Introduction
The Monorchiidae Odhner, 1911 is a major family of trematodes of marine fishes, presently comprising over 50 genera and over 260 species (Cribb & Gibson, Reference Cribb and Gibson2010; Atopkin et al., Reference Atopkin, Besprozvannykh, Ngo, Van Ha, Van Tang, Ermolenko and Beloded2017). This study commences an analysis of the monorchiid trematodes of fishes of Moreton Bay, Queensland Australia. Despite the richness of the fish fauna in this region, 1190 species according to Johnson (Reference Johnson2010), just two monorchiid species have been reported: Ovipusillus mayu Dove & Cribb, 1998 and Hurleytrematoides loi McNamara & Cribb, 2011 (see Dove & Cribb, Reference Dove and Cribb1998; McNamara & Cribb, Reference McNamara and Cribb2011). In the present article, in addition to describing a new species and reporting new records of a known species on the basis of morphology, we commence a systematic programme of reporting second internal transcribed spacer (ITS2) and 28S rDNA sequences as routine for as many taxa as possible. ITS2 sequences have proven valuable in the distinction of trematode species in general and monorchiids in particular (McNamara et al., Reference McNamara, Miller and Cribb2014; Bray et al., Reference Bray, Palm, Cutmore and Cribb2017) and 28S rDNA sequences have proven widely informative for the inference of phylogenetic relationships of many trematode taxa (e.g. Bray & Cribb, Reference Bray and Cribb2012; Cutmore et al., Reference Cutmore, Miller, Curran, Bennett and Cribb2013). There has been remarkably little phylogenetic analysis of the relationship of monorchiids, and we begin here to build upon the preliminary analyses reported by Searle et al. (Reference Searle, Cutmore and Cribb2014) and Atopkin et al. (Reference Atopkin, Besprozvannykh, Ngo, Van Ha, Van Tang, Ermolenko and Beloded2017).
We report here a new species from the surf bream, Acanthopagrus australis (Günther, 1859) (Sparidae), together with sequence information for one previously described species.
Materials and methods
Collection and morphological analysis
Fishes of the families Carangidae and Sparidae were collected from Moreton Bay (Queensland) by line fishing and seining, as well as being sourced from commercial tunnel-netting fisheries. Fishes were euthanized with AquiS® and examined following the recommendations of Cribb & Bray (Reference Cribb and Bray2010). Trematodes were fixed by pipetting into near-boiling saline and were preserved in 70% ethanol for parallel morphological and molecular characterization. Specimens for morphological analysis were washed in fresh water, stained with Mayer's haematoxylin, destained in a solution of 1.0% hydrochloric acid and neutralized in 0.5% ammonium hydroxide solution. Specimens were then dehydrated through a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Measurements were made using an Olympus SC50 digital camera mounted on an Olympus BX-53 compound microscope using cellSens Standard imaging software (Olympus, Tokyo, Japan). Measurements are in micrometres (μm) unless otherwise stated, and are presented as a range, followed by the mean in parentheses. Where length is followed by width, the two measurements are separated by ‘×’. Drawings were made using an Olympus BX-53 compound microscope and drawing tube. Type and voucher specimens are lodged in the Queensland Museum (QM), Brisbane, Australia.
We tested the significance of differences in the prevalence of monorchiids in Moreton Bay sparids with the Unconditional Exact Test in the software Quantitative Parasitology 3 (Rozsa et al., Reference Rozsa, Reiczigel and Majoros2000; Reiczigel et al., Reference Reiczigel, Abonyi-Toth and Singer2008).
