Introduction
Hookworms are soil-transmitted nematodes that cause infections through active skin-penetration of the host by third-stage larvae (L3) (Bethony et al., Reference Bethony2006; Hossain and Bhuiyan, Reference Hossain and Bhuiyan2016). They are widely distributed in the tropics and in subtropical regions (Bethony et al., Reference Bethony2006), where environmental and climatic conditions, including rainfall, surface temperature, altitude and soil temperatures, are conducive for transmission (Hotez et al., Reference Hotez2008). According to Bethony et al. (Reference Bethony2006), hookworm infections are ranked third in terms of disease burden in humans, after common roundworm (Ascaris suum) and whipworm (Trichuris trichuria) infections. Hookworm infection causes severe protein malnutrition and iron-deficiency anaemia and intestinal blood loss through the haematophagous effect of the adult parasites in the intestines as well as bleeding from the feeding sites in the intestines (Zhan et al., Reference Zhan2001; Liu et al., Reference Liu2015; Xie et al., Reference Xie2017).
Most hookworm infections in humans are attributed to Ancylostoma duodenale and Necator americanus (Hawdon, Reference Hawdon1996; Zhan et al., Reference Zhan2001; Phosuk et al., Reference Phosuk2013; Hasegawa et al., Reference Hasegawa2014, Reference Hasegawa2017; Tun et al., Reference Tun2015; George et al., Reference George2016; Smout et al., Reference Smout2017), with N. americanus being the predominant species infecting humans and non-human primates (Hasegawa et al., Reference Hasegawa2014, Reference Hasegawa2017). The common species that infect companion animals such as dogs include Ancylostoma caninum, Ancylostoma ceylanicum, Ancylostoma braziliense and Uncinaria stenocephala, whereas those that commonly infect cats include A. braziliense, Ancylostoma tubaeforme, A. ceylanicum and U. stenocephala (Taylor et al., Reference Taylor, Coop and Wall2007; Bowman et al., Reference Bowman2010; Liu et al., Reference Liu2015). However, A. caninum, A. ceylanicum and A. braziliense may also infect humans and cause zoonotic diseases such as eosinophilic enteritis (EE) and cutaneous larva migrans (CLM) (Ngui et al., Reference Ngui2012; Liu et al., Reference Liu2015). Hookworm species that have been reported in sub-Saharan Africa include N. americanus, A. duodenale (Mabaso et al., Reference Mabaso2003; Hotez and Kamath, Reference Hotez and Kamath2009), A. caninum (Minnaar and Krecek, Reference Minnaar and Krecek2001; Lamb et al., Reference Lamb, Napier and Mukaratirwa2012) and A. braziliense (Minnaar and Krecek, Reference Minnaar and Krecek2001; Bowman et al., Reference Bowman2010; Lamb et al., Reference Lamb, Napier and Mukaratirwa2012; Shepherd et al., Reference Shepherd, Wangchuk and Loukas2018).
Due to rapid urbanization and increased human–wildlife interaction (in conservation centres and zoological gardens for endangered and valuable animals), wildlife are emerging as significant helminthic zoonotic agents (Xie et al., Reference Xie2017). According to Seguel and Gottdenker (Reference Seguel and Gottdenker2017), hookworms in wildlife have been documented mostly through necropsies of culled animals or animals incidentally found dead. Globally, at least 68 hookworm species have been reported in nine orders, 22 families and 108 species of wild mammals (Seguel and Gottdenker, Reference Seguel and Gottdenker2017). Hookworm species with the largest host range in wildlife include A. tubaeforme, Ancylostoma pluridentatum, A. braziliense and the human nematode N. americanus (Seguel and Gottdenker, Reference Seguel and Gottdenker2017). Of 218 studies worldwide describing hookworm infections in wildlife hosts, only 16.4% were conducted in Africa (Seguel and Gottdenker, Reference Seguel and Gottdenker2017). Hookworm species infecting wildlife reported in Africa include Ancylostoma somaliense (jackal), Ancylostoma genettae (genet), Ancylostoma protelesis (aardwolf) and Necator gorillae (gorilla) (Shepherd et al., Reference Shepherd, Wangchuk and Loukas2018).
With regards to public health, it is important to know the prevalence and distribution of hookworms (Palmer et al., Reference Palmer2007). Previously, hookworm species identification was based on morphological characteristics of the adult worms (post-mortem) or coprological examination of hookworm-like eggs recovered from faeces of infected hosts (Palmer et al., Reference Palmer2007; Lamb et al., Reference Lamb, Napier and Mukaratirwa2012). Identification of Ancylostoma spp. based on morphological characters is labour intensive, time consuming and requires skilled personnel, but even then, there is always the possibility of overlooking mixed infections (Traub et al., Reference Traub2004; Lamb et al., Reference Lamb, Napier and Mukaratirwa2012). Lamb et al. (Reference Lamb, Napier and Mukaratirwa2012) reported that differentiating between A. caninum and A. braziliense in dogs using egg morphology is problematic.
Recently, species-specific molecular-based techniques for rapid differentiation of developmental stages of hookworms have been developed (Hawdon, Reference Hawdon1996; Zhan et al., Reference Zhan2001; Traub et al., Reference Traub2004). According to Lamb et al. (Reference Lamb, Napier and Mukaratirwa2012), these molecular-based techniques had hardly been applied in the study of hookworms in southern Africa. Thus, there is a paucity of information on the application of molecular tools for the identification of hookworm species in this region, more especially in humans, dogs and wild canids. This study therefore aimed to determine the hookworm species of humans (school-going children) and stray dogs from KwaZulu-Natal province and selected wild canids and felids from Mpumalanga province of South Africa, and their prevalence and phylogenetic relationships. Techniques applied ranged from coproscopy, coproculture, polymerase chain reaction (PCR) amplification, PCR-restriction fragment length polymorphism (RFLP) and sequencing of the nuclear ribosomal internal transcribed spacer (ITS) and 5.8S rRNA region from the larval isolates.
