Introduction
The subfamily Telotylenchinae, which contains Trophurus Loof, 1956, and related genera, represents a large group of plant-parasitic nematodes (Siddiqi, Reference Siddiqi2000; Geraert, Reference Geraert2011). According to Geraert (Reference Geraert2011), the subfamily Telotylenchinae contains nine genera: Histotylenchus Siddiqi, 1971, Neodolichorhynchus Jairajpuri & Hunt, 1984, Paratrophurus Arias, 1970, Quinisulcius Siddiqi, 1971, Sauertylenchus Sher, 1974, Telotylenchus Siddiqi, 1960, Trichotylenchus Whitehead, 1960, Trophurus and Tylenchorhynchus Cobb, 1913. These nematodes are known as stunt nematodes, and are obligate root ectoparasites of a large variety of plants (Siddiqi, Reference Siddiqi2000; Handoo et al., Reference Handoo, Palomares-Rius, Cantalapiedra-Navarrete, Liébanas, Subbotin and Castillo2014). In the subfamily Telotylenchinae, the female reproductive system of most genera is didelphic; however, Trophurus is the only genus where the posterior branch of the female reproductive system is reduced to a sac (Siddiqi, Reference Siddiqi2000; Geraert, Reference Geraert2011; Handoo et al., Reference Handoo, Palomares-Rius, Cantalapiedra-Navarrete, Liébanas, Subbotin and Castillo2014). Trophurus is a small genus, and only 15 species have been detected and described worldwide (Siddiqi, Reference Siddiqi2000; Geraert, Reference Geraert2011; Sen et al., Reference Sen, Chatterjee and Manna2012). According to the literature, the species of Trophurus are found mostly in cultivated soils in Africa, Asia, Europe, North and South America (Kleynhans & Cadet, Reference Kleynhans and Cadet1994; Sen et al., Reference Sen, Chatterjee and Manna2012). For example, Trophurus impar Ganguly & Khan, 1983 was described from the rhizosphere soil of the betel vine (Piper betel) in India (Ganguly & Khan, Reference Ganguly and Khan1983). Trophurus pakendorfi De Waele & Bolton, 1988 was collected from soil associated with sunflowers (Helianthus annuus) in Transvaal (De Waele & Bolton, Reference De Waele and Bolton1988). In China, Trophurus minnesotensis (Caveness, 1958) Caveness, 1959 has been identified and reported from soil associated with tobacco plants (Nicotiana tabacum) in Guizhou province (Li & Zhao, Reference Li and Zhao2012). In addition, several unidentified Trophurus species have been reported from Taiwan, Tianjin and the provinces of Guangdong and Hainan, China (Li & Zhao, Reference Li and Zhao2012). In 2015, a Trophurus species was collected from the soil associated with Cinnamomum camphora in Wuhu, Anhui Province, China, and is described here as a new species, Trophurus wuhuensis n. sp. The internal transcribed spacer sequences of ribosomal DNA (ITS rDNA) and partial 18S ribosomal DNA (18S rDNA) from T. wuhuensis n. sp. were amplified and sequenced. Phylogenetic relationships of the new species with other species in related genera were studied based on the 18S rDNA sequences.
Materials and methods
Collection and examination of nematodes
Soil associated with C. camphora was collected in Wuhu, Anhui Province, China. Nematodes were extracted from the soil sample using a modified Baermann funnel method (Hooper et al., Reference Hooper, Hallmann, Subbotin, Luc, Sikora and Bridge2005), and the Trophurus population was picked out by hand for examination.
Nematodes were killed by gentle heat, fixed in FG solution (formalin : glycerin : water = 10 : 1 : 89), transferred to anhydrous glycerin, using the method described by Seinhorst (Reference Seinhorst1959), and then mounted on permanent slides. The nematodes were measured, photographed and illustrated using a Nikon Eclipse Ti-S microscope equipped with a Nikon DS-Ri2 camera (Nikon, Tokyo, Japan). For scanning electron microscopy (SEM) studies, nematodes were fixed and processed according to the method described previously (Wang et al., Reference Wang, Xie, Li, Xu, Yu and Wang2013), and photographed with a Hitachi S-3400N electron microscope (Hitachi, Tokyo, Japan).
