Introduction
In the past 20 years, several new species of helminth parasites of amphibians and reptiles have been described in the Neotropics, proving that the diversity of such parasites remains unknown. Among the groups representing this hidden diversity, the genus Rhabdias Stiles & Hassal, 1905 is notable for being the most distinct in the Rhabdiasidae and includes 87 species of which 49 have been described in the past two decades (Kuzmin & Tkach, Reference Kuzmin and Tkach2020). Furthermore, the genus is represented by lung-dwelling parasites of amphibians and certain reptiles, and occurs in every continent, except Antarctica.
According to Poulin et al. (Reference Poulin, Hay and Jorge2019), the use of genetic information is fundamental in systematic parasite taxonomy, especially for descriptions of new species, allowing taxonomic problems among some groups to be solved using molecular phylogenies, description of patterns of gene flow among species and populations, and especially for understanding diversity in complex parasite groups such as Rhabdiasidae (Tkach et al., Reference Tkach, Kuzmin and Snyder2014; Müller et al., Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018).
Considering the exceptionally high diversity of amphibians in Brazil (31 families, 1139 species) it is unsurprising that the country also possesses a great diversity of parasites associated with this host group. Nevertheless, the knowledge of parasite species such as representatives of Rhabdias is poor, and only eight species have been described in amphibians (four in Bufonidae, two in Leptodactylidae, one in Dendrobatidae and one in Hylidae) (Kuzmin & Tkach, Reference Kuzmin and Tkach2020). Among the 18 known species of Rhabdias described from the Neotropical region, until now only Rhabdias breviensis Nascimento, Gonçalves, Melo, Giese, Furtado & Santos, Reference Nascimento, Gonçalves, Melo, Giese, Furtado and Santos2013 and Rhabdias glaurungi Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin & Melo, Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019 have been described using a combination of morphology and molecular data (Nascimento et al., Reference Nascimento, Gonçalves, Melo, Giese, Furtado and Santos2013; Willkens et al., Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019).
The genus Pseudopaludicola Miranda-Ribeiro, 1926 (Anura: Leptodactylidae: Leiuperinae) comprises 25 species widely distributed in South America, from northern Argentina to the east of the Andes in Venezuela, and is associated with open formations close to tropical forests, such as the Chaco, Pampas, Cerrado and Caatinga (Segalla et al., Reference Segalla, Caramaschi, Cruz, Garcia, Grant, Haddad, Santana, Toledo and Langone2019; Frost, Reference Frost2020) biomes. Pseudopaludicola pocoto Magalhães, Loebmann, Nogueira, Kokubum, Baptista, Haddad & Garda, 2014 is commonly found in the Caatinga biome in Brazil, in the states of Ceará, Paraíba, Pernambuco, Piauí and Rio Grande do Norte (Magalhães et al., Reference Magalhães, Loebmann, Kokubum, Haddad and Garda2014; Silva et al., Reference Silva, Lima, Santos, Souza and Pederassi2015, Reference Silva, Roberto, Ávila and Morais2017), while there is a record of this species in the Atlantic Forest biome in the state of Minas Gerais (Andrade et al., Reference Andrade, Barros, Oliveira, Juncá and Magalhaes2015).
During a survey of parasites of P. pocoto in the state of Ceará, Brazil, nematodes were found with characteristics that corresponded to the diagnoses of Rhabdias; however, due to particular morphological characters, these could not be assigned to any known species of the genus. Thus, we herein describe a new species of Rhabdias parasitizing the lungs of P. pocoto using both morphological and molecular phylogenetic analyses, based on the mitochondrial gene cytochrome c oxidase I (coi).
Material and methods
Host collection and morphological study of parasites
During a helminthological survey performed in February 2014 (rainy period), 115 specimens of P. pocoto were collected from the Benguê Reservoir, in the Aiuaba municipality, in the state of Ceará, Brazil (06°35′35″S, 40°08′31″W).
The frogs were anaesthetized with 2% lidocaine hydrochloride (CFMV, 2013) and the internal organs were removed, dissected and analysed under a stereomicroscope. The nematodes found in the lungs were rinsed in saline solution and fixed in pre-heated 70% ethanol. For morphological identification, the specimens were cleared with lactophenol, mounted on temporary slides and observed using a Leica application suite (V3) adapted for a DM5000B light microscope equipped with differential interference contrast optics and a computerized image analysis system (LAS DIC, Leica Microsystems, Wetzlar, Germany).
Some specimens were also analysed using scanning electron microscopy and post-fixed in 1% osmium tetroxide, dehydrated in a graded alcohol series and dried at the critical point of CO2. The specimens were then mounted on an aluminium stub using conductive double-sided tape, coated with gold–palladium and examined using a Vega3 electron microscope (TESCAN, Brno, Czech Republic) at the Laboratory of Embryology and Histology of the Federal Rural University of Amazonia (Universidade Federal Rural da Amazônia, UFRA).