Molecular sequencing
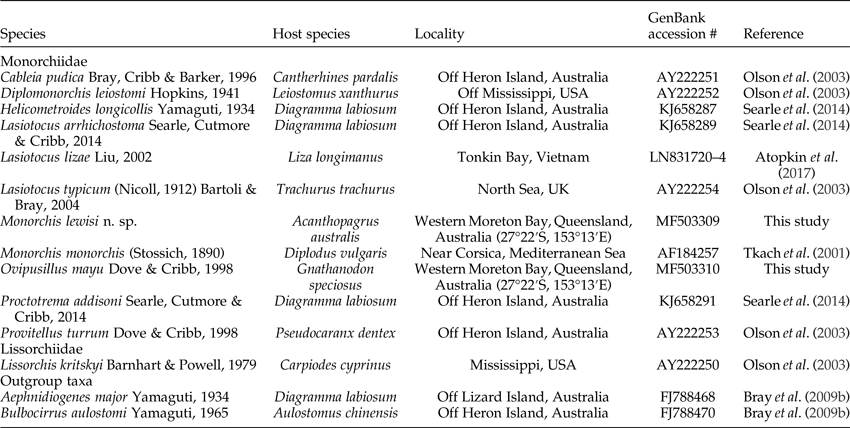
Total genomic DNA was extracted using phenol/chloroform extraction techniques (Sambrook & Russell, Reference Sambrook and Russell2001). Sequence data for two markers of ribosomal DNA (rDNA) were generated for this study, the complete ITS2 rDNA and the partial (D1–D3 region) 28S rDNA region. Cycle sequencing of these regions was carried out following the protocols of Cutmore et al. (Reference Cutmore, Diggles and Cribb2016) using the primers LSU5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC A-3′; Littlewood, Reference Littlewood1994) and 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′; Snyder & Tkach, Reference Snyder and Tkach2001) for the 28S amplification and 3S (3S: 5′-GGT ACC GGT GGA TCA CGT GGC TAG TG-3′; Morgan & Blair, Reference Morgan and Blair1995) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′; Cribb et al., Reference Cribb, Anderson, Adlard and Bray1998) for the ITS2 amplification. Amplified DNA was purified using a Bioline ISOLATE II PCR and Gel Kit according to the manufacturer's protocol (Bioline, London, UK). Sanger sequencing of purified DNA was conducted at the Australian Genome Research Facility using ABI Big Dye™ v.3.1 chemistry (Applied Biosystems, Carlsbad, California, USA) following the manufacturer's recommendations, using the same primers as those used for polymerase chain reaction (PCR) amplification as well as the additional 28S rDNA primers 300 F (5′-CAA GTA CCG TGA GGG AAA GTT G-3′; Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000) and ECD2 (5′-CCT TGG TCC GTG TTT CAA GAC GGG-3′; Littlewood et al., Reference Littlewood, Rohde and Clough1997). Sequencher™ version 4.5 (GeneCodes Corp., Ann Arbor, Michigan, USA) was used to assemble and edit contiguous sequences. The start and the end of the ITS2 region were determined by annotation through the ITS2 Database (Keller et al., Reference Keller, Schleicher, Schultz, Muller, Dandekar and Wolf2009; Ankenbrand et al., Reference Ankenbrand, Keller, Wolf, Schultz and Forster2015) using the ‘Metazoa’ model. GenBank accession numbers for the sequences generated during this study are presented in table 1.
Table 1. 28S rDNA sequence data used in phylogenetic analyses.
Phylogenetic analysis
The partial 28S rDNA sequences generated during this study were analysed together with sequences of monorchiid species available from GenBank (table 1). Sequences were aligned using MUSCLE version 3.7 (Edgar, Reference Edgar2004) with ClustalW sequence weighting and UPGMA clustering for iterations 1 and 2. The resultant alignments were refined by eye using MESQUITE (Maddison & Maddison, Reference Maddison and Maddison2017) and the ends of each fragment were trimmed to match the shortest sequence in the alignment.