Molecular analysis
DNA of one individual nematode was extracted using proteinase K, as described by Wang et al. (Reference Wang, Zhang and Gu2011). Primers used for amplification of the ITS rDNA were as follows: rDNA1 (5′-TTGATTACGTCCCTGCCCTTT-3′) and rDNA2 (5′-TTTCACTCGCCGTTACTAAGG-3′) (Vrain et al., Reference Vrain, Wakarchuk, Levesque and Hamilton1992). The 18S rDNA was amplified as two partially overlapping fragments using two pairs of primers, according to the method described previously, and the primers were as follows: 988 F (5′-CTCAAAGATTAAGCCATGC-3′) and 1912R (5′- TTTACGGTCAGAACTAGGG-3′) for amplification of the first fragment; 1813 F (5′-CTGCGTGAGAGGTGAAAT-3′) and 2646R (5′-GCTACCTTGTTACGACTTTT-3′) for amplification of the second fragment (Holterman et al., Reference Holterman, van der Wurff, van den Elsen, van Megen, Bongers, Holovachov, Bakker and Helder2006). Polymerase chain reaction (PCR) amplifications were performed using KOD FX DNA polymerase (Toyobo, Osaka, Japan) in 25 μl of reaction mixture, as described by Wang et al. (Reference Wang, Xie, Li, Wu and Xu2016). The following PCR cycling steps were used to amplify the ITS rDNA: pre-denaturation at 94°C for 2 min, followed by 35 cycles (denaturation at 98°C for 10 s, annealing at 58.2°C for 30 s, extension at 68°C for 90 s) and final extension at 72°C for 10 min. The PCR conditions for 18S rDNA amplification were as described above, just changing the annealing temperature to 54°C. The PCR products were purified with a gel extraction kit (Omega, Norcross, Georgia, USA), ligated with pJET1.2/blunt cloning vectors (Thermo Scientific, Waltham, Connecticut, USA) and then sequenced by Sangon Biotech Co. Ltd (Shanghai, PR China). The newly obtained sequences in this study were submitted to the GenBank database.
The 18S rDNA sequences of T. wuhuensis n. sp. were compared with sequences of other nematode species in related genera in the GenBank database, using the nucleotide BLAST program from the National Center for Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequence (AY993976) of Boleodorus thylactus was chosen for the outgroup taxon, according to Handoo et al. (Reference Handoo, Palomares-Rius, Cantalapiedra-Navarrete, Liébanas, Subbotin and Castillo2014). Multiple alignments of the 18S rDNA sequences were performed using ClustalW in MEGA 5.05 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). Phylogenetic analysis of the sequence dataset was performed by Bayesian inference (BI) using MrBayes 3.2.6 (Huelsenbeck & Ronquist, Reference Huelsenbeck and Ronquist2001). The best-fit model (GTR+I+G) was obtained by Akaike Information Criterion (AIC) using MrModeltest 2.3 (Nylander, Reference Nylander2004). BI analysis was initiated with a random starting tree. Four Markov chains were run for 1 × 106 generations and sampled at intervals of 100 generations. After discarding burn-in samples, the remaining samples were used to generate a 50% majority rule consensus tree. Posterior probabilities (pp) were given on appropriate clades.
Results
Description of Trophurus wuhuensis n. sp.
Taxonomic summary
Type material. Holotype female, 16 paratype females and 4 paratype males are deposited in the Laboratory of Plant Pathology, Henan Agricultural University, Zhenzhou, Henan Province, PR China. Two paratype females are deposited at the University of California Riverside Nematode Collection (Riverside, California, USA).