Morphological measurements are given as the values of the holotype followed by the mean of the paratypes, and range in parentheses (reported in micrometres, unless otherwise indicated) in accordance with the standardization proposed by Willkens et al. (Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019).
Molecular analyses and phylogenetic study
For molecular analysis, nematodes fixed in heated 70% ethanol were posteriorly transferred to absolute ethanol (Merck®) and stored in a freezer at −20°C for subsequent molecular analysis. Part of the nematode was taken for scanning electron microscopy (anterior part with buccal capsule) and part (posterior or medium part) for DNA extraction. Genomic DNA was extracted using DNeasy® Blood & Tissue Kit (Qiagen) according to the manufacturer's protocol, with a final volume of 30 μl. A Polymerase chain reaction was performed to amplify the partial mitochondrial coi gene using primers and cycling conditions, as well as purification and sequencing procedures in accordance with Müller et al. (Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018). The sequence identity was verified using the Basic Local Alignment Search Tool (BLAST), and contiguous sequences were assembled using Sequencer v. 5.2.4 (Gene Codes, Ann Arbor, MI) and submitted to GenBank (accession numbers presented in table 1).
Table 1. Sequences used in the phylogenetic and distance analysis with GenBank accession numbers.
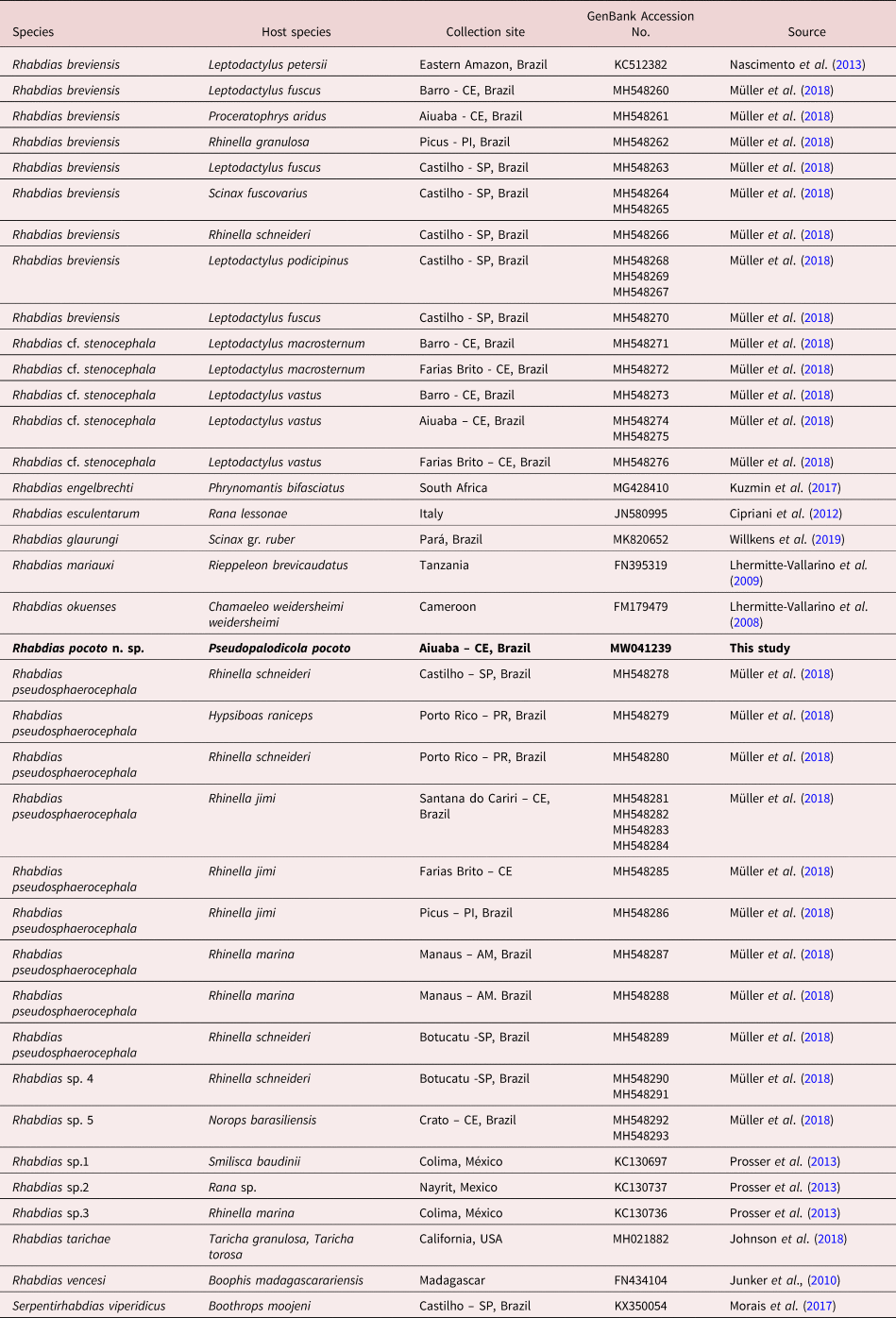
New sequence obtained is expressed in bold.