Bayesian inference and Maximum Likelihood analyses of the 28S dataset were conducted to explore relationships among monorchiid taxa. Bayesian inference analysis was performed using MrBayes version 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Hohna, Larget, Liu, Suchard and Huelsenbeck2012), run on the CIPRES portal (Miller et al., Reference Miller, Pfeiler and Schwartz2010). Maximum Likelihood analysis was performed using RAxML version 7.7.1 (Stamatakis et al., Reference Stamatakis, Hoover and Rougemont2008). The software jModelTest version 2.1.10 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) was used to estimate the best nucleotide substitution model for the dataset. Both the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) in jModelTest predicted the GTR + Γ model as the best estimator. Nodal support in the Maximum Likelihood analysis was estimated by performing 100 bootstrap pseudoreplicates. Bayesian inference analysis was run over 10,000,000 generations (ngen = 10,000,000) with two runs each containing four simultaneous Markov Chain Monte Carlo (MCMC) chains (nchains = 4) and every 1000th tree was saved (samplefreq = 1000). Bayesian inference analysis used the following parameters: nst = 6, rates = gamma, ngammacat = 4 and the priors parameters of the combined dataset were set to ratepr = variable. Samples of substitution model parameters, and tree and branch lengths were summarized using the parameters ‘sump burnin = 3000’ and ‘sumt burnin = 3000’. Species of the family Lepocreadiidae were designated as the functional outgroup.
Results
Monorchiidae Odhner, 1911; Monorchis Monticelli, 1893; Monorchis lewisi n. sp.
Taxonomic summary
Type host
Acanthopagrus australis (Günther, 1859), surf bream (Perciformes: Sparidae).
Type locality
Peel Is., eastern Moreton Bay, Queensland, Australia 27°24′S, 153°20′E.
Other localities
Moreton Bay, Queensland, Australia: Amity Point, North Stradbroke Island, 27°24′S, 153°20′E; Green Is., 27°24′S, 153°20′E; Juminpin, 27°44′S, 153°26′E; Port of Brisbane, 27°23′S, 153°11′E; Wellington Pt, 27°28′S, 153°15′E; Wynnum Nth, 27°25′S, 153°11′E. New South Wales: Iluka, 29°22′S, 153°21′E; New Brighton, 28°31′S, 153°33′E.
Site in host
Intestine.
Prevalence
21/242 (Moreton Bay).
Deposition of specimens
Holotype: QM G236250; paratypes: QM G236251–62.
ZooBank registration
To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature, details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Monorchis lewisi n. sp. is urn:lsid:zoobank.org:act:AAA5838A-4B7D-454A-9E3B-77035ED7A4EC.
Molecular sequence data
ITS2 rDNA, two identical replicates (one submitted to GenBank MF503313); 28S rDNA, one sequence (GenBank MF503309).
Etymology
This species is named for Professor John Lewis, editor of the Journal of Helminthology for more than 20 years, in honour of his major contribution to the field of helminthology.
Description
Measurements based on 13 gravid, unflattened, whole-mount specimens. Body oval to elongate oval, often with distinctly flattened posterior extremity in larger specimens, with maximum width in hindbody, 371–795 (613) × 199–434 (327). Length/width ratio 1.64–2.07 (1.87):1. Tegument prominently spined throughout, except on ventral sucker. Forebody 133–283 (209) long, occupying 32.4–39.6% (34.4%) of body length. Hindbody 159–449 (321) long, occupying 42.9–56.5% (51.8%) of body length. Oral sucker opening ventro-sub-terminally, spherical to sub-spherical, 74–119 (97) × 89–131 (115). Ventral sucker rounded, 57–97 (81) × 62–106 (88). Oral sucker width to ventral sucker width ratio 1.12–1.55 (1.31):1. Prepharynx very short, visibly separating oral sucker and pharynx in only one specimen. Pharynx distinctly smaller than oral sucker, 45–85 (63) × 54–91 (74). Oesophagus short, frequently not distinguishable, 0–21 (6) long. Caeca blind, terminating 23–63 (42) from posterior end of body. Testis single, usually entire, occasionally slightly lobed, usually wider than long, close to but usually slightly posterior to ventral sucker, 71–180 (111) × 88–175 (128). Post-testicular region 94–269 (184) long, occupying 22.3–34.0% (29.5%) of body length. Cirrus sac extends from just posterior to, to slightly anterior to, posterior margin of ventral sucker, opening into median genital atrium (fig. 1a), 68–188 (146) (straight-line length) × 28–85 (67). Internal seminal vesicle single-chambered, prominent; external seminal vesicle absent (fig. 1b). Pars prostatica not distinct, but prostatic cells fill available space outside seminal vesicle and ejaculatory duct. Eversible ejaculatory duct armed with prominent spines. Genital atrium unspined. Common genital pore median, distinctly anterior to ventral sucker, bifurcal. Ovary strikingly variable, ranging from apparently entire to somewhat irregular two- and three-lobed forms (fig. 1c), postero-dextral to and contiguous with ventral sucker, always reaching anterior to posterior margin of ventral sucker but never beyond anterior margin, 46–175 (108) × 60–153 (102). Vitellarium comprising discrete lateral groups of follicles between level of pharynx and ventral sucker, 99–149 (125) from anterior end of body and 218–498 (367) from posterior end of body; number of distinguishable individual follicles increasing with body size from minimum of 3 to at least 8 on each side. Uterus restricted to hindbody where profuse coils extend posteriorly beyond testis and laterally beyond intestinal caeca, enters terminal organ towards its middle. Terminal organ bipartite, comprising posterior unspined and spiny anterior chambers, 53–139 (107) × 31–81 (56); spiny portion equivalent to or shorter than spiny ejaculatory duct. Egg capsules without filament, 21–27 (24) × 9–13 (11). Excretory vesicle I-shaped, extends to about level of ventral sucker, 225–495 (355) long. Excretory pore terminal.

