Introduction
The canine hookworms Ancylostoma caninum, Ancylostoma braziliense, Ancylostoma ceylanicum and Uncinaria stenocephala are well known for their detrimental impacts on canine health. Ancylostoma caninum is a leading cause of acute, potentially fatal haemorrhagic enteritis in young puppies and causes chronic iron-deficiency anaemia in older dogs (Miller, Reference Miller1968; Georgi et al., Reference Georgi, LeJambre and Ractliffe1969). However, in addition to their veterinary significance, all four species of canine hookworms also have zoonotic potential. In humans, all these species are capable of producing cutaneous larva migrans (CLM) or ‘ground itch’, which is usually self-limiting (Bowman et al., Reference Bowman2010). Only A. braziliense can cause a more severe form of CLM (‘creeping eruptions’), resulting in raised, highly pruritic tracks in humans (Malgor et al., Reference Malgor1996), which usually necessitate treatment (Caumes, Reference Caumes2000; Hochedez and Caumes, Reference Hochedez and Caumes2007). Ancylostoma ceylanicum is the only species of canine hookworms capable of causing patent infections in humans, both naturally and experimentally (Traub, Reference Traub2013). This hookworm is the agent of an emerging zoonosis that is being increasingly detected during molecular-based epidemiological surveys in the Asia Pacific region comprising Cambodia (Inpankaew et al., Reference Inpankaew2014), Malaysia (Ngui et al., Reference Ngui2012), Thailand (Traub et al., Reference Traub2008; Jiraanankul et al., Reference Jiraanankul2011), Laos (Sato et al., Reference Sato2010; Conlan et al., Reference Conlan2012), Solomon Islands (Speare et al., Reference Speare, Bradbury and Croese2016; Bradbury et al., Reference Bradbury2017), and Australia (Koehler et al., Reference Koehler2013; Smout et al., Reference Smout2017). In rare cases, A. caninum larvae develop into pre-adult, non-patent worms in the human intestine, causing eosinophilic enteritis (Landmann and Prociv, Reference Landmann and Prociv2003). It is likely that this zoonosis is grossly underdiagnosed due to the obscure and non-specific clinical signs that often accompany the infection coupled with the challenges of diagnosis (Bradbury and Traub, Reference Bradbury, Traub and Loukas2016).
There is little information on the occurrence and public health significance of zoonotic canine hookworms in sub-Saharan Africa, as most reports of CLM are based on cases of tourists diagnosed after returning to their home countries (Jelinek et al., Reference Jelinek1994; Bouchaud et al., Reference Bouchaud2000; Blackwell and Vega-Lopez, Reference Blackwell and Vega-Lopez2001; Kelkar, Reference Kelkar2007; Dhir et al., Reference Dhir, O'Dempsey and Watts2010). However, lack of recognition is the most likely reason for this apparent absence in the local population (Ngui et al., Reference Ngui2012). Previous studies in Kenya have reported the occurrence of adult hookworms in dogs following post-mortem examination, but the species were never identified either morphologically or by molecular methods (Wachira et al., Reference Wachira1993; Buishi et al., Reference Buishi2006). However, in a recent study in Tanzania, four zoonotic hookworm species (A. caninum, A. braziliense, A. ceylanicum and U. stenocephala) were detected in dogs (Merino-Tejedor et al., Reference Merino-Tejedor2018). As the frequency of hookworm infection is associated with climatic conditions, and is usually more frequent in regions with higher rainfall because of better conditions for soil larvae, the sites for this study were regions of Kenya within different climatic zones. We report here the estimated prevalence and species of zoonotic hookworms in dogs from four different ecological regions of Kenya.
Materials and methods
Study sites
Turkana County is situated in north-western Kenya between 1°30′ and 5°30′ N and between 34°30′ and 36°40′ E. The climate is hot and arid to semi-arid, with temperatures ranging between 20°C and 41°C. The county receives 150–400 mm of erratic rainfall annually, leading to recurring drought conditions. Isiolo County is situated in northern-central Kenya between 0°05′ S and 2° N and 36°50′ and 39°30′ E. The temperature range is lower than in Turkana (12–28°C), with similar aridity (300–500 mm annual rainfall). Meru County lies to the immediate east of Mt Kenya, straddling the equator (between 0°6′ N and 0°1′ S, and between 37°5′ E and 38°25′ E). The climate is temperate, with an average temperature between 16°C and 23°C and average annual rainfall of 1600 mm. Narok County is situated in South Rift Valley, sharing borders with Tanzania in the south. The climate is also temperate, with average temperature in the range 12–28°C and average rainfall in the range 500–1800 mm per annum. Its geographical coordinates are 0°50′ to 2°05′ S and 35°28′ to 36°25′ E. Narok County includes the Maasai Mara National Reserve (fig. 1).