For the molecular phylogenetic analyses, we constructed a matrix using sequences of the coi gene of Rhabdias spp. obtained from GenBank (table 1). As an outgroup, the taxon chosen was Serpentirhabdias viperidicus Morais, Aguiar, Muller, Narciso, Silva & Silva, Reference Morais, Aguiar, Müller, Narciso, da Silva and Silva2017 (GenBank accession number KX350054). Sequences were aligned using the default parameters of the Muscle algorithm (Edgar, implemented in Geneious 7.1.3 (Kearse et al., Reference Kearse, Moir, Wilson, Stones-Havas, Cheung, Sturrock and Drummond2012). Stop codons and indels were also checked in Geneious 7.1.3 (Kearse et al., Reference Kearse, Moir, Wilson, Stones-Havas, Cheung, Sturrock and Drummond2012), translation frame 2, table 5 invertebrate mitochondrial. The substitution saturation index was estimated in DAMBE 5 (Xia, Reference Xia2013) to calculate its occurrence. The best-fit model for nucleotide substitution in the resulting matrix was GTR + I + G, determined by the Akaike information criterion (AIC) in a jModelTest (Posada, Reference Posada2008).
Phylogenetic reconstructions were performed using maximum likelihood (ML) and Bayesian inference (BI). ML inference was carried out using RAxML (Guindon & Gascuel, Reference Guindon and Gascuel2003) with bootstrap support values of 1000 repetitions. BI analyses were run using MrBayes (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003), with Markov chain Monte Carlo chains run for 10 million generations and sampling tree topologies for every 1000 generations. ‘Burn-in’ parameters were set to the first 25,000 generations. Both ML and BI phylogenetic analyses were performed on CIPRES (Miller et al., Reference Miller, Pfeiffer and Schwartz2010). The trees were visualized and edited using the FigTree v1.3.1 software program (Rambaut, Reference Rambaut2009). Genetic divergence was calculated for the coi gene matrix using the Kimura 2-parameter model, with 1000 bootstrap replicates in MEGA7 software (Kimura, Reference Kimura1980; Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011).
Results
Systematics
Order: Rhabditida Chitwood, 1933
Family: Rhabdiasidae Railliet, 1915
Rhabdias pocoto Morais, Melo & Müller n. sp.
Taxonomic summary
Type locality: Reservoir Benguê, Aiuaba municipality, state of Ceará, Brazil (06°35′35″S, 40°08′31″W).
Type host: Pseudopaludicola pocoto Magalhães, Loebmann, Nogueira, Kokubum, Baptista, Haddad & Garda, 2014 (Amphibia: Leptodactylidae: Leiuperinae).
Site of infection. Lung (mainly the right).
Numbers of specimens/hosts, prevalence, mean intensity of infection and range: a total of 125 nematodes were found in 115 frogs, P = 100%; Mean infection intensity (MII) = 1.09 (1–2).
Type material. Holotype and 22 paratypes were deposited at the CHIOC, under number: Holotype: CHIOC 38948 and paratypes: CHIOC 38942 – CHIOC 38963.
GenBank Accession number: MW041239.
ZooBank registration. The Life Science Identifier for R. pocoto n. sp. is 175D3EDE-61DD-4B1E-A5F6-34E13A8CD6B5.
Etymology. The new species is named after the specific epithet of the type-host.
Description
Based on the holotype and 22 paratypes, all gravid hermaphrodites. Body 5.88; 5.62 (3.41–7.43) mm long, curved dorsally, gradually tapering from mid-region to anterior end and expanding gradually from mid-region to posterior part of body, with a gradual narrowing of the caudal end (fig. 1a). Anterior end rounded, posterior end extremely pointed. Outer layers of body cuticle prominently swollen, especially at anterior end, with irregular transverse folds along entire body. Presence of lateral pores and ducts (figs 1a and 2a).

Fig. 1. Line drawings of Rhabdias pocoto n. sp.(a) Entire body, lateral view; (b) anterior end of body, en face view; (c) optical section through vestibulum; (d) optical section through anterior part (buccal capsule); (e) optical section through posterior part of buccal capsule; (f) anterior end of body, in lateral view; (g) mid-body region with vulva and (h) caudal end, lateral view.

Fig. 2. Rhabdias pocoto n. sp. scanning electron micrographs. (a) entire gravid hermaphrodite; (b) anterior end, lateral view (circle dashed lines, firm rounded dilation between inflation and body wall, Lp, lateral pore); (c) apical view, (arrowheads, cephalic papillae; asterisks amphids; Lp, lateral pore); (d) posterior end, lateral view.