Fig. 1. Monorchis lewisi n. sp.: (a) adult worm, ventral; (b) terminal genitalia, ventral; (c) variation in body size and shape, and conformation of ovary and testis.
Remarks
This species showed a surprising level of variation in that the total length of gravid specimens ranged from 371 to 795 μm, greater than we have seen in other monorchiids with which we are familiar. Relative to the smallest specimens, larger specimens are noticeably more elongate, tend towards a more flattened posterior extremity, frequently have a distinctly lobed rather than entire ovary, and have a larger number of vitelline follicles. We explored the possibility that more than one species was present but found evidence that these features formed a continuum from the smallest to the largest specimens (fig. 1c).
We note that the prevalence of this species in A. australis in Moreton Bay (21/242) is relatively low, but contrasts with the complete absence from the other two sparids examined in the area (71 Rhabdosargus sarba (Forsskål, 1775) and 27 Chrysophrys auratus (Forster, 1801); Unconditional Exact Test P = 0.006 and 0.1467, respectively, relative to prevalence in A. australis).
Ovipusillus Dove & Cribb, 1998; Ovipusillus mayu Dove & Cribb, 1998
New records: taxonomic summary
Host
Gnathanodon speciosus (Forsskål, 1775), Golden Trevally (Perciformes: Carangidae).
Localities
Eastern Moreton Bay, Queensland, Australia – Peel Is., 27°24′S, 153°20′E; Green Is., 27°24′S, 153°20′E.
Site in host
Intestine.
Prevalence
4/8.
Deposition of voucher specimens
QM G236263–236267.
Molecular sequence data
ITS2 rDNA, three identical replicates (one submitted to GenBank MF503314); 28S rDNA, one sequence (GenBank MF503310).
Remarks
New specimens of this species from the type host and locality agreed with the original description (Dove & Cribb, Reference Dove and Cribb1998) in all respects (fig. 2). This species has not been reported since its original description and no sequence data are available. Here we report identical ITS2 rDNA sequences from specimens taken from three individual fishes. We analysed a single partial 28S rDNA sequence for phylogenetic position (see below).

Fig. 2. Ovipusillus mayu Dove & Cribb, 1998. New record from intestine of Gnathanodon speciosus from Moreton Bay.
Phylogeny
Maximum Likelihood and Bayesian inference analyses of the partial 28S rDNA dataset produced identical topologies in which many clades were poorly supported (fig. 3). Only two clades had strong support, that comprising species of Lasiotocus Looss in Odhner, 1911, Monorchis, Ovipusillus, Proctotrema Odhner, 1911, Provitellus Dove & Cribb, 1998 and Diplomonorchis Hopkins, 1941, and that of the two species of Monorchis plus two of the species of Lasiotocus. Only two genera in the analysis have more than one species (Monorchis and Lasiotocus) and in neither case did the congeneric species form a clade.