Materials and methods
Sample collection
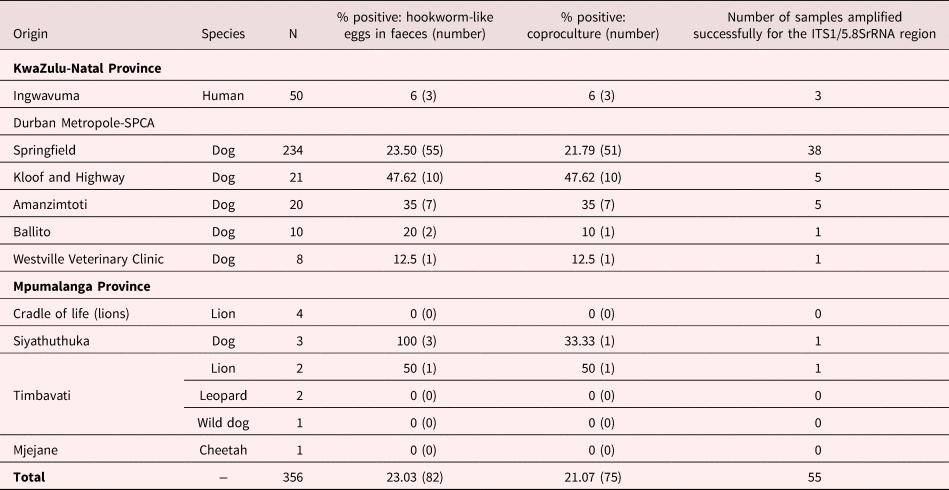
A total of 356 faecal samples were collected from stray dogs housed in five centres of the Society for the Prevention of Cruelty to Animals (SPCA) in Durban metropole, and Siyathuthuka community, Belfast, Mpumalanga province (table 1). Human samples were collected from two primary schools (Mbadleni primary school (n = 25) and Madeya intermediate primary school (n = 25)) in the Ingwavuma area of KwaZulu-Natal province of South Africa and from wild canids and felids in Mpumalanga game reserves (Cradle of Life (n = 4 lions), Timbavati (n = 2 lions, n = 2 leopards and n = 1 wild dog) and Mjejane (n = 1 cheetah)) (table 1). Selection of schools was based on risk of infection as reported from a previous study on soil-transmitted helminths of school-going children in the area (Kabuyaya et al., Reference Kabuyaya2017). The history of the stray dogs and wild animals was unknown and they were of mixed sex, different breeds, and varying ages. Fresh faecal samples were collected in universal sample bottles. Depending on how far from the laboratory the samples were collected, transportation times for the faecal samples varied. For instance, it took less than 30 minutes to transport samples to the laboratory from the SPCA centres in the Durban metropole, whereas it took 4–6 hours to transport faecal samples from game reserves in Mpumalanga province. Samples collected from Ingwavuma area were individually mixed with semi-wet charcoal in 500 ml plastic jars and then stored in cooler boxes for transport to the laboratory.
Table 1. Prevalence of hookworms from faecal samples of dogs, wild canids and humans from the Durban metropole and selected areas of Mpumalanga province, South Africa.
Faecal sample examination
Upon arrival of samples in the laboratory, the modified Wisconsin sugar flotation method was used to process the samples in order to detect the presence of hookworm-like eggs microscopically (Pittman et al., Reference Pittman2010). Immediately after coproscopy, faeces from each positive sample (faeces with hookworm-like eggs) were individually cultured, and larvae recovered using the Baermann technique as described by Reiss et al. (Reference Reiss2007). Culturing of faecal samples involved mixing 5 g of faeces with sterile semi-wet crushed charcoal in 500 ml plastic jars and incubating for 7 days at 27°C (Reiss et al., Reference Reiss2007). Third-stage larvae (L3) were recuperated using the Baermann apparatus and preserved in 70% ethanol for molecular analysis.
Molecular analysis
DNA was extracted from four pooled larvae from each coproculture positive sample using the Genomic DNA™ Tissue MiniPrep (Zymo Research Corporation, Irvine, CA, USA) following the manufacturer's instructions. Pooled tissue samples of no fewer than four larvae were microcentrifuged at 14,000 ×g for 5 minutes to facilitate the removal of alcohol. The extracted DNA was subjected to PCR targeting the internal transcribed spacer and 5.8S rRNA (ITS1-5.8S) region using primers AF (5′-CTTTGTCGGGAAGGTTGG-3′) and AR (5′-TTCACCACTCTAAGCGTCT-3′) designed by Liu et al. (Reference Liu2015). This primer pair is reported to amplify a 404 bp region of A. ceylanicum, a 404 bp region of A. caninum, a 408 bp region of A. braziliense and a 406 bp region of U. stenocephala DNA (supplementary table S1). Each sample was amplified in reactions of 25 μl containing 12.5 μl DreamTaq PCR master mix (2X), 5 μl of DNA, 1 μl of each primer (forward and reverse, concentration 10 μm) and 5.5 μl of nuclease-free H2O. The PCR cycle parameters were as follows: 1 cycle of 96°C for 5 minutes for initial denaturation, followed by 35 cycles at 96°C for 30 seconds, 54°C for 30 seconds and 72°C for 50 seconds, and 1 final extension cycle at 72°C for 7 minutes. PCR products were separated by electrophoresing 4 μl of amplicons at 80 V for 80 minutes in 2% gels (in 0.5X TBE buffer). The electrophoresis buffer was 0.5X TBE, to which 100 μl 0.5 μg/ml ethidium bromide was added to allow visualization of bands by UV transillumination. Restriction fragment length polymorphism (RFLP) analysis was performed by digesting 7 μl of each ITS PCR product with 0.2 μl of EcoRII in a total volume of 20 μl for 3 h at 37°C. Using the same conditions, the PCR products were also digested directly with BsuRI. EcoRII differentiates A. ceylanicum from A. braziliense, whereas BsuRI differentiates U. stenocephala from the other hookworm species (A. ceylanicum, A. braziliense and A. caninum) (supplementary table S1). Restriction fragments were separated by electrophoresis in 3% agarose stained with ethidium bromide under the conditions described above.