Body width at vulva 287; 256 (135–380), width at esophago-intestinal junction 183; 195 (112–187). At anterior extremity, subapical lateral pores surrounded by rounded cuticular expansions on lateral sides of body. Both expansions filled with amorphous gland-like material (figs 1b, f and 2a, b). Oral opening round. Six small lips similar in shape and size; submedian lips located closer to edge of oral opening, lateral lips some distance away (figs 1b and 2c). Each lip associated with one papilla, amphidial openings located at base of lateral lips. Two external pores situated at the edge of oral region. Each pore connected to an amorphous material, glandular-like internal structure below the cuticular inflation (figs 1b, f and 2b, c). Vestibulum cylindrical, cuticularized, with narrow lumen (fig. 1c). Buccal capsule small, cup-shaped (fig. 1c–f). Anterior part of buccal capsule circular in shape, with smooth internal wall (fig. 1d), posterior part with irregularly folded internal surface (fig. 1e). Maximum diameter of buccal capsule 12; 12 (11–17), total depth of both parts 12; 12 (9–17); depth-to-width ratio 1; 1.1 (0.6–1.1). Oesophagus club shaped, 605; 598 (475–677) long, representing 11.6%; 10.8% (8.9–13.9%) of body length, with posterior bulb. Width of oesophagus 48; 53 (43–64) in anterior part; width of middle part 53; 57 (41–76), and 96; (65–137) in maximum width. Nerve-ring surrounding oesophagus, approximately at its mid-length, 303; 299 (238–339) from anterior end of body (fig. 1f). Excretory pore not observed. Intestine thick walled. Rectum short, funnel shaped and lined with thin cuticle. Contents of intestine brown throughout length (fig. 1a).
Genital system typical of Rhabdiasidae, amphidelphic with anterior and posterior ovaries (fig. 1a). Vulva equatorial, small, vulvar lips distinct, not salient, slightly prominent in some specimens, aperture short and transverse, weakly cuticularized (fig. 1h). Distance from anterior end to vulva 3.0; 2.8 (1.6–4.1) mm, representing 53% (39–64%) of body length. Uteri joined, sac-like, containing numerous eggs (>100 in total); eggs near vulva with fully developed larvae (fig. 1h). Eggs 83–101 × 45–54 (N = 9, measured in uteri close to vulva of holotype). Oviducts long, straight, thick walled, slightly shorter than uterus. Ovaries wide, elongated and reflected in oocyte zone, sometimes with one or two bends closer to the beginning of the oviducts (fig. 1a).
Tail comparatively short, conical (figs 1a, g and 2a, d), length 137; 135 (98–163) and representing 2.3%; 2.8% (2.8–5.8%) of body length. Presence of a pore-shaped phasmid located approximately 26; 27 (17–67) from the tail.
Remarks
The new species have been assigned to the genus Rhabdias based on molecular data and the following morphological characters: inflated body cuticle, small buccal capsule, amphidelphic reproductive system with a short transverse vagina and joined uteri; and due to its parasitism in the lungs of amphibian hosts. The number, shape, structure and position of the circumoral lips or pseudolabia, the relative position of the vulva and the shape of the tail are among the essential characters that can be used for specific diagnosis of the genus (Kuzmin et al., Reference Kuzmin, Tkach and Snyder2003; Nascimento et al., Reference Nascimento, Gonçalves, Melo, Giese, Furtado and Santos2013).
Rhabdias pocoto n. sp. differs from all congeneric species through the combination of a unique set of morphological characters: (1) body gradually expanding from mid-region to posterior part of the body and gradual narrowing of the caudal end; (2) at anterior end, two lateral pores connected to amorphous material, gland-like internal structure inside the cuticular inflation; (3) relatively short tail.
Therefore, considering the peculiar set of morphological characters in R. pocoto n. sp., the new host, record in the Caatinga biome and molecular data, we herein propose a new species, and will compare the new taxon with only those species reported in the Neotropical region which are morphologically similar to R. pocoto n. sp. However, as proposed by Willkens et al. (Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019), we will not use the species Rhabdias mucronata Schuurmans-Stekhoven, Reference Schuurmans-Stekhoven1952, and Rhabdias truncata Schuurmans-Stekhoven, Reference Schuurmans-Stekhoven1952 for comparison, as the author described those species based only on juvenile forms (Schuurmans-Stekhoven, Reference Schuurmans-Stekhoven1952).
Based on the oral structure arrangement, the new species resembles those from the group are characterized by having six lips (four submedian and two lateral lips): Rhabdias androgyna Kloss, Reference Kloss1971, Rhabdias breviensis, Rhabdias fuelleborni Travassos, 1926, Rhabdias galactonoti Kuzmin, Melo, Filho & Santos, Reference Kuzmin, Melo, Silva Filho and Santos2016, R. glaurungi, Rhabdias manantlanensis Martínez-Salazar, Reference Martínez-Salazar2008, and Rhabdias stenocephala Kuzmin, Melo, Filho & Santos, Reference Kuzmin, Melo, Silva Filho and Santos2016 (Martínez-Salazar, Reference Martínez-Salazar2008; Nascimento et al., Reference Nascimento, Gonçalves, Melo, Giese, Furtado and Santos2013; Kuzmin et al., Reference Kuzmin, Du Preez and Junker2015; Kuzmin et al., Reference Kuzmin, Melo, Silva Filho and Santos2016; Willkens et al., Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019). We will also compare R. pocoto n. sp. with the Neotropical species Rhabdias elegans Gutiérrez, Reference Gutiérrez1945 and Rhabdias hermaphrodita Kloss, Reference Kloss1971 as information about the arrangement of the circumoral structures was not reported by the authors.