Type habitat and locality. The new species was collected from the soil associated with C. camphora in Wuhu, Anhui Province, China.
Morphological description
Measurements. See table 1.
Table 1. Morphometics (μm) of Trophurus wuhuensis n. sp. from China. All measurements are in the form: mean ± SD (range); n, number of specimens observed; L, body length; a, L/max. width; b, L/pharyngeal length; c, L/tail length; c′ = tail length/anal body diameter; V, distance of vulva from anterior end × 100/L; V′, distance from anterior end to vulva × 100/distance from anterior end to anus; T, distance between cloaca and anterior-most part of testis × 100/L; m, metenchium length × 100/stylet length.
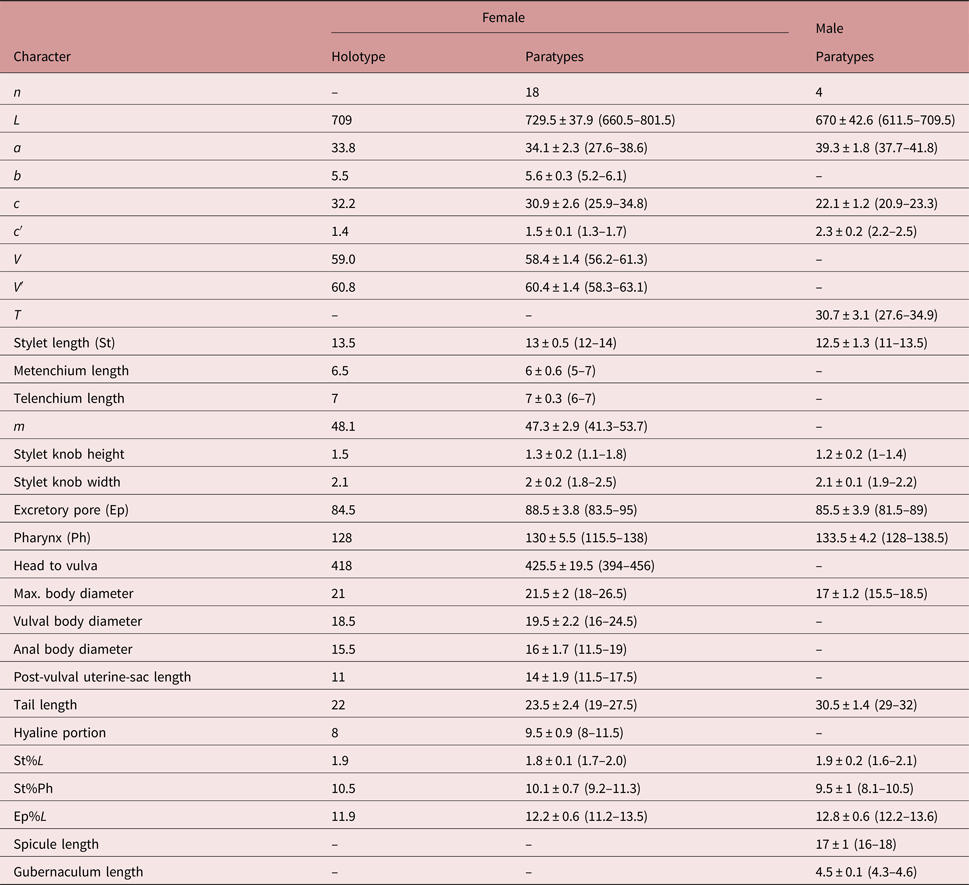
Female (figs 1a, c–i, 2a–l, 3a–i). Body vermiform, slender, straight to ventrally curved after heat relaxation. Body annuli very fine, 0.8 ± 0.1 (0.7–0.9) μm wide at mid-body. Lateral field 6 ± 0.4 (5–7) μm wide, with four distinct lines forming three bands. SEM photographs show that the three lateral bands are not smooth, but are marked by short and scattered grooves. Head sub-truncate, without transverse striae, and continuous with body contour. Cephalic framework weak. SEM view of the head shows a kind of rim on the top of the head of about 1.5 μm in diameter, surrounding a sunken area with apparently six papillae around an indistinct mouth opening; amphidial apertures not distinct, outside the rim, on the lateral side of the head. Stylet slender and straight, 13 ± 0.5 (12–14) μm long; stylet knobs rounded, directed laterad; cone occupying 47.3 ± 2.9 (41.3–53.7)% of stylet length. Isthmus slender, 29 ± 1.8 (23.5–31.5) μm long. Nerve ring at middle or slightly anterior region of isthmus. Basal bulb pyriform in shape, abuts intestine or the dorsal gland slightly overlaps intestine; cardia prominent. Excretory pore situated opposite middle of isthmus. Hemizonid situated 3–8 annuli posterior to excretory pore, 3–4 annuli wide. Vulva 425.5 ± 19.5 (394–456) μm from anterior end. Ovary