Sequencing and phylogenetic analysis
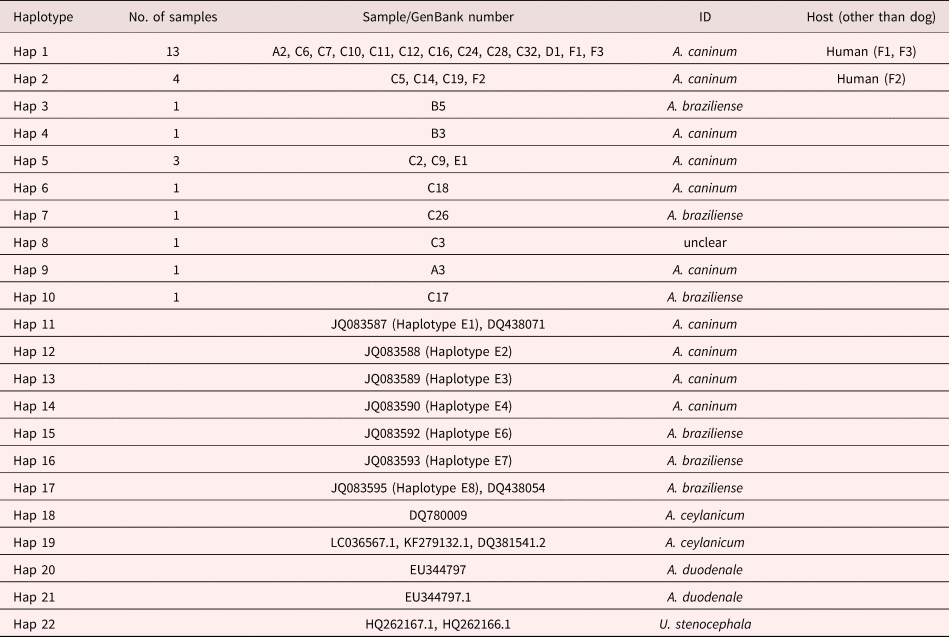
Thirty-four amplicons from samples from stray dogs (30), humans (3) and a lion (1) were selected and sent for sequencing at Inqaba Biotechnical Industries (Pty) Ltd., Pretoria, South Africa. Obtained sequences were BLAST searched in the GenBank database of the National Center for Biotechnology Information (NCBI) to identify the closest matches of each sequence. Sequences were downloaded, edited and aligned together with GenBank-derived sequences of A. caninum, A. braziliense, A. duodenale, A. ceylanicum, A. tubaeformae and U. stenocephala using the BioEdit Sequence Alignment Editor (Hall, Reference Hall1999).
Trees were created using neighbour-joining and maximum likelihood methods in PAUP 4.0b10 (Swofford, Reference Swofford2002) and Bayesian Inference as implemented in MrBayes version 3.0b4 (Huelsenbeck and Ronquist, Reference Huelsenbeck and Ronquist2001), based on the most likely model of nucleotide substitution (HKY) determined in MEGA 7 (Posada, Reference Posada2008). In maximum likelihood analyses, starting trees were obtained by neighbour-joining followed by tree bisection and reconnection (TBR) branch swapping. One thousand bootstrap replicates were carried out in the neighbour-joining and maximum likelihood analyses in order to assess node reliability. Bayesian analyses were run using four Markov chains and sampling every 100 generations until the standard deviation of the split frequencies was less than 0.01. The chains were heated with the temperature scaling factor T = 0.02. We discarded the first 50,000 trees as burn-in, in each case having checked in a preliminary run that this was more than sufficient to achieve stationarity, and constructed a 50% majority rule consensus tree from the remaining trees.
Individual pairwise genetic p-distances between the sequence haplotypes were determined using MEGA7. Between and within clade (species) genetic distances, based on the clades present in the phylogenetic analysis, were also calculated.
Data analysis
The number of samples positive for hookworm-like eggs was used in calculating the prevalence of infection (%) using the formula Prevalence = (Total number of infected animals divided by the total number of animals examined) × 100.
Results
Faecal sample examination
Of the 356 faecal samples from humans and other animal species that were collected and screened by coproscopy for hookworm-like eggs, 23.03% (82/356) were positive (table 1). Of the 82 positive samples, 21.07% (75/356) were found to be positive through coproculture. In the Durban metropole, prevalence of hookworm-like eggs determined by coproscopy was highest in stray dogs from the Kloof and Highway SPCA (47.62%), followed by Amanzimtoti (35%), Springfield (23.50%) and Ballito (20%), whereas the Westville clinic had the lowest prevalence (12.5%). Only three stray dogs were screened in Siyathuthuka community, Mpumalanga province and they were all found to be positive through coproscopy; however, only one was positive through coproculture and was successfully amplified. One lion out of the 13 wildlife samples screened was positive for hookworm-like eggs. Of the 75 samples that were deemed positive by coproculture, the ITS1/5.8S rRNA region of 55 samples was successfully amplified.