Fig. 3. Phylogenetic relationships of Monorchiidae based on partial 28S rDNA sequences. Bayesian inference analysis support is given above the nodes; Maximum Likelihood support below the nodes. Support <85% is not shown. O., outgroup; L., Lissorchiidae.
Discussion
Taxonomy
The species reported here from A. australis agrees well with the concept of Monorchis as recognized by Madhavi (Reference Madhavi, Bray, Gibson and Jones2008). Key features in this respect are the oval body, vitellarium in the forebody, single testis, absence of an external seminal vesicle and unfilamented eggs. According to Gibson (Reference Gibson2013), and our analysis of the literature, 12 species of Monorchis were recognized prior to the present work. (One further species requires mention. Monorchis polyipini Reimer, 1985 was reported from two salmoniform fishes from off Mozambique by Reimer (Reference Reimer1985). This species was later transferred to Bathymonorchis Bray & Gaevskaya, 1993 as the type- and only species of that genus (Bray & Gaevskaya, Reference Bray and Gaevskaya1993). However, Madhavi (Reference Madhavi, Bray, Gibson and Jones2008), in her review of the family, continued to recognize this species as belonging to Monorchis. This species has a body shape completely different from the present species and confluent rather than separate vitelline follicles.) The 12 species generally recognized as belonging to Monorchis are reported from a wide range of fishes and incorporate significant morphological variation, to the point that we think it unlikely that all will ultimately prove to belong to a single genus. The consistently long I-shaped excretory vesicle reaching to the level of the ventral sucker differentiates the present form from seven species in which the vesicle is reported, or clearly shown, as V- or Y-shaped: M. monorchis (Stossich, 1890) (type species), M. bengalensis Dutta & Manna, 1997, M. blennii Jousson & Bartoli, 2002, M. hermani Issa, 1963, M. latus Manter, 1942, M. parvus Looss, 1902 and M. xiamenensis Liu, 1995. Just three species have I-shaped excretory vesicles: M. diplovarium Mamaev, 1971, M. fusiformis Wang, 1982 and M. minutus Madhavi, 1977. The condition of the excretory vesicle of M. heterorchis Bilqees, 1980 and M. japonicus Zhukov, 1970 remains unreported. Monorchis heterorchis is clearly distinct from the present form (and perhaps from the genus as a whole) in having the ovary deeply lobed to the point that it is almost follicular. Monorchis japonicus is clearly distinct from the present form in having a massive testis which is at the posterior end of the body and is not exceeded posteriorly by the intestinal caeca. The excretory vesicle of M. fusiformis is very short, reaching only to the mid-hindbody, and thus quite different from the condition in the present form. Distinction of the present form relative to M. diplovarium and M. minutus requires more extended consideration.
Monorchis diplovarium and M. minutus, both reported from haemulids, generally resemble the present form. Monorchis diplovarium, from the silver grunt Pomadasys argenteus (Forsskål) (as P. hasta) in the Gulf of Tonkin, resembles the present form in most features. Its reported size range, 450–580 μm (Mamaev, Reference Mamaev, Oshmarin, Mamaev and Lebedev1971), is within that recorded here. However, we detect three significant points of difference. First, the caeca of M. diplovarium terminate well short of the posterior end of the body, rather than close to it as in the present specimens. Second, the cirrus sac occupies a noticeably larger proportion of the body length in M. diplovarium than in the present species. Finally, the pharynx of M. diplovarium is significantly smaller, only 40–45 in diameter relative to 45–85 × 54–91 for M. lewisi n. sp. Monorchis minutus, from the saddle grunt Pomadasys maculatus (Bloch) in the Bay of Bengal, is reported to reach only 387–560 μm in length (Madhavi, Reference Madhavi1977), comparable to, but with a smaller range, than the present form. In line with the relatively small size of the ‘numerous’ specimens examined by Madhavi (Reference Madhavi1977), most measured features are slightly smaller than those for M. lewisi n. sp. Notably, there is no indication that the ovary is ever other than entire, as seen in the smaller specimens reported here. The intestinal caeca are reported to reach to close to the posterior extremity, as in the present specimens. The only clear distinction relative to M. lewisi n. sp. is a striking difference in the size of the pharynx; 33–39 × 43–51 in M. minutus relative to 45–85 × 54–91 in M. lewisi n. sp. This distinction translates into a dramatic difference in oral sucker width to pharynx width ratio, 2.45 in the figured holotype of M. minutus relative to 1.44–1.77 (1.55) in M. lewisi n. sp.