The new species can easily be distinguished from R. androgyna by body length, as R. pocoto n. sp. is much smaller than R. androgyna (3.41–7.43 × 9.35–13.4 mm) (Kloss, Reference Kloss1971), smaller buccal capsule (11–17 × 11–17 μm in the new species vs. 19–27 × 7–9 μm wide in R. androgyna) and the particular shape of the anterior end of the body, as according to Kloss (Reference Kloss1971) and Kuzmin et al. (Reference Kuzmin, Du Preez and Junker2015), R. androgyna has a shoulder-like circular dilatation with an extended cuticular swelling arranged in two layers, while the new taxon has two lateral inflations of cuticle containing an amorphous gland-like structure around the sub-apical lateral pores.
Rhabdias breviensis parasitizing Leptodactylus petersii Steindachner, 1864 and Leptodactylus macrosternum Miranda-Ribeiro, 1926 resembles the new species by its dorsally curved body and parasitism of the hosts of the same family (Nascimento et al., Reference Nascimento, Gonçalves, Melo, Giese, Furtado and Santos2013). However, R. breviensis has a shorter and significantly wider body (2.63–3.63 mm long and 370–543 μm wide at vulva level). Additionally, R. breviensis has a smaller buccal capsule (7–13 width × 4–9 μm depth), a nerve-ring situated closer to the anterior end, and a post-equatorial vulvar opening. Although R. pocoto n. sp. and R. breviensis (139–191 μm) share a short and conical tail, the new taxon has a shorter tail (98–163 μm).
The description of R. elegans found parasitizing Rhinella arenarum Hensel, 1867 toads from Argentina, is not complete in Gutiérrez (Reference Gutiérrez1945). Furthermore, while Kloss (Reference Kloss1974) provided additional characters and measurements for the species, the author did not describe the structure of the oral aperture and lips. Rhabdias elegans and the new species possess an approximately similar body length and position of the vulva, though R. pocoto n. sp. has larger buccal capsule (11–17 μm in R. pocoto n. sp. vs. 7 × 9 μm in R. elegans; after Kloss, Reference Kloss1974) and longer oesophagus (475–677 μm) than R. elegans (314–490 μm) (Kloss, Reference Kloss1971). Additionally, according to Gutiérrez (Reference Gutiérrez1945) and Kloss (Reference Kloss1974), R. elegans do not exhibit cuticular inflation at the anterior end, which was observed in the new species.
Rhabdias fuelleborni parasitic in bufonid hosts (Kuzmin et al., Reference Kuzmin, Du Preez and Junker2015) is distinguished by being larger than R. pocoto n. sp. (10–12 mm), and despite the two species sharing a morphology of the internal surface of the anterior and posterior segments of the buccal capsule, R. fuelleborni has a larger buccal capsule (18–19 μm, based on the description of Kuzmin et al., Reference Kuzmin, Du Preez and Junker2015). Furthermore, according to the description of Kuzmin et al. (Reference Kuzmin, Du Preez and Junker2015), R. fuelleborni has a slightly narrower portion of the body at the level of the oesophageal apex, which was not observed in the new species.
Rhabdias galactonoti resembles the new species by its total length and dimensions of the buccal capsule. However, R. galactonoti differs in size and in the morphology of the internal surface (Kuzmin et al., Reference Kuzmin, Melo, Silva Filho and Santos2016) of the buccal capsule; in this species, the anterior part is transparent, circular in the apical view, with a regularly folded inner surface, while in R. pocoto n. sp. the anterior part is smooth. The position of the vulva in R. galactonoti is slightly pre-equatorial vs. the equatorial vulva of R. pocoto n. sp., and in the caudal region the cuticular inflation does not reach the tip of the tail in R. glactonoti (Kuzmin et al., Reference Kuzmin, Melo, Silva Filho and Santos2016), whereas in the new species it does. The former species is also more elongated and larger in size (229–333 vs. 98–163 μm) (Kuzmin et al., Reference Kuzmin, Melo, Silva Filho and Santos2016).
Rhabdias glaurungi found parasitizing the lungs of Scinax gr. ruber Laurenti, 1768 is similar to the new species in body length. The two species can easily be distinguished, however, because R. pocoto n. sp. has a larger buccal capsule (width × depth: 11–17 × 9–17 μm vs. 10–16 × 6–9 μm), larger relative proportions of the oesophagus, a different vulva position (equatorial in the new species vs. post-equatorial in R. glaurungi), and a short tail (98–163 μm (8.9–13.9%) vs. 151–216 μm (4.9–8.2%)) (Willkens et al., Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019).