PCR amplifications
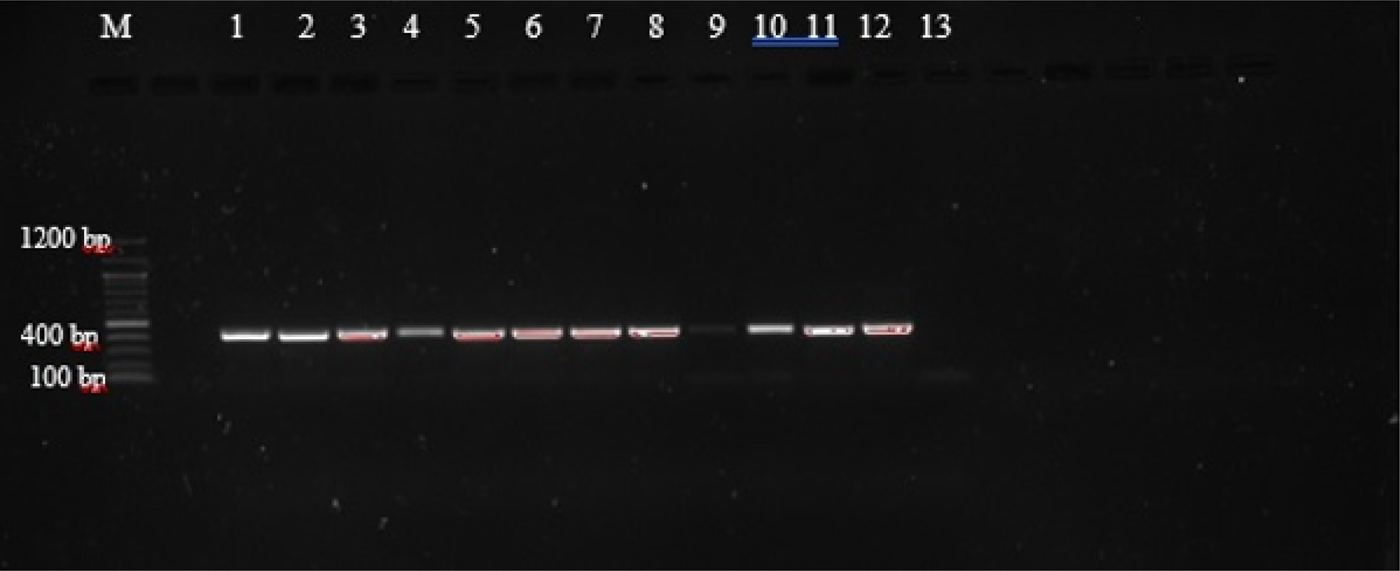
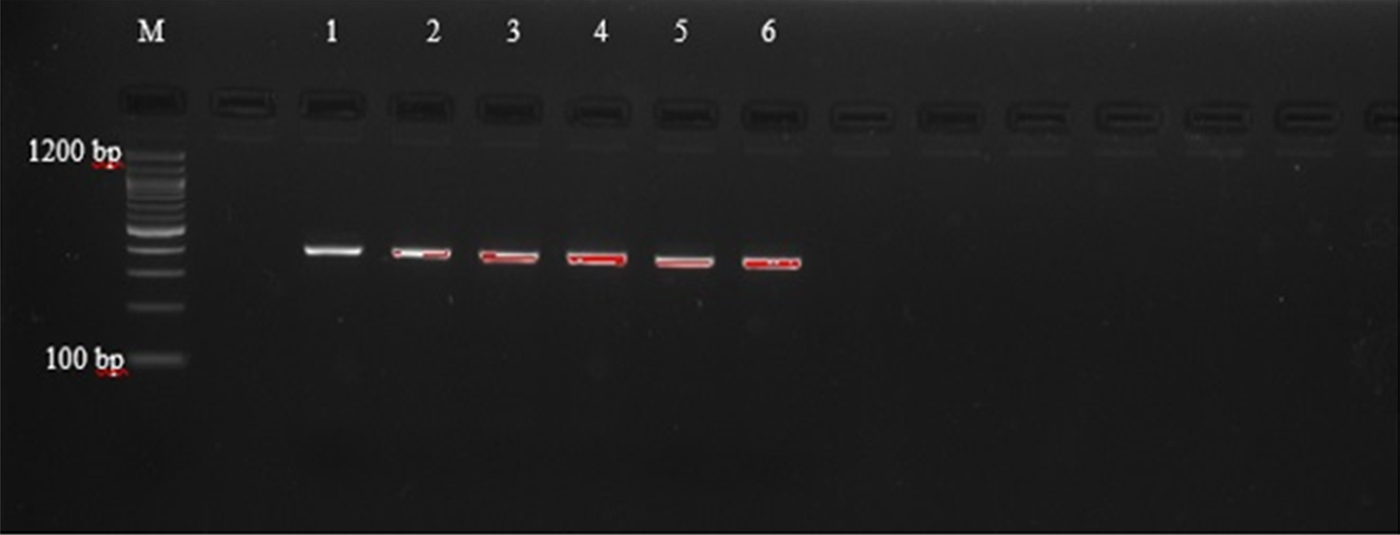
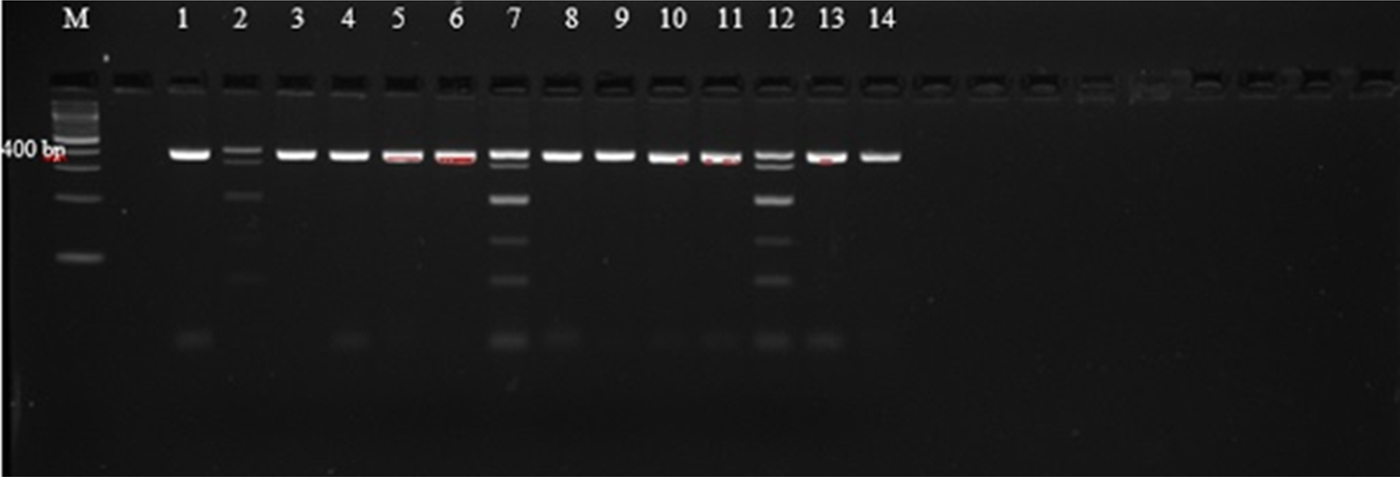
Agarose gel electrophoresis of PCR amplicons generated with primers AF and AR yielded single bands approximately 400 bp in length, corresponding with those reported by Liu et al. (Reference Liu2015), as shown in supplementary table S1, and indicating that amplification was successful (fig. 1). However, these primers did not allow for easy differentiation between the species, as they amplify regions that are of very similar size (404 bp, A. caninum; 404 bp, A. ceylanicum; 408 bp, A. braziliense; 406 bp, U. stenocephala). The amplicons were therefore subjected to PCR-RFLP using restriction endonucleases BsuRI and EcoRII (figs 2 and 3) in order to allow differentiation between the species by size and number of restriction endonuclease cleavage products (supplementary table S1).
