Introduction
Lungworms of the genus Metastrongylus Molin, 1861 are parasitic nematodes in the respiratory tract of swine. The infection with these nematodes leads to verminous pneumonia and secondary disorders of the hosts resulting in weight loss and abortions (Alcaide et al., Reference Alcaide, Frontera, Rodríguez, Sáez, Domínguez–Alpízar, Reina and Navarrete2005). The infection may also cause immunodepression of the hosts, making them more susceptible to negative health impacts (Gassó et al., Reference Gassó, Rossi and Mentaberre2014). Importantly, although rarely seen, Metastrongylus infection in humans has been reported (Miloshev, Reference Miloshev1956; Calvopina et al., Reference Calvopina, Caballero, Morita and Korenaga2016). Thus, Metastrongylus nematodes can be potential health risks to both animals and humans. There are numerous studies on Metastrongylus infections in wild boars in Europe, the Americas and Africa, reporting high prevalence, while surveys in domestic pigs are few (Nssien & Adesehinwa, Reference Nssien and Adesehinwa1999; Adedokun et al., Reference Adedokun, Adejinmi and Ukoikpoko2001; Carstensen et al., Reference Carstensen, Vaarst and Roepstorff2002; Li et al., Reference Li, Luo and Zhang2016, Reference Li, Shahzad, Zhang, Mehmood, Jiang, Luo, Zhang, Dong and Li2018). At present, six Metastrongylus species are considered to be valid, including Metastrongylus apri (Gmelin, 1790) Vostokov, 1905 (Synonym Metastrongylus elongatus (Dujardin, 1845) Railliet & Henry, Reference Railliet and Henry1911, Metastrongylus salmi (Gedoelst, 1823), Metastrongylus pudendotectus (Wostokow, 1905), Metastrongylus confusus (Jansen, 1964), Metastrongylus asymetricus (Noda, 1973) and Metastrongylus madagascariensis Chabaud & Gretillat, 1956. Three species – M. apri, M. salmi and M. pudendotectus – are reported worldwide and are usually present in mixed infections with variable frequencies, while the distribution of three other species – M. confusus, M. asymetricus and M. madagascariensis – is restricted to specific areas (Epe et al., Reference Epe, Spellmeyer and Stoye1997; Adedokun et al., Reference Adedokun, Adejinmi and Ukoikpoko2001; Carstensen et al., Reference Carstensen, Vaarst and Roepstorff2002; García–González et al., Reference García–González, Pérez–Martín, Gamito–Santos, Calero–Bernal, Alcaide–Alonso and Frontera–Carrión2013; Gassó et al., Reference Gassó, Rossi and Mentaberre2014; Poglayen et al., Reference Poglayen, Marchesi, Dall'Oglio, Barlozzari, Galuppi and Morandi2015).
Metastrongylus species are distinguished by the following key morphological features: copulatory bursa and spicules in males; and prevulvar cuticular valve (swelling), dilatation, position of the vulva and anus in females. Gassó et al. (Reference Gassó, Rossi and Mentaberre2014) provided a key to the identification of five species, which excludes M. madagascariensis. However, mis-identifications when observing morphological features under microscope are possible, especially in the case of similar species, such as M. confusus, M. apri and M. salmi (Gassó et al., Reference Gassó, Rossi and Mentaberre2014). Molecular data have been used to distinguish species and to study the genetic variation of Metastrongylus species based on Random Amplified Polymorphic DNA (RAPD) analysis or Polymerase chain reaction - Restriction Fragment Length Polymorphism (PCR–RFLP) (Leignel et al., Reference Leignel, Humbert and Elard1997; Conole et al., Reference Conole, Chilton, Järvis and Gasser1999). Nucleotide sequence data, with the majority from European countries, have been used to analyse the phylogenetic relationships of few individual Metastrongylus species with other nematode taxa (Conole et al., Reference Conole, Chilton, Jarvis and Gasser2001; Carreno & Nadler, Reference Carreno and Nadler2003; Jex et al., Reference Jex, Hall, Littlewood and Gasser2010; Li et al., Reference Li, Luo and Zhang2016, Reference Li, Shahzad, Zhang, Mehmood, Jiang, Luo, Zhang, Dong and Li2018; Yong et al., Reference Yong, Song, Eamsobhana and Lim2016). In Vietnam, as well as in other Southeast Asian countries, studies on swine lungworms are extremely scarce and do not include any sequence data (Arambulo et al., Reference Arambulo, Hernandez and Soria-Abaga1967), even though domestic pigs are commonly raised in outdoor systems in many locations throughout the region. Therefore, the aim of this study is to survey Metastrongylus infection in domestic pigs in Dien Bien Province, Northern Vietnam, where pigs are raised freely, and to analyse the phylogenetic relationships of Metastrongylus species.