single, outstretched, oocytes arranged in two rows. Spermatheca rounded or oval, 11 ± 1.2 (9–13) μm long and 10 ± 1.4 (7.5–13) μm wide, filled with round spermatozoa. Post-vulval uterine sac 14 ± 1.9 (11.5–17.5) μm long, about seven-tenths of vulval body diameter. Post-rectal intestinal sac absent. Tail cylindroid, hyaline tail portion 9.5 ± 0.9 (8–11.5) μm long. Tail terminus not smooth, slightly rough when being observed by the light microscope. SEM photographs show a broadly rounded terminus with deep wrinkles.

Fig. 1. Morphology of Trophurus wuhuensis n. sp. Female: (a) entire body, (c) anterior part, (d) anterior end and basal bulb, (e–f) reproductive system and lateral lines, (g–i) tails. Male: (b) entire body, (j) posterior part.

Fig. 2. Light micrographs of Trophurus wuhuensis n. sp. Female: (a) entire body, (b) anterior part, (c–d) anterior end, (e) oocytes in two rows, (f) spermatheca, (g) post-uterine sac, (h) lateral lines, (i–l) tails. Male: (m) entire body, (n) anterior end, (o) tail and spicule. Scale bars: (a, m) 50 μm; (b–l, n–o) 10 μm.

Fig. 3. Scanning electron micrographs of Trophurus wuhuensis n. sp. Female: (a) en face view; (b–c) head, oblique view; (d) vulva, lateral view; (e) vulva, ventral view; (f) lateral field marked by short and scattered grooves; (g) tail terminus with deep wrinkles; (h–i) tails. Scale bars: (a–c) 1 μm; (d–i) 5 μm.
Male (figs 1b, j, 2m–o). Similar to female except for reproductive system and tail shape. Testis outstretched, with small spermatozoa. Spicules distinct, 17 ± 1 (16–18) μm long. Tail tapering gradually to a pointed terminus. Bursa large, surrounding tail tip.
Diagnosis and relationships
Trophurus wuhuensis n. sp. is characterized by having females with a slender body 660.5–801.5 μm in length, stylet 12–14 μm long, knobs directed laterad, lateral field marked by short and scattered grooves, post-vulval uterine sac shorter than vulval body diameter, post-rectal intestinal sac absent and tail terminus with deep wrinkles; and by males with spicules 16–18 μm long.