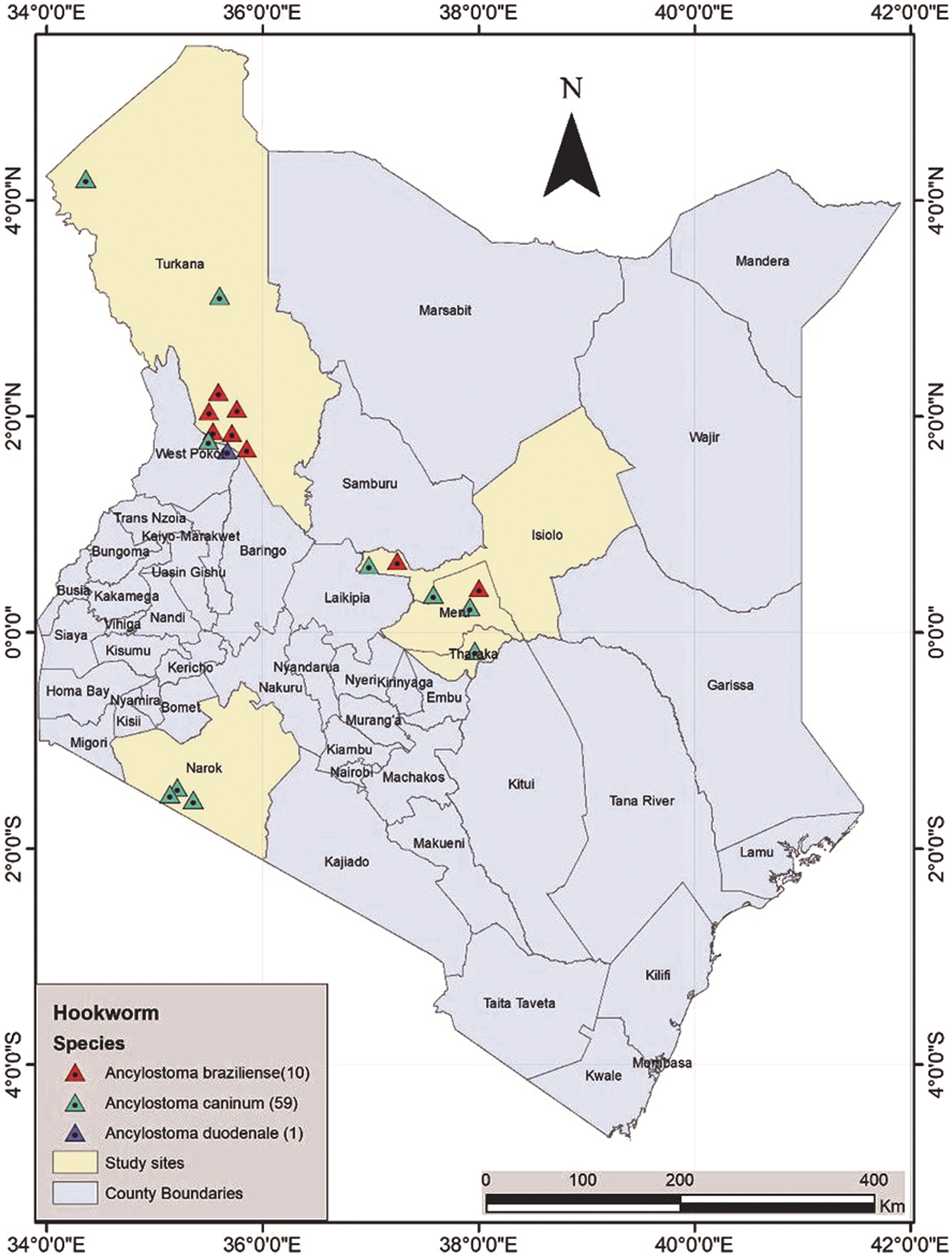
Fig. 1. A map showing the study sites of Turkana, Meru, Isiolo and Narok counties, in Kenya, and the distribution and frequency of hookworm species.
Dog faecal sample collection
Single canine faecal samples were collected from the environment, as described previously (Mulinge et al., Reference Mulinge2018), between September 2013 and June 2016. Samples were collected from households with dogs, from pathways leading to the homes, and in some rural and urban centres. Faecal samples were mainly identified according to field signs, as described previously (Verster, Reference Verster1965), and in some cases with the help of the household members. To minimize multiple sampling from the same dog, only very fresh samples (up to one day old) were collected. The faecal samples (~20–30 g) were preserved in 80% ethanol immediately after collection.
Isolation of hookworm eggs
Briefly, the ethanol was drained, 2 ml (~2 g) of stool was transferred into 15 ml falcon tubes and the sample was rinsed with 8 ml of distilled water. Helminth eggs were recovered using the zinc chloride flotation/sieving method (specific density: 1.45) (Mathis et al., Reference Mathis, Deplazes and Eckert1996). The concentrate/pellet was examined microscopically for the presence of helminth eggs.