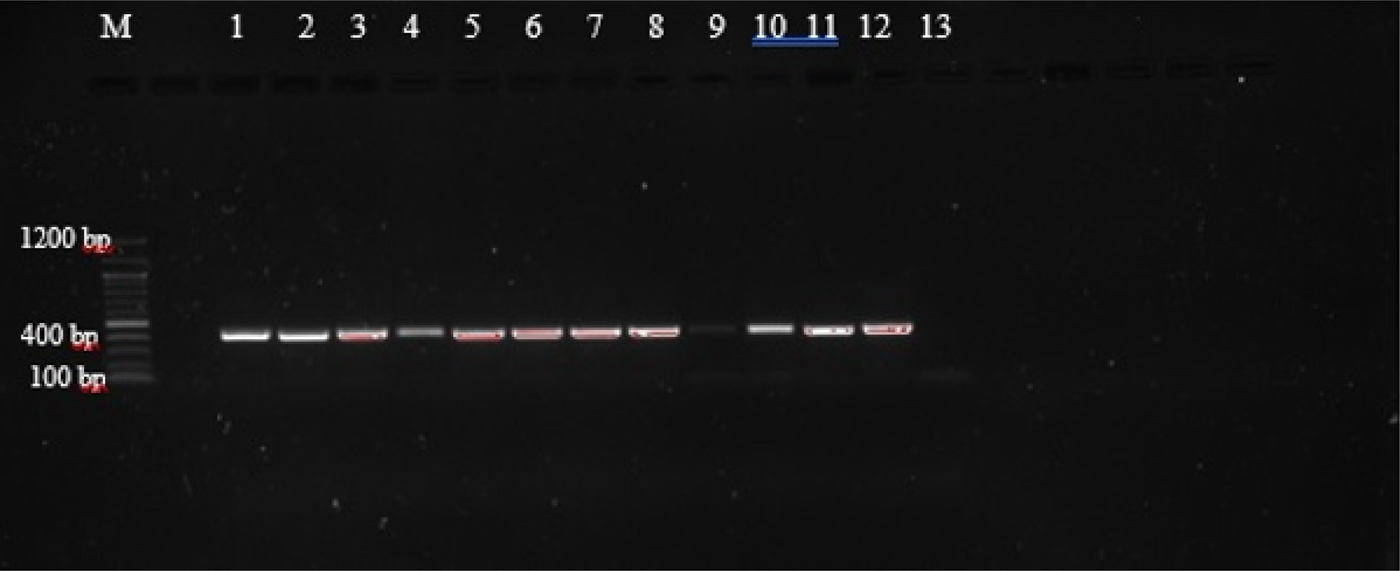
Fig. 1. PCR amplification with ITS1/5.8S primers of third-stage larvae (L3) from selected hookworm spp. isolates from dogs and lion. (1) C26; (2) B4; (3) H1; (4) NL1; (5) C12 (6) C11; (7) C2; (8) C22; (9) C1; (10) C7; (11) C25; (12) C6; (13) negative control.

Fig. 2. PCR-RFLP with BsuRI restriction endonuclease of selected third stage larvae (L3) of hookworm spp. isolates from dogs. (1) C24; (2) C5; (3) C9; (4) C20; (5) C8; (6) B5.