As discussed by Willkens et al. (Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019), the description of R. hermaphrodita is superficial and incomplete, with no measurement of the buccal capsule or the structure of the apical region. The species described by Kloss (Reference Kloss1971) is similar in its equatorial vulva position (49% and 54% of the body length vs. 51% in R. pocoto n. sp.). However, R. hermaphrodita is larger than R. pocoto n. sp. (up to 12 mm total length), with a larger tail length (226–524 μm).
When compared with R. manantlanensis, the new species has a smaller body size (up to 6.48–9.64 mm long in R. manantlanensis), the cuticular swelling is more prominent at the anterior part of the body and present along the entire body, while according to the description of Martínez-Salazar (Reference Martínez-Salazar2008), the body cuticle of R. manantlanensis is not swollen or only slightly swollen, thin, and smooth. Furthermore, the two species differ in the buccal capsule (11–17 μm in R. pocoto n. sp. vs.19–27 μm wide in R. manantlanensis), and while both have a similar, comparatively short tail, the shape of the tail in the new species is remarkably different.
Rhabdias stenocephala also described from leptodactilyd frogs (Leptodactylus pentadactylus Laurenti, 1768 and Leptodactylus paraensis Heyer, 2005) is easily distinguished from the new species by the morphology of its anterior end (Kuzmin et al., Reference Kuzmin, Melo, Silva Filho and Santos2016). In this species, the anterior end has a narrow anterior part separated from the remainder of the body by a distinct constriction, which was not observed in R. pocoto n. sp. Additionally, they have almost identical buccal capsule dimensions (11–17 × 9–17 μm, in R. pocoto n. sp. vs. 9–11 × 15–18 μm, wide in R. stenocephala), and morphologies of the internal surface of the buccal capsule; the anterior part of the buccal capsule has a smooth internal wall in the new species, while the inner surface of the anterior part is irregularly folded in R. stenocephala (Kuzmin et al., Reference Kuzmin, Melo, Silva Filho and Santos2016).
Molecular analyses and phylogenetic study
We obtained the partial coi sequence (376 bp fragment) of R. pocoto n. sp., which for phylogenetic analysis was aligned with 44 other sequences retrieved from the GenBank database. From table 1 it can be seen that just 15 species were included in the ingroup identified as Rhabdias, and also in the outgroup Serpentirhabdias viperidicus (KX350054). All the sequences used for phylogenetic analyses are presented in table 1.
DAMBE revealed lower Iss values than Iss.cAsym and Iss.cSym values, which mean the lack of a saturation signal in the matrix. The final trimmed alignment comprised 298 characters with 46 sequences, and both ML and BI converged, with similar results clustering Rhabdias pocoto n. sp. with low support (0.85 for BI and 49 for ML) in the nodes within the Rhabdias clade A2″ (0.85 for BI and 18 for ML), closely related to the Rhabdias pseudosphaerocephala Kuzmin, Tkach & Brooks, 2007 species complex clade, grouped with R. glaurungi (MK820652) from Scinax gr. ruber, Rhabdias sp. (KC130736) from Rhinella marina Linnaeus, 1758 from Colima, Mexico, and Rhabdias sp. 5 (MH548293, MH548292) from Rhinella schneideri (=Rhinella diptycha Cope, 1862) (Clade A2″) (fig. 3).

Fig. 3. Maximum Likelihood phylogenetic topology of Rhabdias spp. of coi gene using Serpentirhabdias viperidicus as outgroup. Support values are above or below nodes: posterior probabilities <0.90 and bootstrap scores <70 are not shown or are represented by a dash. Branch-length scale bar indicates number of substitutions per site.
The genetic divergence using the coi gene of R. pocoto n. sp. compared to the Rhabdias spp. sequences used in the present study varied from 2% to 16%, with the lowest divergence observed within species of the R. pseudosphaerocephala complex (2%), and 16% within the outgroup (supplementary table S1).
Discussion
The morphological and biological features of the new species correspond to the diagnosis of Rhabdias proposed by Tkach et al. (Reference Tkach, Kuzmin and Snyder2014). Our morphological data identified several characters specific to the new taxon, mainly body shape, the structure of the apical end, and the length of the tail, which differentiate the species of our study from its congeners. Additionally, our molecular data grouped the new species with the R. pseudosphaerocephala species complex and identified divergence from the sequences previously deposited in GenBank.
In the first molecular phylogenetic study of Rhabdiasidae, Tkach et al. (Reference Tkach, Kuzmin and Snyder2014) inferred that the oral arrangement (presence or absence of various structures [e.g. lips, pseudolabia and submedian lips], variations in shape and position) represents one of the best characters for the differentiation of Rhabdias species, despite its limited usefulness for phylogenetic inferences.