DNA extraction
A total of 78/490 microscopically positive faecal samples were selected randomly for DNA extraction (table 1). All the faecal samples selected had parasite intensity of at least 300 eggs per gram, based on the McMaster method (results not shown here), and these included 20 from three study sites (Turkana, Meru, Narok) and 18 samples from Isiolo. The eggs from each sample were settled by centrifugation at 8000 rpm for 1 minute, and the excess water was removed by decanting. The QIAamp® DNA Stool Mini Kit (Qiagen) was used to extract total DNA according to the manufacturer's protocol, with slight modification. Briefly, the eggs were lysed through three rounds of freezing (−80°C for 30 minutes) and thawing (80°C for 15 minutes). DNA was eluted in 50 μl elution buffer and stored at −20°C awaiting PCR processing. Positive control genomic DNA for A. caninum and A. ceylanicum was provided by Prof. Rebecca Traub (University of Melbourne, Australia).
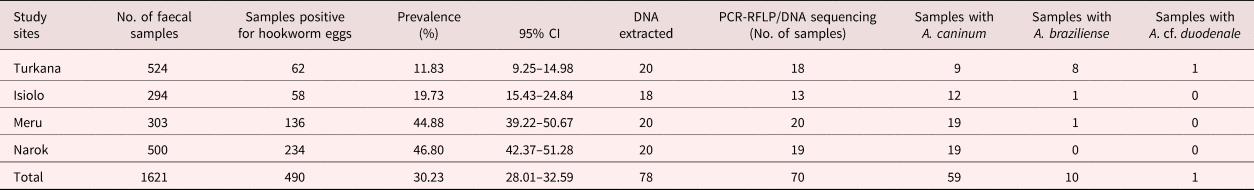
Table 1. Faecal prevalence of hookworm eggs in dog faeces, and molecular species determination.
Polymerase chain reaction of internal transcribed spacer (ITS) 1 and 2, 5.8S and 28S rRNA
Direct PCRs were carried out using primers RTGHF1 (5′-CGT GCT AGT CTT CAG GAC TTT G-3′) and RTABCR1 (5′-CGG GAA TTG CTA TAA GCA AGT GC-3′) targeting the internal transcribed spacer (ITS)-1, 5.8S and ITS 2 regions, yielding a 544 base pairs (bp) PCR product for A. caninum, A. ceylanicum and A. duodenale and 552 bp for A. braziliense (Traub et al., Reference Traub2004). With negative samples, a second separate PCR was performed using the primer pair NC1 (5′-ACG TCT GGT TCA GGG TTC TT-3′) and NC2 (5′-TTA GTT TCT TTT CCT CCG CT-3′) to amplify an approximately 310 bp region of the internal transcribed spacer-2 (ITS-2), 5.8S and 28S ribosomal RNA gene (Gasser et al., Reference Gasser1993). PCR was done in a final reaction volume of 50 μl, consisting of 5 μl genomic DNA, 2 mm MgCl2, 0.2 mm dNTPs, 0.5 μm of each primer, 1 × DreamTaq™ Green Buffer (Thermo Fisher Scientific) and 1.25 units of DreamTaq™ Green DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA). The cycling conditions included initial denaturation at 94°C for 5 minutes, followed by 50 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 30 s and a final extension at 72°C for 5 minutes. Ten microliters of the PCR products were detected on 2% agarose gel stained in ethidium bromide.
Host species identification
To identify the host (source) of a faecal sample containing A. duodenale eggs, genomic DNA was extracted from the faecal sample as described by Dinkel et al. (Reference Dinkel1998) and subjected to a nested PCR to amplify the canid and felid specific cytochrome b gene using the primer pairs and methods previously described by Huttner et al. (Reference Huttner2009) and Kagendo et al. (Reference Kagendo2014).
Amplification of cytochrome c oxidase I
A single PCR reaction of the mitochondrial cytochrome c oxidase I (cox1) was performed on hookworm isolate identified as A. duodenale by the ITS regions PCR. The forward primer AceyCOX1F (5′-GCT TTT GGT ATT GTA AGA CAG-3′) and reverse primer AceyCOX1R (5′-CTA ACA ACA TAA TAA GTA TCA TG-3′) amplified a fragment of 377 bp (Inpankaew et al., Reference Inpankaew2014). The 50 μl PCR reaction consisting of 5 μl genomic DNA, 2 mm MgCl2, 0.2 mm dNTPs, 0.25 μm of each primer, 1 × DreamTaq™ Green Buffer (Thermo Fisher Scientific) and 1.25 units of DreamTaq Green DNA Polymerase (Thermo Fisher Scientific). The cycling conditions were the same as those of the ITS 1 and 2 regions PCR except for the annealing temperature, which was increased to 58°C. Ten microlitres of the PCR products were detected on 2% agarose gel stained in ethidium bromide.