Materials and methods
Sample collection
The lungs of 739 domestic pigs from slaughterhouses in Dien Bien Province, Northern Vietnam, were examined for lungworms. The lungs and trachea were removed from each animal and placed in a washbasin containing water. The respiratory pathways were opened from the trachea into the bronchi and into the bronchioles using sharp scissors. The lungs were washed with water to remove contents in the respiratory pathways. The contents in the washbasin were filtered through a 100-mesh (149 μm) sieve. Lungworms in the sieve were collected and washed in 0.9% physiological saline. They were checked under a stereo-microscope and separated based on morphology. Depending on the number of specimens of each morphological type of lungworm, one to five worms were preserved in 96% ethanol for molecular analyses, and the other specimens were treated with hot 4% formaldehyde solution, then preserved in 4% formaldehyde solution for further morphological examination.
Morphological studies
Metastrongylus nematodes were immersed in lactophenol solution (phenol, lactic acid, glycerol and distilled water in a volume of 1:1:2:1) until the specimens became transparent for observation of taxonomic characteristics under a light microscope. Morphological examination and photographs were taken using a light microscope (ECLIPSE Ni, Nikon, Tokyo, Japan). The main taxonomic morphometrics of 30 adult nematodes (15 males and 15 females) of each species were measured. A paired-samples t-test was performed to examine the significance of differences.
Molecular analyses
Total genomic DNA was extracted from individual worms from different hosts using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). The internal transcribed spacer 2 (ITS2) region of the nuclear ribosomal DNA and a partial region of the mitochondrial cytochrome c oxidase subunit 1 (cox1) were chosen for analysis. They were amplified via the polymerase chain reaction (PCR) method with primer set 3S and A28 (Bowles et al., Reference Bowles, Blair and McManus1995), and JB3 and JB4.5 (Bowles et al., Reference Bowles, Hope, Tiu, Liu and McManus1993), respectively. The amplification reactions were carried out in a total volume of 25 μl, containing: 13 μl of Dream Taq Green PCR master mix (2X) (Thermo Fisher Scientific, Foster City, California, USA), 2 μl of Template DNA of 10 ng/μl, 1 μl of each forward and reverse primer (working concentration: 10 pmol/L), 8 μl of nuclease-free water. The PCR was performed with the following cycling protocol: initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 54°C for 1 min and elongation at 72°C for 1 min. After cycling, a final elongation step at 72°C for 10 min was performed. The PCR products were visualized by gel electrophoresis and purified using a Qiaquick PCR Purification Kit (Qiagen, Hilden, Germany), and were subsequently sequenced by an Ab3730 sequencer using a Big-Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Weiterstadt, Germany). Nucleotide sequence data obtained in this study were deposited in the GenBank™, EMBL and DDBJ databases under the accession numbers: LC625785–LC625790 (cox1 sequences) and LC625791–LC625793 (ITS2 sequences).
The ITS2 and cox1 sequence data obtained in the present study were aligned with sequences downloaded from GenBank, using ClustalW with default options in MEGA7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Short or abnormal sequences from GenBank were excluded. The final data set included 20 ITS2 and 18 cox1 sequences of three species (M. apri, M. salmi and M. pudendotectus) in the analyses. The sequence (KF316481) of Aelurostrongylus abstrusus (Metastrongyloidea) was used as an outgroup for the cox1 tree, whereas no outgroup was used for the ITS2 tree due to the Metastrongylus sequences being too phylogenetically distant from those of the other genera of nematodes. Phylogenetic trees were constructed using the Maximum Likelihood method based on the best model: Tamura 3-parameter model for the ITS2 tree, and Tamura-Nei model combined with the proportion of invariable sites (+I) for the cox1 tree. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbour-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood approach and then selecting the topology with superior log likelihood value. All positions containing gaps and missing data were eliminated. We evaluated the statistical confidence of branching patterns using 1000 bootstrap replicates.