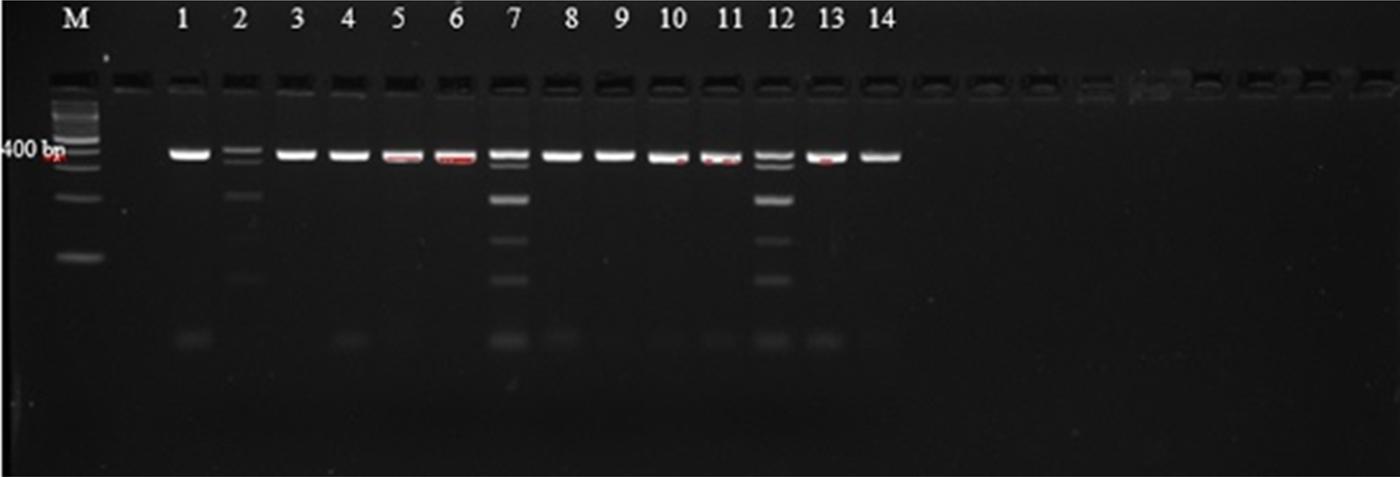
Fig. 3. PCR-RFLP with EcoRII restriction endonuclease of selected third-stage larvae (L3) of hookworm spp. isolates from dogs. (1) C28; (2) C26; (3) C2; (4) C27; (5) B1; (6) C12; (7) C3; (8) A2; (9) C25; (10) C11; (11) C10; (12) C17; (13) A1; (14) E1.
PCR-RFLP analysis
An attempt was made to digest all 55 successfully amplified samples with the restriction endonucleases EcoRII and BsuRI. None of the amplicons were digested by BsuRI enzyme (fig. 2 and supplementary table S2), leading to the conclusion that U. stenocephala was not present in the sample set. Forty of the samples were not digested by either restriction enzyme (EcoRII and BsuRI), yielding a single band of 404 bp, consistent with an identification as A. caninum (supplementary table S2) (Liu et al., Reference Liu2015). Fifteen samples were digested by EcoRII (fig. 3 and supplementary table S2). These samples yielded complex banding patterns not consistent with those reported by Liu et al. (Reference Liu2015) for either A. ceylanicum only (two bands of 76 and 328 bp) or A. braziliense only (76, 122 and 210 bp) (supplementary table S1). Rather, seven of the samples contained bands equivalent to 404, 328, 210, 122 and 76 bp (fig. 3). This combination of bands is consistent with the presence in the sample of a mixture of A. caninum (404 bp), A. ceylanicum (76 and 328 bp) and A. braziliense (76, 122 and 210 bp) (supplementary table S1). Probably, there were also mixtures of A. caninum and A. ceylanicum in four other samples. Four of the samples yielded bands that were consistent with A. caninum and one or two more band patterns that did not correspond with any of the bands that were expected, thus the parasite was unidentifiable (unknown) (supplementary table S2). The restriction results therefore revealed an overall prevalence of 72.5% (40/55) of A. caninum, 12.7% (7/55) of mixed infection of A. caninum, A. braziliense and A. ceylanicum, 7.3% (4/55) A. caninum and A. ceylanicum mixed infection and 7.3% (4/55) A. caninum and the unreadable bands.
Sequencing and phylogenetic analysis
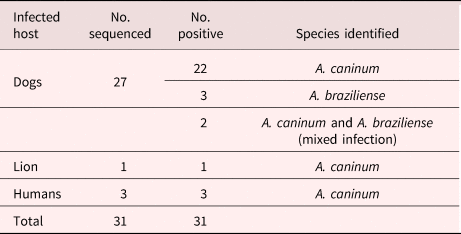
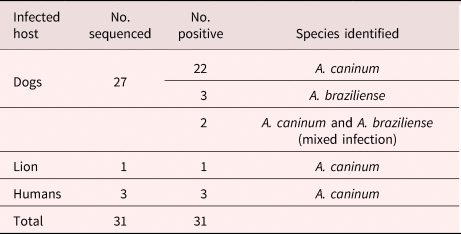
Thirty-four amplicons were selected and sent for sequencing using only the forward primer (AF). Samples sent for sequencing included those that had more than one band after restriction with EcoRII and were suspected to be species other than A. caninum. However, only 31 of the sequences were readable and GenBank BLAST searches of obtained sequences showed that 28 of the samples matched with A. caninum, whereas three were A. braziliense. After sequencing it was also revealed that of the 15 EcoRII restricted samples, three matched with A. braziliense and the remaining 12 were A. caninum, indicating the prevalence to be 94.4% (52/55) A. caninum and 5.45% (3/55) A. braziliense in the samples sequenced. Results of sequencing and phylogenetic analysis were therefore not consistent with the findings of the PCR-RFLP analysis.
Of the 27 sequenced sample isolates from stray dogs, 22 were shown to be A. caninum, three were A. braziliense and the remaining two were shown to have a mixed infection of A. caninum and A. braziliense. The hookworm-positive wildlife (1) and human (3) sequenced samples matched with A. caninum. Ancylostoma braziliense infections were only detected in dog samples from the Durban metropole (table 2).
Table 2. Summary of hookworm species from dogs, humans and wildlife from the Durban metropole and Mpumalanga provinces identified through BLAST searches after sequencing.