We suspect that the nature of the host specificity of the present specimens, apparently restricted to one sparid fish species, supports the distinction between it and M. diplovarium and M. minutum. The evidence for trematodes in general (Miller et al., Reference Miller, Bray and Cribb2011), monorchiids in general (e.g. McNamara & Cribb, Reference McNamara and Cribb2011) and some species of Monorchis in particular (Jousson & Bartoli, Reference Jousson and Bartoli2002), is that their host specificity tends to be high. However, several monorchiids have been reported to infect both haemulids and sparids: Diplomonorchis leiostomi Hopkins, 1941 by Nahhas & Powell (Reference Nahhas and Powell1965) and Overstreet (Reference Overstreet1969); Lasiotocus beauforti (Hopkins, 1941) Thomas, 1959 by Nahhas & Powell (Reference Nahhas and Powell1965); Lasiotocus lintoni (Manter, 1931) Thomas, 1959 by Nahhas & Powell (Reference Nahhas and Powell1965); and Lasiotocus truncatus (Linton, 1910) Thomas, 1959 by Nahhas & Cable (Reference Nahhas and Cable1964). However, if the present species was indeed conspecific with either M. diplovarium or M. minutum, it would be surprising that it was not found in the other sparids found co-occurring with A. australis or the 12 species of haemulids we have examined in Moreton Bay and on the Great Barrier Reef. Further compounding the identification problem is the uncertain significance of the geographical separation of the present species and those previously reported from the Bay of Bengal (M. minutus) and the Gulf of Tonkin (M. diplovarium). As reviewed recently (Cribb et al., Reference Cribb, Bray, Diaz, Huston, Kudlai, Martin, Yong and Cutmore2016), there is conflicting evidence for both widespread and narrowly distributed species in the Indo-West Pacific. Overall, however, there is so little evidence based on molecular analysis of identity over range that we are unable to have any expectation that these species will be either widespread or narrowly distributed.
In the light of these deliberations, we conclude that it is best to propose a new species for the present form. In our view, realistic testing of this hypothesis will depend on the production of molecular data for all the forms concerned. Molecular data was critical to distinguishing closely related Mediterranean species of Monorchis (Jousson & Bartoli, Reference Jousson and Bartoli2002) and we hope that the sequence information (especially ITS2 rDNA) provided here will prove similarly useful in the future.
Phylogenetic relationships
The present analysis adds 28S rDNA sequence data for a second species of Monorchis, in addition to that of M. monorchis reported by Tkach et al. (Reference Tkach, Pawlowski, Mariaux, Swiderski, Littlewood and Bray2001), and for Ovipusillus mayu, the sole species of the genus. The coverage of monorchiid genera, of which there are nearly 50, remains weak, with only 11 species putatively relating to eight genera represented in the 28S rDNA dataset. Overall resolution from the analysis is poor; it seems likely that additional markers will be needed in addition to the present partial 28S rDNA sequences if the relationships of these taxa are to be fully resolved. The Monorchiidae as a whole is weakly supported relative to Lissorchis kritskyi Barnhart & Powell, 1979. Lissorchis kritskyi is the sole species of Lissorchiidae Magath, 1917 for which a substantial amount of 28S rDNA data is available. Besprozvannykh et al. (Reference Besprozvannykh, Ermolenko and Atopkin2012) reported relatively short 28S rDNA sequences for Asymphylodora perccotti Besprozvannykh, Ermolenko & Atopkin, 2012, which formed a strongly supported clade with L. kritskyi to the exclusion of four of the monorchiids analysed here. The sister relationship between the Monorchiidae and the Lissorchiidae thus seems relatively well established, especially in the light of the restriction of lissorchiids to freshwater fishes and monorchiids to marine fishes.