Species identification by restriction fragment length polymorphism
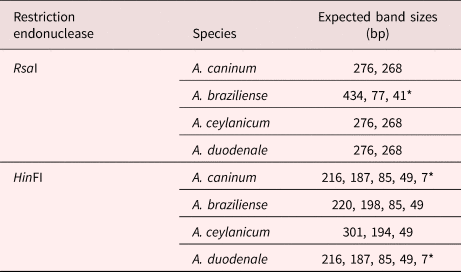
Restriction digests were performed on the 544–552 bp PCR products only; restriction endonucleases (HinFI and RsaI) (New England Biolabs, Ipswich, MA, USA) were used (Traub et al., Reference Traub2004). Briefly, 10 μl of the PCR product was digested separately in a 20 μl final volume in a reaction consisting of 5 units’ enzymes, 1 × CutSmart® Buffer (NEB). The digests were incubated overnight at 37°C, and separated on 3% agarose gel to identify hookworm species, according to Palmer et al. (Reference Palmer2007). PCR products from known A. caninum and A. ceylanicum were digested alongside the test samples as controls.
Sequencing of PCR products
Thirty-five PCR products generated by NC1 and NC2 (15) and RTGHF1 and RTABCR1 (20) primers were purified using Wizard® SV Gel and PCR Clean-Up (Promega, Fitchburg, WI, USA) and sequenced with reverse primers RTABCR1 (5′-CGG GAA TTG CTA TAA GCA AGT GC-3′) and NC2 (5′-TTA GTT TCT TTT CCT CCG CT-3′), respectively, at GATC Biotech AG, Germany. The cox1 PCR product was also purified and sequenced using both primers (AceyCOX1F and AceyCOX1R). The chromatograms were checked manually and edited using GENtle v. 1.9.4 (http://gentle.magnusmanske.de), and species were confirmed through identity search of the National Center for Biotechnology Information (NCBI) reference sequences, using the Basic Local Alignment Search Tool (BLAST).
Data analysis
The prevalence and exact binomial confidence intervals (95% CI) were calculated for each study site. Statistical differences between the study sites were assessed using Fisher's exact chi-square test on SPSS version 22.0. Significance was indicated by a probability of P < 0.05.
Ethical approval
This study was approved by the Scientific Ethics Review Unit (SERU) and the Animal Care and Use Committee at Kenya Medical Research Institute (SSC. No. 2658). Permission was also obtained from the Department of Veterinary Services, Kenya.
Results
Prevalence of hookworm eggs in dog faecal samples
Hookworm eggs were detected by microscopy in 490/1621 (30.23%; 95% CI 28.01–32.59%) faecal samples. Narok county (Maasai Mara region) had the highest prevalence (46.80%; 95% CI 42.37–51.28%) (234/500), followed by Meru (44.88%; 95% CI 39.22–50.67%) (136/303), Isiolo (19.73%; 95% CI 15.43–24.84%) (58/294) and Turkana (11.83%; 95% CI 9.25–14.98%) (62/524) (table 1). There was a significant difference in hookworm prevalence in the four counties (P < 0.0001).