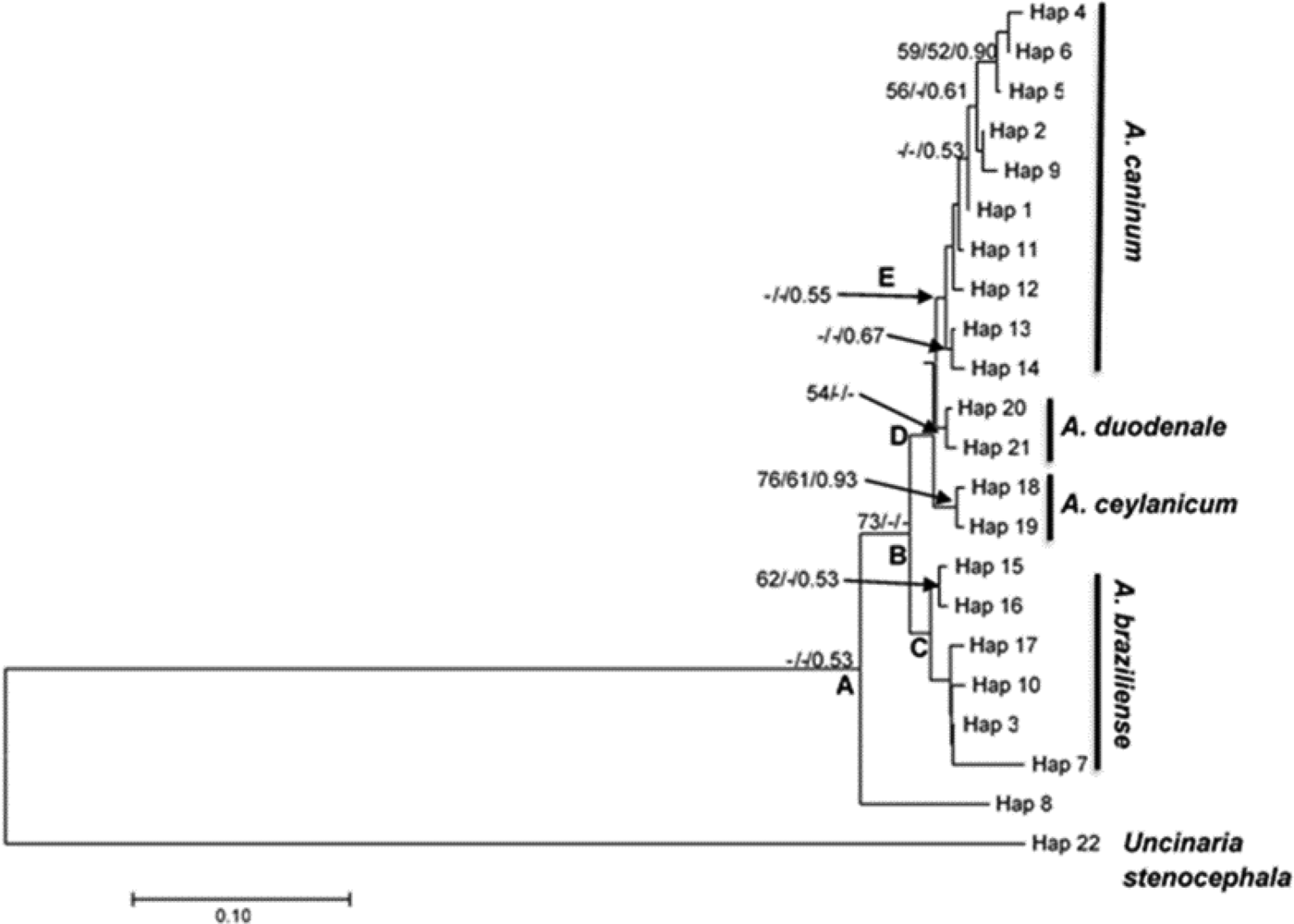
Sequenced samples were identified by their cladal affiliation with reference to other identified sequences downloaded from the NCBI GenBank and were included in phylogenetic analyses of the nuclear ribosomal ITS1/5.8S DNA region (table 3 and fig. 4). All experimental samples grouped together to form a monophyletic clade (A) with reference to the outgroup represented by U. stenocephala (fig. 4). However, Clade A was not supported. Clade B consisted of all haplotypes except haplotype 8 and was moderately supported (bootstrap value of 73% from neighbour-joining analysis). Haplotype 8 represented sample C3, which was one of those with a complex banding pattern in the RFLP analysis (fig. 3), suggestive of a mixed infection by more than one (and possibly three) hookworm species (A. caninum, A. braziliense and A. ceylanicum). It did not yield an identifiable sequence, possibly because it was a mixture of more than one species. Clade B was an Ancylostoma clade and included haplotypes identified as A. caninum, A. duodenale, A. braziliense and A. ceylanicum. The haplotypes further clustered to form clade D (A. caninum, A. ceylanicum and A. duodenale), which was sister to clade C (A. braziliense). There was little bootstrap support for the above clades, except for the A. ceylanicum clade, which was moderately supported.