Based on the set of morphological and molecular data produced (Tkach et al., Reference Tkach, Kuzmin and Snyder2014; Müller et al., Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018; Willkens et al., Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019) so far, we can observe three patterns of oral arrangements in species in the Neotropical region parasitizing amphibians and for which information about the oral arrangement is available: (1) four submedian lips and two lateral pseudolabia (five spp. R. manantlanensis, R. savage, R. kuzmini, R. pseudosphaerocephala and R. elegans]); (2) six equal lips (seven spp. [R. androgyna, R. fuelleborni, R. breviensis, R. galactonoti, R. glaurungi, R. stenocephala and R. tobagoensis]); and (3) absence of lips (two spp. R. alabialis and R. paraensis). Using molecular tools, in a recent study of Rhabdias populations of amphibians and lizards in Brazil, including those also from the Caatinga biome, Müller et al. (Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018) reported that all studied species (five species, 26 specimens) exhibited an oral arrangement with four submedian lips and two lateral pseudolabia (pattern 1). However, this pattern was not observed in the morphology of species with no molecular data; although pattern 1 includes most known species. Kuzmin (Reference Kuzmin2013) produced a schematic figure and the position of the circumoral structures and suggested that the transformation of the lateral lips into lateral pseudolabia is due to an evolutionary pattern.
Rhabdias pocoto n. sp. is the 19th species from the Neotropical region, the ninth from Brazil, the eighth species exhibiting pattern two (six lips) in the Neotropical region, and the first species with a unique feature that has not previously been observed in any of the known species of amphibian Rhabdias (two lateral cuticular inflations containing an amorphous gland-like structure around the sub-apical lateral pores). Lhermitte-Vallarino et al. (Reference Lhermitte-Vallarino, Junker and Bain2009) described a similar structure in Rhabdias brevicorne Lhermitte-Vallarino, Junker & Bain, Reference Lhermitte-Vallarino, Junker and Bain2009, found in a lizard from Madagascar, reported by the authors as a ‘dense internal circular tissue surrounding the mouth’ and called ‘cephalic pad’; however, they did not mention the presence of sub-apical lateral pores.
According to Langford & Janovy (Reference Langford and Janovy2013), host specificity varies greatly among species of Rhabdias, and the same authors suggest that ecological fitting plays a role in shaping such host specificity. Rhabdias pocoto n. sp. is the first Rhabdias found in frogs of the genus Pseudopaludicola and has a unique set of morphological characters. These features may indicate specific specialization in the host-parasite lineage and suggest a strict host specificity in the Rhabdias pocoto n. sp. system.
Moreover, these relationships can be observed at another taxonomic level, such as the R. pseudosphaerocephala species complex, which is commonly found associated with bufonids (Müller et al., Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018). Müller et al. (Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018) suggested the evidence for host–parasite cophylogeny between the R. pseudosphaerocephala complex and toads (Bufonidae). However, these authors also found a host spillover of this parasite in Boana raniceps Cope, 1862 (Hylidae) in southern Brazil. On the other hand, the R. breviensis species complex, found by Müller et al. (Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018), appears to be more generalist in terms of host family (associated with the Leptodactylidae, Bufonidae, Odontophrynidae and Hylidae species), suggesting that ecological fitting may be related to the host specificity of the Rhabdias species.
Tkach et al. (Reference Tkach, Kuzmin and Snyder2014) suggested that the colonization of reptiles has occurred more than once in the evolutionary history of Rhabdias and presume that host spillover and ecological fitting were more evolutionarily important than the association with particular host taxa. Thus, these findings suggest that host switching appears to be a hallmark of Rhabdias, which included numerous exchanges between different lineages of anurans.
The geographic distribution of the species of Rhabdias seems to follow a pattern where the species are restricted to their zoogeographic region (Tkach et al., Reference Tkach, Kuzmin and Snyder2014). Two lineages of Rhabdias (species complexes of R. pseudosphaerocephala and R. breviensis) are distributed across the south, southeast, and north-east of Brazil, and according to Müller et al. (Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018) and Willkens et al. (Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019), there are cryptic species in the Caatinga biome, in the north-east of Brazil, such as R. cf. stenocephala (in L. macrosternum Miranda-Ribeiro, 1926 and L. vastus Lutz, 1930) and Rhabdias sp. (in the lizard Norops brasiliensis Vanzolini & Williams, 1970).