Molecular identification of species
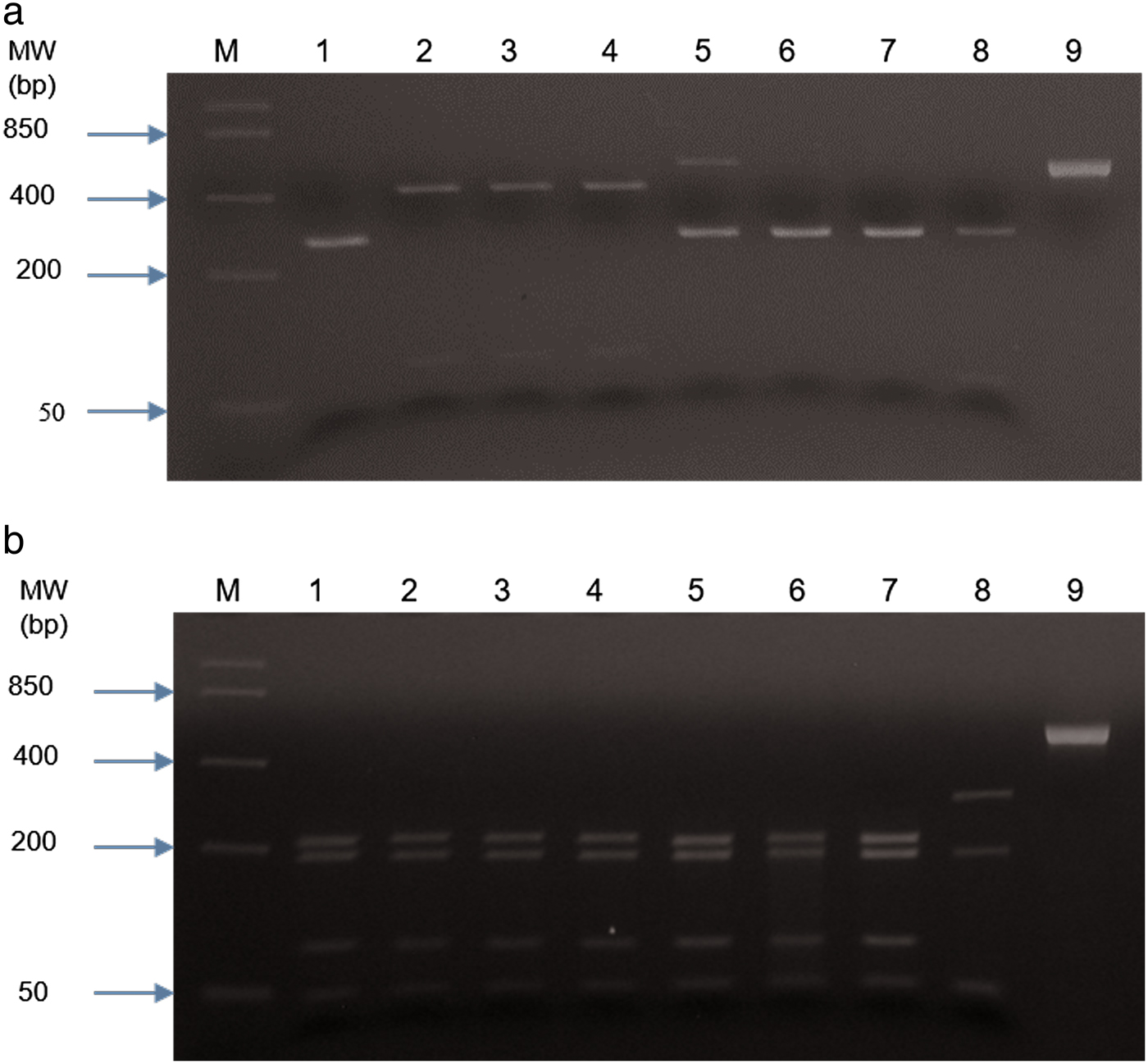
PCR products were obtained from 70/78 samples with either of the two sets of primers; eight DNA samples failed to yield a product with either of the two PCR methods (table 1 and supplementary material). The first PCR successfully amplified DNA from 55/78 faecal samples, whereas 15/78 PCR products were obtained with the second PCR (NC1/2 primers). RFLP identified hookworm species in all 55 amplified products from the first PCR. The restriction profiles following digestion with both endonuclease RsaI and HinFI are shown in table 2 and fig. 2. Hookworm species were identified by sequencing of all 15 PCR products (second PCR). To further confirm the RFLP results, 20 of 55 PCR products (first PCR) were selected randomly for sequencing. Hookworm species identified included A. caninum (n = 59), A. braziliense (n = 10) and A. cf. duodenale (n = 1) (table 1 and supplementary material). Neither of the detection methods gave indications of multiple species infections (e.g. double peaks). All A. caninum (n = 25) and A. braziliense (n = 10) sequences were 100% identical to references DQ438071 and DQ438061 (e Silva et al., Reference e Silva2006), respectively. All the nucleotide sequences obtained from A. braziliense and A. caninum isolates were identical for each species, and therefore only representative sequences for each species were deposited in GenBank, under accession numbers MG271917, MG271918 and MG271920. The A. cf. duodenale sequence showed 99% identity with A. duodenale accession number EU344797 (Wang et al., unpubl. data), with three single nucleotide polymorphisms (SNPs) occurring at ITS 1 (2) and ITS 2 (1) regions. This nucleotide sequence was deposited in GenBank under accession number MK271367. Amplification and partial sequencing of mitochondrial cytochrome c oxidase I (cox1) confirmed the A. duodenale isolate. The nucleotide sequence of this isolate was 99% identical to the reference sequence accession number AJ417718 (Hu et al., Reference Hu, Chilton and Gasser2002) and was deposited in GenBank under accession number MK288015.