Results
Morphology
The nematode specimens collected from the lungs of domestic pigs in Dien Bien Province, Vietnam, were identified as M. apri and M. pudendotectus based on the morphological key of Gassó et al. (Reference Gassó, Rossi and Mentaberre2014). The two species varied in size and in morphology with the following main features.
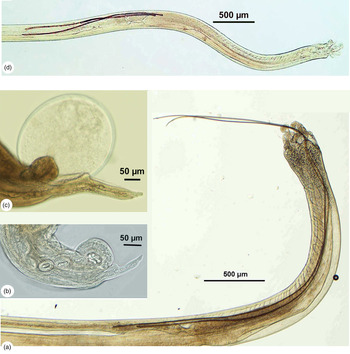
Metastrongylus apri: the body length of females (39.4 ± 5.0 mm) is greater than that of males (19.8 ± 1.7 mm). In males, two long spicules (4.2 ± 0.5 mm in length) are usually protruded out the body (fig. 1a). In females, prevulva cuticular dilatation is absent. Prevulva swelling is 108.2 ± 8.0 × 84.3 ± 6.7 μm in size. The distance from the anus and from the vulva to the posterior end of the body is 89.5 ± 2.8 μm and 109.4 ± 7.0 μm, respectively (fig. 1b).

Fig. 1. The caudal ends of Metastrongylus apri and Metastrongylus pudendotectus collected from domestic pigs in Vietnam: (a) M. apri male showing two long spicules protruded out the body; (b) M. apri female showing prevulvar cuticular swelling, but no dilatation; (c) M. pudendotectus female showing prevulvar cuticular swelling and well-developed dilatation; (d) M. pudendotectus male showing two medium spicules indented into the body..
Metastrongylus pudendotectus: the body length of females (20.8 ± 0.9 mm) is greater than that of males (14.2 ± 0.8 mm). In females, the prevulva swelling is 92.0 ± 1.1 × 52.3 ± 1.9 μm in size, surrounded by well-developed spherical cuticular dilatation of 259.9 ± 2.9 × 238.6 ± 5.0 μm. The vulva is posterodorsal to prevulvar dilatation. The distance from the anus and from the vulva to the posterior end of the body is 221.7 ± 6.0 μm and 328.0 ± 4.5 μm, respectively (fig. 1c). In males, two medium spicules, 1.6 ± 0.05 mm in length, were usually indented in the body, distant to the posterior end of the body 1.5–2.0 mm (fig. 1d)
The statistical analyses revealed that the body length, spicule length, prevulva swelling, distance from the vulva and from the anus to the posterior end of the body of the two species were all significantly different (P < 0.001).
Molecular analyses
We obtained three cox1 sequences of each species, which were morphologically identified as M. apri and M. pudendotectus, and three ITS2 sequences of M. apri, but we did not obtain any ITS2 sequence of M. pudendotectus from the 17 pigs infected with this species.
For ITS2, three sequences of M. apri collected from Vietnam were completely identical to each other but differed from the Danish and Estonian M. apri sequences (Estonian sequences registered as M. elongatus) at a distance of 2.0–2.3%. Intraspecific nucleotide variation within M. apri (including M. elongatus as a synonym) ranged from 0.0 to 2.3%. Interspecific nucleotide distances between M. apri and M. salmi ranged from 1.3 to 3.6%, while the distances between M. pudendotectus and M. apri were much greater (from 13.1 to 15.0%), almost similar to that between M. pudendotectus and M. salmi (from 13.0 to 13.4%). In the phylogenetic tree (fig. 2a), three species belonged to distinct clades: M. apri was close to M. salmi, and separated from M. pudendotectus.

Fig. 2. Phylogenetic relationships of Metastrongylus species reconstructed from ITS2 sequences (a) and from cox1 sequences (b). Nucleotide sequences downloaded from GenBank are shown with accession number, species name and country name of their geographical origin. The sequences obtained in the present study are printed in bold. Bootstrap values are shown at the nodes. Scale bar shows the number of substitutions per site.