Nevertheless, considering the distribution records of the P. pocoto frog, which is mainly found in the Caatinga domain (Silva et al., Reference Silva, Roberto, Ávila and Morais2017) and the results of helminth records associated with amphibians of other species in the biome (Teles et al., Reference Teles, Araujo-Filho, Ávila and Almeida2014, Reference Teles, Sousa, Teixeira, Silva, Oliveira, Silva and Ávila2015; Reference Teles, Pinto, Teixeira and Araujo-Filho2018a, Reference Teles, Brito, Araujo-Filho, Ribeiro, Teixeira, Mesquita and Almeidab; Araujo-Filho et al., Reference Araujo-Filho, Brito, Almeida, Morais and Ávila2015; Lins et al., Reference Lins, Aguiar, Morais, Silva, Ávila and Silva2017; Alcantara et al., Reference Alcantara, Ferreira-Silva, Silva, Lins, Ávila, Morais and Silva2018; Müller et al., Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018; Silva et al., Reference Silva, Ávila and Morais2018; Amorim et al., Reference Amorim, Olivera, Dyna, Sousa, Santos, Lima, Pinto and Ávila2019; Oliveira et al., Reference Oliveira, Ávila and Morais2019; Madelaire et al., Reference Madelaire, Franceschini, Morais, Gomes and Silva2020), we suggest the potential endemism of Rhabdias pocoto n. sp. in this biome, and considering the unique morphological character and specificity for the right lung of the host, suggest that the new taxon may have strict specificity for this species of host or host family.
Studies on the ecological aspects of the Rhabdias genus in Brazil are scarce, especially in the Caatinga biome. However, Silva et al. (Reference Silva, Ávila and Morais2018) studied the ecology of Rhabdias sp., and this species is finally described herein as Rhabdias pocoto n. sp. It was observed that the environmental characteristics of the type locality support the occurrence of larvae throughout the year, and parasitized frogs were collected in the dry and rainy seasons; additionally, it was noted that the host species can be exposed and act as a potential host for this helminth. The authors also observed that temperature does not influence the parasitological descriptors of this parasite, while high precipitation rates positively influence the infection rates of the Rhabdias population.
A priori, regions with high diversity are the best areas for studying a new parasite species and should be key targets of parasite discovery efforts (Jorge & Poulin, Reference Jorge and Poulin2018). The Caatinga biome has approximately 53 anuran species, approximately 12% of which are endemic (Garda et al., Reference Garda, Stein, Machado, Lion, Juncá, Napoli, Silva, Leal and Tabarelli2017), and most of which have unknown helminth fauna (Campião et al., Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Silva2014). We suggest that additional species of Rhabdias will be discovered in this region because of this large number of anuran species and the potential hosts for lungworms.
According to Poulin (Reference Poulin2007), host phylogeny is one determinant for host spillover and/or host switching. Therefore, it was expected that Rhabdias pocoto n. sp. would be a sibling group of the R. breviensis species complex, where both nematodes parasitize Leptodactylidae host specimens (see Nascimento et al., Reference Nascimento, Gonçalves, Melo, Giese, Furtado and Santos2013). However, we observed that the phylogenetic analysis using the coi gene positioned the new species as a sister group of the R. pseudosphaerocephala species complex + R. glaurungi (from Scinax gr. ruber) and Rhabdias sp. (KC130736) (from Rhinella marina from Colima, Mexico) (Clade A2″).
These findings suggest that the new species represent a distinct phylogenetic lineage that evolved among the R. pseudosphaerocephala species complex. However, nodal support was low and considered not supported (below 0.9 posterior probability for Bayesian inference and 70% bootstrap for ML inference), and this phylogenetic position might change in future studies when more taxa will be added to the phylogeny. The main clades A and B, despite the general low support in the nodes (apparently a common characteristic of the coi gene for Rhabdias spp.), corroborate the results demonstrated in recent studies (Müller et al., Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and Silva2018; Willkens et al., Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2019).
This record represents the first of Rhabdias described in frogs of the genus Pseudopaludicola, which is the basal taxon of Leptodactylidae (Pyron & Wiens, Reference Pyron and Wiens2011) and first Caatinga biome species. The evidence of the low diversity of Rhabdias (nine species) compared with the high diversity of amphibians from Brazil (1038 species) reveals the limited attempts to map global parasite diversity in Brazilian biomes, especially in the Caatinga biome.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X20000929.
Acknowledgements
We would like to thank our laboratory colleagues who helped us.
Financial support
Financial support for this study was provided by Fundação de Amparo a Pesquisa do Estado de São Paulo (the Sao Paulo State Research Support Foundation) – FAPESP (2011/20186-6 and 2012/24945-1), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (the Coordination for the Improvement of Higher Education Personnel) – CAPES, M.I.M. (grant number CAPES AUX-PE-PNPD 3005/2010, and the Young Researcher Program PROPE-UNESP 02/2016, FAPESP 2017/16546-3) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (the National Council for Scientific and Technological Development), (grant number 431809/2018-3 Universal) and for the research fellowships to DHM (# 313241/2018-0), FTVM (#304955/2018-3), RJS (#309125/2017-0) and RWA (# 303622/2015-6; 305988/2018-2).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work complied with all applicable institutional, national and international guidelines for the care and use of animals. Specimens were collected under license number ICMBio/SISBIO 29613-1; 55467-1) and authorized by the Ethics Committee of the Universidad Regional do Cariri (Cariri Regional University) (URCA no 00260/2016.1).