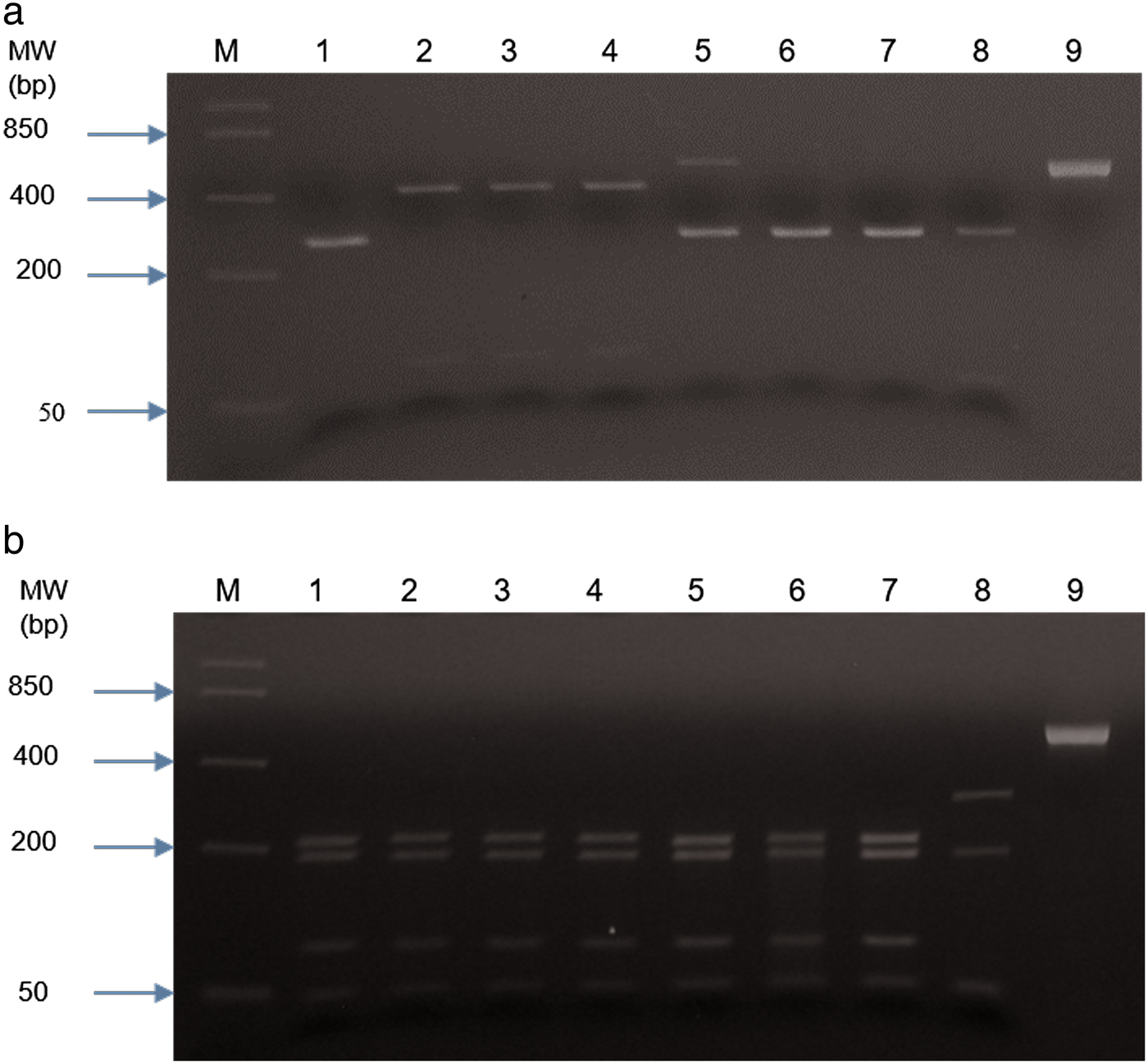
Fig. 2. RFLP of PCR product RTGHF1-RTABCR1 following digestion with restriction endonucleases (a) RsaI and (b) HinFI. Digests were separated on 3% (w/v) agarose gel and stained in ethidium bromide. Lane M: molecular weight marker; lane 1: Ancylostoma duodenale (TNU 331); lanes 2–4: Ancylostoma braziliense; lanes 5 & 6: Ancylostoma caninum; lane 7: Ancylostoma caninum positive control; lane 8: Ancylostoma ceylanicum positive control; lane 9: uncut PCR product.
Table 2. Restriction profile of RTGHF1-RTABCR1 PCR products following digestion with restriction endonucleases (RsaI & HinFI).

*Product too small to visualize on gel
Geographical distribution of hookworm species
Ancylostoma caninum was the most common species in all study sites and the only species detected in Narok (Maasai Mara). Ancylostoma braziliense was common in Turkana, with 8/20 positive faecal samples, but was found in only one faecal sample from Isiolo and one sample from Meru. Ancylostoma cf. duodenale was detected in only one faecal sample from Turkana (table 1 and fig. 1).
Discussion
This study is the first to report the prevalence and species of hookworms from dog faecal samples in Kenya. The overall prevalence of hookworms in this study (30.23%) is in the range of prevalence reported from Nigeria (34.6%) (Sowemimo, Reference Sowemimo2009) and Gabon (34.8%) (Davoust et al., Reference Davoust2008). Within Kenya, there are only two previous studies available for comparison, both with a small number of dogs examined from single locations. One study was done in Nairobi, where 88% of 156 dogs were found to be infected (Wachira et al., Reference Wachira1993). The second study was done in Turkana, giving a prevalence (11.8% of 42 dogs; Buishi et al., Reference Buishi2006) that is remarkably close to our data (11.9%). However, these studies were based on post-mortem examination, which would be expected to give higher sensitivities due to the identification of pre-patent infections. Also, hatching of hookworm eggs in the faeces before collection is likely to decrease the sensitivity of our method, as is desiccation of eggs in the environment (Levecke et al., Reference Levecke2011).