In the cox1 region of Vietnamese Metastrongylus, there was a single nucleotide difference among three M. apri specimens. Similarly, there was one single nucleotide difference among three M. pudendotectus sequences. At present, there are no cox1 sequences of M. apri available in GenBank for comparison. For M. pudendotectus, the specimens from Vietnam differed from the Chinese, French and Estonian sequences from 7.2 to 8.8%. Intraspecific nucleotide distance within M. pudendotectus specimens ranged from 0.0 to 8.8%. In comparison between species, nucleotide distances between M. apri and M. salmi ranged from 7.7 to 9.0%, smaller than that between M. pudendotectus and M. salmi (from 12.6 to 13.3%), and between M. pudendotectus and M. apri (from 13.8 to 14.9%). In the phylogenetic tree (fig. 2b), similar to the ITS2 tree, each species belonged to distinct clades, with M. apri and M. salmi close to each other, and separated from M. pudendotectus.
Prevalence of infection
Of the 739 domestic pigs examined, M. apri was found in 178 (24.1%) pigs with an intensity ranging from 7 to 113 (average: 30.1 ± 20.6) nematodes per infected pigs, and M. pudendotectus was found in 17 (2.3%) pigs with an intensity ranging from 2 to 10 (average: 5.3 ± 2.4) nematodes per infected pigs. There was a statistically significant difference (P < 0.0001) between the two species in terms of the infection rate and the intensity. We found pigs being infected with only M. apri or co-infected with the two species (M. apri and M. pudendotectus), but there were no pigs found to be infected with only M. pudendotectus.
Discussion
The morphological taxonomic features of the two lungworm species, M. apri and M. pudendotectus, found in domestic pigs in Vietnam were compatible with that described by Gassó et al. (Reference Gassó, Rossi and Mentaberre2014). The two species are distinguishable as follows: in females, M. pudendotectus bears a well-developed prevulvar cuticular dilatation, whereas M. apri does not; and in males, M. apri bears two spicules much longer than those of M. pudendotectus (4.2 ± 0.2 mm vs. 1.38 ± 0.07 mm) (Gassó et al., Reference Gassó, Rossi and Mentaberre2014). In addition to these diagnostic features, we found that the body size of M. apri was greater than M. pudendotectus, and the spicules of M. apri usually protruded out the body, whereas those of M. pudendotectus usually indented into the body, distant to the posterior end of the body 1.5–2.0 mm (about the length of the spicules). The protruded or indented status of the spicules should be a typical feature of each species because the specimens of the two species were all preserved in 4% formaldehyde solution by the same method. To our knowledge, this particular feature has not been mentioned previously. The spicules of Metastrongylus males should be examined in more detail to see if indentation of the spicules is a typical feature of M. pudendotectus or just a unique feature of Vietnamese specimens.
In the present study, we did not obtain ITS2 sequences of M. pudendotectus. However, as expected, the cox1 sequences of M. pudendotectus and the ITS2 sequences of M. apri from the specimens collected in Vietnam supported the morphological identification of the two species. They were clustered with reference sequences of these two species from other countries with some differences. It should be noted that almost all ITS2 sequences of M. elongatus from Estonia were completely identical to the M. apri sequence (KP890022) from Denmark and were placed in the same clade in the ITS2 tree. It is not surprising, because the two species names are considered synonyms based on morphology. The issue is that the two names are used inconsistently; some scientists use M. apri as the validated name and consider M. elongatus a synonym (Gassó et al., Reference Gassó, Rossi and Mentaberre2014; Poglayen et al., Reference Poglayen, Marchesi, Dall'Oglio, Barlozzari, Galuppi and Morandi2015), whereas others accept M. elongatus and place M. apri as a synonym (Nssien & Adesehinwa, Reference Nssien and Adesehinwa1999; Nosal et al., Reference Nosal, Kowal and Nowosad2010). Furthermore, the nucleotide sequences under accession numbers AJ305373–AJ305381, registered in GenBank with the name M. elongatus, were originated from the source organism of M. apri (Conole et al., Reference Conole, Chilton, Jarvis and Gasser2001). Taxonomically, however, the validated name of this species should be used correctly and consistently. Historically, Railliet & Henry (Reference Railliet and Henry1911) used the name M. elongatus in place of M. apri without explanation (Dougherty, Reference Dougherty1944). Since then, this proposal was not always but usually adopted. Importantly, based on the basis of priority of the International Code of Zoological Nomenclature, M. elongatus (Dujardin, 1845) Railliet & Henry, Reference Railliet and Henry1911 should be a synonym of M. apri (Gmelin, 1790) Vostokov, 1905. The molecular analysis supported this synonymization, as ITS2 sequences of M. elongatus were completely identical to that of M. arpi. Genetically, M. apri was found to be closely related to M. salmi but far distant from M. pudendotectus in both ITS2 and cox1 regions. Currently, there are relatively few nucleotide sequences of few Metastrongylus species in comparison to their abundance and wide distribution. Therefore, more sequence data of Metastrongylus species from various geographical locations should be obtained and analysed for a full understanding of their molecular phylogenetic relationships.
