. These results coincide with those of Erbas et al. (Reference Erbas, Pala, Pala, Oltulu, Aktug, Yavasoglu and Taskiran2014), who noted a significant reduction in PTX3 levels in RSV-treated diabetic animals compared to untreated diabetic animals.
Due to the noticeable inflammation that occurs in different tissues during the course of T. spiralis infection, anti-inflammatory drugs are a basic part of the management protocol of this disease (Shimoni et al., Reference Shimoni, Klein, Weiner, Assous and Froom2007). Interestingly, the current study revealed that RSV ameliorated the severe inflammatory reaction induced by T. spiralis infection, as evidenced by the improvements in histopathological changes in the intestines and muscles and the decreased levels of PTX3. Accumulating data have strongly demonstrated the anti-inflammatory activities of RSV in different disease models and different affected organs (Cong et al., Reference Cong, Li, Lu, Dai, Zhang, Zhao and Liu2014; Li et al., Reference Li, Xie, Zhuang, Li, Yao, Shao and Wang2015; Liu et al., Reference Liu, Bao, Zeng and Wei2016; Wang et al., Reference Wang, Li, Li, Miao and Xiao2017; Yan et al., Reference Yan, Sun and Xu2018). Its inhibitory effects on the expression of inflammatory mediators, including IL-1β, matrix metallopeptidase 13, cyclooxygenases 2, nuclear factor kappa B and many other molecules, have been proposed as mechanisms of its anti-inflammatory actions (Yar et al., Reference Yar, Menevse and Alp2011; Coutinho et al., Reference Coutinho, Pacheco, Frozza and Bernardi2018).
Angiogenesis is one of the requirements for the maintenance of viability and the proper development of muscle larvae within nurse cells. Circulatory rete formation around nurse cells aids in larval nutrition and the efficient disposal of waste products (Ock et al., Reference Ock, Cha and Choi2013). During this process, VEGF acts as the chief vascular endothelial stimulating factor (Capó et al., Reference Capó, Despommier and Polvere1998). Hypoxia is the key stimulator of VEGF expression (Sang et al., Reference Sang, Fang, Srinivas, Leshchinsky and Caro2002). In the present study, the RSV-treated group showed weak VEGF expression in skeletal muscles compared with the strong expression in the positive control group. To the best of our knowledge, this is the first study to investigate the effect of resveratrol administration on the expression of VEGF during T. spiralis infection. Therefore, we compared our results with those of studies that investigated the effect of resveratrol administration on the expression of VEGF in other disease models. Our results are consistent with those of Liu et al. (Reference Liu, Li and Yang2012), who reported that RSV exerts anti-osteosarcoma activity through its inhibitory effect on VEGF expression, and those of Yan et al. (Reference Yan, Sun and Xu2018), who concluded that RSV improves cardiovascular function in rats with diabetes-related myocardial infarction via the inhibition of VEGF expression. In the current work, we assume that the reduced expression of VEGF can interrupt the process of angiogenesis, thus depriving developing muscle larvae of necessary nutrients and causing the accumulation of waste products. Altogether, the drug could induce dysontogenesis of muscle larvae, which may be a leading cause of the reduction in larval burden in muscles.
In conclusion, our results collectively show the protective effects of orally administered RSV against several aspects of the pathological consequences of T. spiralis infection. To the best of our knowledge, this study demonstrates for the first time that RSV has effects against T. spiralis infection in vivo. The drug also exhibits notable anti-oxidant and anti-inflammatory activities. Therefore, RSV can be considered a useful adjuvant for the treatment of trichinellosis, and further studies on its usage as an adjuvant in experimental animals and in humans are worth consideration.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. The study protocol was approved by the Laboratory Animal Centre for Research Ethics Committee, Faculty of Medicine, Tanta University (code number 33555/12/19).