When using the key to the species of Trophurus proposed by Kleynhans & Cadet (Reference Kleynhans and Cadet1994) and Geraert (Reference Geraert2011), and using the body length, stylet length and post-rectal intestinal sac absence as a guide, T. wuhuensis n. sp. is close to Trophurus clavicaudatus Sen, Chatterjee & Manna, 2012, Trophurus deboeri Kleynhans & Cadet, 1994, Trophurus lomus Saha, Chawla & Khan, 1974, Trophurus longimarginatus Roman, 1962, Trophurus marathwadensis Suryawanshi, 1971, Trophurus scognamiglii Talamé, 1974, Trophurus sculptus Loof, 1956 and Trophurus similis Khan & Nanjappa, 1971. Trophurus wuhuensis n. sp. can be separated easily from these eight species by having a rough tail terminus with deep wrinkles in the female. Some other characters separating T. wuhuensis n. sp. from these species are described below. The new species differs from T. clavicaudatus by having a shorter stylet length (12–14 μm vs. 17–17.5 μm), a shorter distance from excretory pore to anterior end (83.5–95 μm vs. 103–110 μm), a shorter tail length (19–27.5 μm vs. 51.5–59 μm), a higher c value (25.9–34.8 vs. 12.5–15.5) and a lower c′ value (1.3–1.7 vs. 3.0–3.9) in the female; and a shorter spicule length (16–18 μm vs. 22.5–24.5 μm) in the male (Sen et al., Reference Sen, Chatterjee and Manna2012). It differs from T. deboeri by having a lower a value (27.6–38.6 vs. 42–56), a lower c′ value (1.3–1.7 vs. 1.7–2.9), stylet knobs directed laterad vs. back-sloped, spermatheca rounded or oval vs. lobed, and post-vulval uterine sac shorter than vulval body diameter vs. about equal to vulval body diameter in the female (Kleynhans & Cadet, Reference Kleynhans and Cadet1994). It differs from T. lomus by having a shorter stylet length (12–14 μm vs. 16–18 μm), a shorter pharynx length (115.5–138 μm vs. 160 μm), a shorter tail length (19–27.5 μm vs. 33 μm) and a shorter distance from excretory pore to anterior end (83.5–95 μm vs. 105 μm) in the female; and a shorter spicule length (16–18 μm vs. 20–22 μm) and a shorter gubernaculum length (4.3–4.6 μm vs. 7–8 μm) in the male (Geraert, Reference Geraert2011). It differs from T. longimarginatus by having a shorter body length (660.5–801.5 μm vs. 840–1050 μm), a shorter stylet length (12–14 μm vs. 14–16 μm), a shorter tail length (19–27.5 μm vs. 32 μm) and a lower a value (27.6–38.6 vs. 41–50) in the female; and a longer spicule length (16–18 μm vs. 11–15 μm) in the male (Geraert, Reference Geraert2011). It differs from T. marathwadensis by having a shorter body length (660.5–801.5 μm vs. 1030–1210 μm), a shorter stylet length (12–14 μm vs. 15–16 μm), a shorter tail length (19–27.5 μm vs. 37 μm), oocytes arranged in two rows vs. in a single row, a lower a value (27.6–38.6 vs. 43–50) and a lower b value (5.2–6.1 vs. 6.2–7.4) in the female; and a shorter spicule length (16–18 μm vs. 19–22 μm) in the male (Kleynhans & Cadet, Reference Kleynhans and Cadet1994; Geraert, Reference Geraert2011). It differs from T. scognamiglii by having a shorter body length (660.5–801.5 μm vs. 850–1010 μm), a shorter stylet length (12–14 μm vs. 14.5–17 μm), a shorter pharynx length (115.5–138 μm vs. 160 μm) and ovary outstretched vs. plicate in the female; and a shorter spicule length (16–18 μm vs. 22 μm) in the male (Geraert, Reference Geraert2011). It differs from T. sculptus by having a shorter tail length (19–27.5 μm vs. 29–44 μm), a higher c value (25.9–34.8 vs. 16–24), spermatheca rounded or oval vs. bilobed, and vagina without epiptygma vs. with small double epiptygma in the female (Geraert, Reference Geraert2011); and from T. similis by having a lower a value (27.6–38.6 vs. 40–58), a longer stylet length (12–14 μm vs. 9–11 μm), a shorter tail length (19–27.5 μm vs. 36 μm) and a higher c value (25.9–34.8 vs. 18–25) in the female (Geraert, Reference Geraert2011).