As in the analysis of Searle et al. (Reference Searle, Cutmore and Cribb2014), the single species of Helicometroides Yamaguti, 1934 for which sequence data are available is identified as sister to all other monorchiids, but with poor support. This genus was included by Madhavi (Reference Madhavi, Bray, Gibson and Jones2008) in the Hurleytrematinae Yamaguti, 1958, one of three subfamilies characterized in part by the presence of filamented eggs. Noticeably, this subfamily includes Provitellus turrum Dove & Cribb, 1998, which the present analysis shows to be distant from Helicometroides. Resolution of the status of the Hurleytrematinae will be dependent on the generation of sequence data for other hurleytrematines, but especially species of Hurleytrema Srivastava, 1939.
Cableia Sogandares-Bernal, 1959 was first recognized as having monorchiid affinities by Cribb et al. (Reference Cribb, Bray, Littlewood, Pichelin, Herniou, Littlewood and Bray2001) and Olson et al. (Reference Olson, Cribb, Tkach, Bray and Littlewood2003). The present analysis confirms this position and suggests that, ultimately, Cableia is likely to require separate status, at least at the subfamily level, within the Monorchiidae. At present, there is no indication of a close relationship with any other monorchiid genus, either by morphology or host association. The three species of Cableia lack the typical monorchiid appearance of restricted vitelline follicles and spiny male and female terminal genitalia, and are exclusively parasites of tetraodontiforms (Bray et al., Reference Bray, Justine and Cribb2009a). Notably, this order of fishes is surprisingly depauperate for monorchiids. Our records show just eight other monorchiids reported from tetraodontiforms, none of which resemble species of Cableia.
Species of the remaining genera in our analysis form a strongly supported clade which includes the type species of the type genus, Monorchis. All the genera within this clade are currently included in the Monorchiinae Odhner, 1911, except for Provitellus which, as discussed above, is placed in the Hurleytrematinae on the basis of the possession of filamented eggs. The Monorchiinae is currently the largest of the monorchiid subfamilies, with 27 genera. Just two genera in the present analysis, Lasiotocus and Monorchis, are represented by more than one species; neither is recovered as monophyletic in the present analysis. In the case of Lasiotocus, L. arrhichostoma Searle, Cutmore & Cribb, 2014, L. lizae Liu, 2002 and L. typicum (Nicoll, 1912) Bartoli & Bray, 2004 are all phylogenetically distant from each other. Presently, Lasiotocus has over 50 accepted species and, as such, is the largest monorchiid genus. It appears that its present diagnosis is too generalized to allow the recognition of a monophyletic assemblage. Noticeably, the three species for which sequence data are available show dramatic differences in overall body shape, the shape of the oral sucker, the length of the intestinal caeca, the shape of the ovary and conformation of the vitellarium. It is thus unsurprising that they do not appear to be closely related. Also, M. monorchis and M. lewisi n. sp. are not each other's closest relatives in our analyses. Madhavi (Reference Madhavi1977) commented that the variation in the form of the excretory vesicle in species of Monorchis, extremes of which are represented by the two species in the present analysis, might form the basis for recognition of a new genus distinct from Monorchis. Our molecular analyses suggest that she was right. The present analysis suggests that the concepts of both Lasiotocus and Monorchis require reconsideration, but we think that the addition of further sequence data is needed before this can be done reliably.
Acknowledgements
This work was supported by an Australian Biological Resources Study grant to explore the parasites of fishes of Moreton Bay. We thank Mr John Page, Mr David Thompson and the many students who assisted with the collection of the fishes. We are grateful to Dr Olena Kudlai for assistance with translation of Russian literature. Finally, we thank the staff of the Moreton Bay Research Station for their support of our work.
Financial support
T.H.C. and S.C.C. acknowledge the Australian Biological Resources Study (ABRS) for their ongoing support. This study was funded by the ABRS National Taxonomy Research Grant RF215-40.
Conflict of interest
None.
Ethical standards
All fish were caught and handled according to University of Queensland Animal Ethics Approval Certificate SBS/248/15/ABRS/ARC.