Narok and Meru counties had far higher prevalence than Isiolo and Turkana counties. This may be attributed to the hot (> 40°C) and dry conditions (annual average rainfall 250 mm) in Turkana and Isiolo, which are known to negatively affect the survival of hookworm larvae in the environment (Crompton, Reference Crompton2001; Mudenda et al., Reference Mudenda2012), while high-rainfall conditions in Meru and Narok are favourable for larval development in contaminated soil. Eggs of hookworms do not develop below 15°C and larvae prefer shady and moist areas with temperatures at or above 30°C, only being killed at temperatures above 45°C. Larvae also survive for longer periods at lower temperatures (20–25°C) than at temperatures above 30°C (Udonsi and Atata, Reference Udonsi and Atata1987; Brooker et al., Reference Brooker, Clements and Bundy2006; Pullan and Brooker, Reference Pullan and Brooker2012).
In this study three hookworm species, namely A. caninum, A. braziliense and A. cf. duodenale, were identified in canine faecal samples. Ancylostoma caninum was the most common species, found in 84.3% of positive faecal samples, an observation also reported in Australia (Palmer et al., Reference Palmer2007), Brazil (Coelho et al., Reference Coelho2011; Oliveira-Arbex et al., Reference Oliveira-Arbex2017), China (Liu et al., Reference Liu2015), India (Traub et al., Reference Traub2003; George et al., Reference George2016) and Thailand (Traub et al., Reference Traub2008). The failure to amplify hookworm DNA in eight samples could be as a result of microscopy misdiagnosis of hookworm eggs with other related nematode eggs, such as Trichostrongylus and Oesophagostomum, which are morphologically similar to hookworm eggs (Jozefzoon and Oostburg, Reference Jozefzoon and Oostburg1994; Ziem et al., Reference Ziem2006). Occasionally mite eggs are also confused with hookworm eggs (Werneck et al., Reference Werneck2007). Genomic DNA extracted from faecal samples is known to contain PCR inhibitors, which results in false negatives (Traub et al., Reference Traub2004; Repetto et al., Reference Repetto2013). Furthermore, low egg counts are also linked with limited or undetectable DNA by PCR (Palmer et al., Reference Palmer2007; Hu et al., Reference Hu2016). Ancylostoma caninum is the most widely distributed hookworm species in the tropics, subtropics and in warm temperate areas in southern Europe (Galanti et al., Reference Galanti, Fusco and Nardiello2002; Rinaldi et al., Reference Rinaldi2006). This is not surprising given the biological advantage of A. caninum in relation to the other hookworm species in the ability of its larvae to undergo transmammary transmission and arrested development in tissue during seasons unfavourable for its survival in the environment. Although eosinophilic enteritis caused by A. caninum has not been reported in humans in Kenya, this study provides a basis for future investigation. A recent study in India detected A. caninum eggs in human stool; however, the authors could not provide evidence that these eggs were the result of natural infection or an inadvertent passage (George et al., Reference George2016). Moreover, as A. caninum and A. duodenale are closely related and appear to have recently diverged, it is possible that the ITS region of the rRNA is too conserved to allow confident differentiation of the two species (Traub et al., Reference Traub2007).
Ancylostoma braziliense was the second most abundant species of canine hookworms in this study (found in 14.3% of faecal samples). This parasite is limited to tropical and subtropical regions, including Central and South America, the Caribbean, south-eastern USA, Africa, Malaysia, and northern Australia (Del Giudice et al., Reference Del Giudice2002; Schaub et al., Reference Schaub, Perruchoud and Buechner2002). It is responsible for causing ‘creeping eruptions’ caused by cutaneous larva migrans, often reported in travellers returning from endemic areas (Bowman et al., Reference Bowman2010). Two patients in the UK and two in France were treated for cutaneous larva migrans after visiting Kenya (Bouchaud et al., Reference Bouchaud2000; Kelkar, Reference Kelkar2007; Dhir et al., Reference Dhir, O'Dempsey and Watts2010). Furthermore, 32% of 44 patients treated for cutaneous larva migrans at the Hospital for Tropical Diseases, London, originated from Africa (Blackwell and Vega-Lopez, Reference Blackwell and Vega-Lopez2001), whereas in Germany 10.2% of patients treated for creeping eruption at a travel-related-disease clinic had visited East Africa (Jelinek et al., Reference Jelinek1994). Hookworm-related cutaneous larva migrans has been reported in other areas where A. braziliense is endemic in dogs and cats (Hochedez and Caumes, Reference Hochedez and Caumes2007; Bowman et al., Reference Bowman2010). Human cases of cutaneous larva migrans in Kenya are limited or not documented; therefore, the public health significance of this hookworm may be underestimated, possibly due to misdiagnosis for other skin conditions, as observed elsewhere (Mahdy et al., Reference Mahdy2012).