Molecular characterization and phylogenetic relationships
Two ITS rDNA sequences of T. wuhuensis n. sp. were obtained and submitted to the GenBank database under accession numbers MF139731–MF139732. The length of the two ITS sequences was 1045–1046 bp with 6 bp variation. At present, no other ITS sequences of Trophurus species are available in the GenBank database.
Four 18S rDNA sequences of T. wuhuensis n. sp. were obtained and submitted to the GenBank database under accession numbers MF139733–MF139736. The length of the four 18S sequences was 1748 bp with 1–3 bp variation. The BLAST search showed that the 18S sequences of the new species were closest to the sequence from T. imperialis Loof, 1956 (FJ969144) within the sequenced Trophurus species. Identity between the 18S sequences from T. wuhuensis n. sp. and T. imperialis was 95%. Alignment of the 18S rDNA contained 19 sequences with 1685 positions in length. The 50% majority rule consensus tree reconstructed from the 18S dataset by the Bayesian analysis is shown in fig. 4. In this tree, four 18S rDNA sequences of T. wuhuensis n. sp. clustered together, and formed a 100% supported clade with that of T. imperialis. Other selected nematode species in the subfamily Telotylenchinae, including Neodolichorhynchus microphasmis, Sauertylenchus maximus, Telotylenchus ventralis and Tylenchorhynchus claytoni were in a 89% supported monophyletic clade and sister to the two Trophurus species (T. wuhuensis n. sp. and T. imperialis) with high support (pp = 100%).

Fig. 4. The 50% majority rule consensus tree inferred from the 18S rDNA sequences of Trophurus wuhuensis n. sp. and some other species in related genera under the GTR+I+G model; posterior probabilities more than 50% are given for appropriate clades; newly obtained sequences are indicated in bold font.
Discussion
In this study, a new species, T. wuhuensis n. sp. was obtained from C. camphora in Anhui Province, China, and thus the current number of Trophurus species is increased to 16. Kleynhans & Cadet (Reference Kleynhans and Cadet1994) gave a dichotomous key to species of Trophurus in which body length, stylet length, tail shape, shape of stylet knobs, a value, c value, etc. were used to differentiate Trophurus species. These species usually show considerable variation in many characters. For example, the basal bulb is usually offset from the intestine, but the dorsal gland may extend slightly over the intestine; the posterior branch of the female reproductive system is completely regressed and represented by a uterine sac, but often carries rudiments of posterior ovary; spermatheca oval, rounded, lobed or bilobed; post-rectal intestinal sac absent or present; female tail terminus may be distinctly annulated, appearing smooth or rough with deep wrinkles (Kleynhans & Cadet, Reference Kleynhans and Cadet1994; Siddiqi, Reference Siddiqi2000; Geraert, Reference Geraert2011). Sher & Bell (Reference Sher and Bell1975) reported that Trophurus sp. did not show any structures on the lip region, not even the amphid apertures or oral opening, when examined by SEM. In the observation of T. wuhuensis n. sp., the SEM view of the head showed a kind of rim on the top of the head, surrounding a sunken area with apparently six papillae around an indistinct mouth opening; amphidial apertures were outside the rim, on the lateral side of the head. In addition, the tail terminus of the new species was slightly rough when observed by light microscopy; however, SEM photographs showed a broadly rounded terminus with deep wrinkles. Thus, we believe that the SEM micrographs will provide many useful characters with which to identify Trophurus species.
Molecular data become more and more valuable in the identification of closely similar species. However, there is no molecular information available on most Trophurus species. Therefore, we believe that, in the future, the use of molecular data will also make the identification of Trophurus more accurate.
Financial support
This work was supported by the China Postdoctoral Science Foundation (no. 2015M582185), National Natural Science Foundation of China (no. 31601619) and Special Fund for Agro-Scientific Research in the Public Interest of China (no. 201503112).
Conflict of interest
None.