Regarding prevalence, most investigations focused on Metastrongylus spp. in wild boars in Europe, the Americas and Africa, reporting high infection rates up to 100% (Forrester et al., Reference Forrester, Porter, Belden and Frankenberger1982; Epe et al., Reference Epe, Spellmeyer and Stoye1997; Poglayen et al., Reference Poglayen, Marchesi, Dall'Oglio, Barlozzari, Galuppi and Morandi2015; Amayour et al., Reference Amayour, Alaoui, Alkhali, Hassouni, Kharrim and Belghyti2016). There are few reports on lungworms in domestic pigs (Nssien & Adesehinwa, Reference Nssien and Adesehinwa1999; Adedokun et al., Reference Adedokun, Adejinmi and Ukoikpoko2001; Carstensen et al., Reference Carstensen, Vaarst and Roepstorff2002; Li et al., Reference Li, Luo and Zhang2016, Reference Li, Shahzad, Zhang, Mehmood, Jiang, Luo, Zhang, Dong and Li2018). In the present study in Vietnam, we found the infection rate of M. apri in the pigs studied was much higher than that of M. pudendotectus. Our data agrees with previous studies that M. apri was the dominant species and usually co-infected with M. pudendotectus (Nssien & Adesehinwa, Reference Nssien and Adesehinwa1999; Nosal et al., Reference Nosal, Kowal and Nowosad2010), although the composition of Metastrongylus spp. and the prevalence varies from place to place (Nosal et al., Reference Nosal, Kowal and Nowosad2010). Moreover, we did not find M. pudendotectus infecting any pig individuals alone. Similarly in Florida, USA, a single infection with M. apri and concurrent infections with M. apri and M. pudendotectus or with M. apri and M. salmi were found, but a single infection with only M. pudendotectus or M. salmi was not observed (Forrester et al., Reference Forrester, Porter, Belden and Frankenberger1982). This may suggest that infection of M. pudendotectus is dependent on or requires prior infection with M. apri, as previously speculated on the mutualistic association between M. apri and M. pudendotectus (Ewing et al., Reference Ewing, Ewing, Keener and Mulholland1982). On the other hand, Poglayen et al. (Reference Poglayen, Marchesi, Dall'Oglio, Barlozzari, Galuppi and Morandi2015) found random infections of different Metastrongylus species within the same host, they did not observe dependence or competition among them. Thus, more surveys for Metastrongylus nematodes should be conducted to confirm this ecological point.
In conclusion, the present study provided the prevalence, morphological characteristics and molecular data of M. apri and M. pudendotectus from domestic pigs in Northern Vietnam, with a slight morphological and genetic variation compared to those from other countries. Our analyses also reveal a close relationship between M. apri and M. salmi, but far distant from M. pudendotectus, and highlight the needs for further studies to clarify the morphological characteristics, ecological and molecular phylogenetic relationships of Metastrongylus species at the global scale.
Financial support
None.
Conflicts of interest
None.
Ethical standards
The present study was approved by the rector of Thai Nguyen University of Agriculture and Forestry (decision no. 225/QĐ-ĐHNL-ĐT). The lungs of domestic pigs were purchased from the local owners in Dien Bien province where pork meat and visceral parts of pigs are sold in local markets. The use of the lungs of domestic pigs for research purposes was informed to the owners with their approval.