Ancylostoma cf. duodenale was characterized by DNA sequencing in a single faecal sample originating from Turkana. However, in the absence of adult worms to confirm this finding, we postulate that this observation could be a result of coprophagy of human stools containing A. duodenale eggs, which is common in the area (personal observation). Furthermore, detection of Echinococcus felidis (Mulinge et al., Reference Mulinge2018) and Taenia saginata (Mulinge et al., unpubl. data) in dog faeces in related studies confirms that dog coprophagy is common. The mechanical transmission of human parasites by dogs has been demonstrated by the passive passage of viable Toxoplasma gondii oocysts after feeding on cat faeces (Lindsay et al., Reference Lindsay1997; Frenkel et al., Reference Frenkel2003), and passage of Ascaris lumbricoides eggs in dog faeces (Joshi and Sabne, Reference Joshi and Sabne1977; Traub et al., Reference Traub2002). Dogs were also involved in transient passage of other canid parasites, such as Toxocara canis, as a result of coprophagy (Fahrion et al., Reference Fahrion2011); this phenomenon has often led to an overestimation of the occurrence of patent helminth infections in dogs (Nijsse et al., Reference Nijsse2014). Although the ITS region is thought to be too conserved for accurate differentiation of A. caninum and A. duodenale (Traub et al., Reference Traub2007), this is unlikely to be the case in this study because the isolate was confirmed as A. duodenale by amplification and partial sequencing of the cytochrome c oxidase gene. The possibility that this isolate originated from human faeces as a result of misidentification as dog faeces was ruled out after detecting the latter's DNA from the faecal sample. This finding is interesting because human infections with A. duodenale are rare in Kenya and have been identified through morphological characterization (Miller, Reference Miller1970; Macpherson and Craig, Reference Macpherson, Craig, Macpherson and Craig1991). Moreover, recent molecular-based studies have identified Necator americanus as the only hookworm species infecting humans in Kenya (Arndt et al., Reference Arndt2013; Easton et al., Reference Easton2016).
The zoonotic canine hookworm A. ceylanicum was not reported in this study. However, only 70 faecal samples of the 490 microscopically positive hookworm samples were characterized by PCR-RFLP and/or DNA sequencing. The failure to detect this species could be attributed to the inability of the PCR to detect mixed infections (George et al., Reference George2016), especially where the primary hookworm A. caninum is dominant, and would necessitate the use of species-specific primers (Merino-Tejedor et al., Reference Merino-Tejedor2018). Additionally, despite co-infection with different hookworm species, eggs are not shed equally. Dogs experimentally infected with A. caninum excreted more eggs than those infected with A. braziliense; however, more adult worms of the latter were detected than those of A. caninum (Loukas et al., Reference Loukas2005; Dias et al., Reference Dias2013). Ancylostoma ceylanicum has been reported in humans and cats in African countries, including Sierra Leone, Madagascar and South Africa (Traub, Reference Traub2013). Ancylostoma ceylanicum was recently detected in four dog faecal samples in Tanzania, and this was only possible after using species-specific primers to show a coinfection with the dominant A. caninum (Merino-Tejedor et al., Reference Merino-Tejedor2018). This observation confirms our postulation that A. ceylanicum may be prevalent in dogs in Kenya, as both countries experience similar climatic conditions. In previous post-mortem studies in Kenya, A. ceylanicum might have been overlooked due to the morphological similarities with A. braziliense, which was reported from Turkana (Wachira et al., Reference Wachira1993; Buishi et al., Reference Buishi2006; Traub, Reference Traub2013).
In conclusion, the findings of this study highlight the need to consider the public health importance of domestic dogs as reservoirs of zoonotic ancylostomiasis. As environmental hygiene is poorly regulated in Kenya, public areas, including beaches, parks, shared water sources (humans and animals) and playing grounds for children, can be significant sources of such zoonoses. Ideal effective control measures against zoonotic ancylostomiasis would include regular deworming of community dogs by integrated anthelmintic treatment, with ongoing dog population control and vaccination programmes implemented by the county governments in collaboration with the department of veterinary services and other partners. The public should be educated in relation to responsible pet ownership (prohibiting free roaming), the importance of at least monthly deworming protocols, and appropriately disposing of dog faeces as advocated by the Tropical Council for Companion Animal Parasite (TroCCAP) “Guidelines for the diagnosis, treatment and control of canine endoparasites” (www.troccap.com). The public and tourists should also be educated on basic hygienic principles, including the use of protective footwear in parks, playgrounds or beaches.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X1900018X
Author ORCIDs
E. Mulinge, 0000-0003-2402-7913
Acknowledgements
We wish to acknowledge participants in this study with whose consent we collected faecal samples from their dogs. We acknowledge field assistants and veterinary officers for their support during fieldwork. We appreciate the undergraduate students and interns who offered technical support. This work is published with permission from the Director, Kenya Medical Research Institute (KEMRI).
Financial support
This study was funded by Deutsche Forschungsgemeinschaft (DFG) RO3753-1/1, -2/1, -3/1 through the Cystic Echinococcosis in sub-Saharan Africa Research Initiative (CESSARi).
Conflict of interest
None.
Ethical standards
See Materials and methods section.