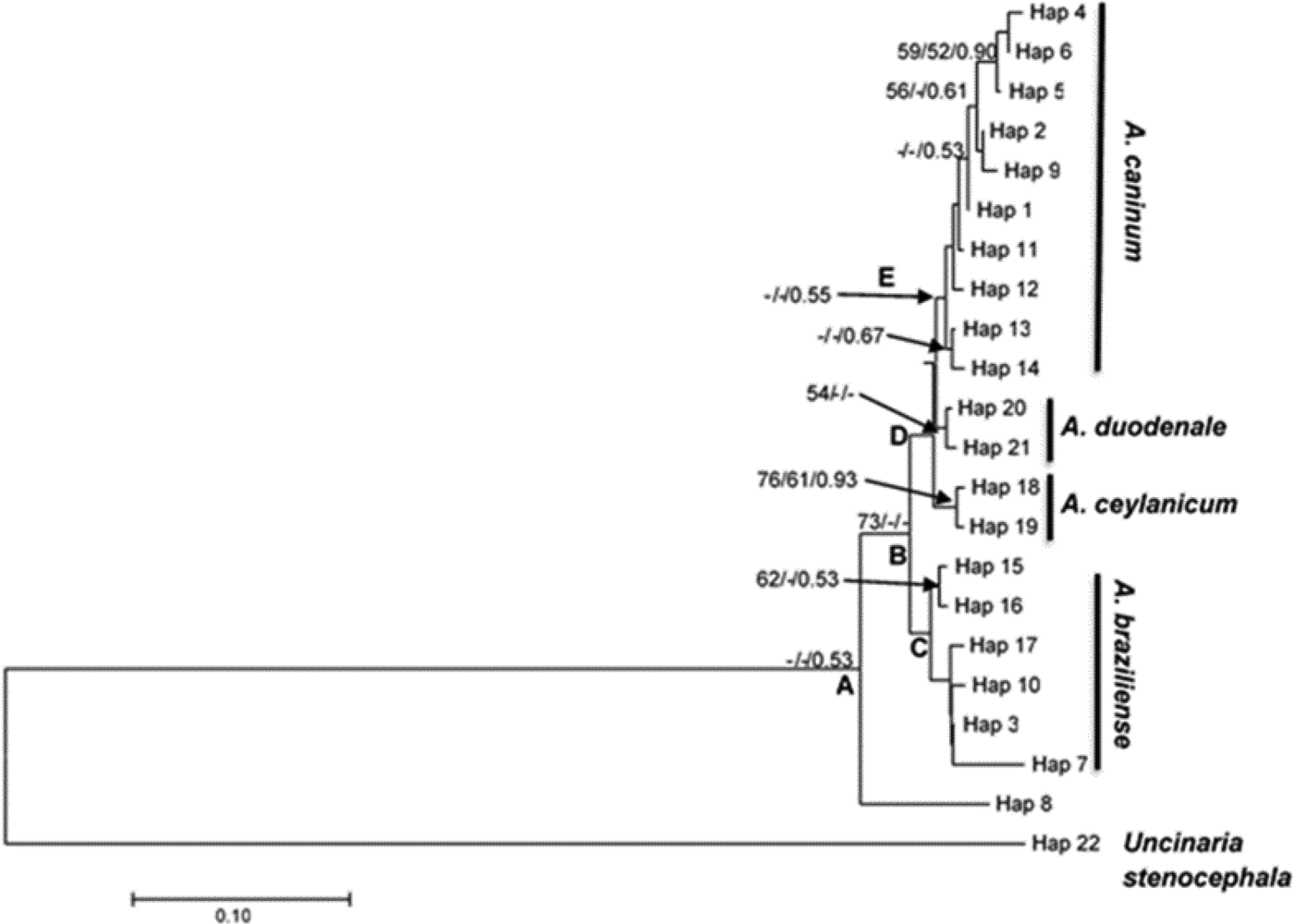
Fig. 4. Neighbour-joining tree based on haplotypes of the ITS1/5.8S rDNA region of experimental samples obtained from dog, human and selected wildlife samples (Hap 1–Hap 10), GenBank-derived haplotypes of the Ancylostoma species (A. caninum, A. braziliense, A. ceylanicum and A. duodenale) and Lamb et al. (Reference Lamb, Napier and Mukaratirwa2012) haplotypes of A. caninum and A. braziliense as the in groups. GenBank-derived Uncinaria stenocephala haplotypes were used as the outgroup (Hap 22). Support values indicated at the nodes are, in order, derived from neighbour-joining (NJ) analyses and congruent maximum likelihood (ML) and Bayesian inference analyses.
Table 3. Haplotype status of hookworm species from experimental samples and GenBank used in molecular identification of hookworms infecting dogs, humans and wildlife from the Durban metropole and Ingwavuma area of KwaZulu-Natal, and Mpumalanga province of South Africa, as shown in fig. 4.
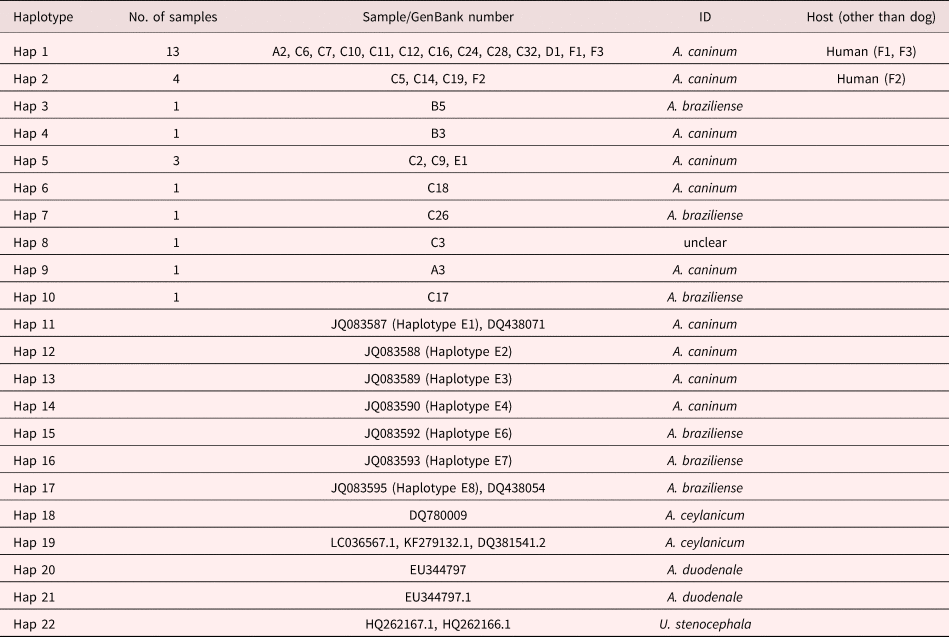
Identification of experimental samples was based on cladal affiliation with samples of known identity downloaded from GenBank, according to the phylogenetic species concept (Cracraft, Reference Cracraft and Johnston1983). Thus experimental haplotypes 1, 2, 4, 5, 6 and 9 were identified as A. caninum based on their presence in a clade that contained GenBank-derived haplotypes 11, 12, 13 and 14, identified as A. caninum (table 3 and fig. 4). Similarly, experimental haplotypes 3, 7 and 10 were identified as A. braziliense based on their co-occurrence in a clade (C) with three GenBank haplotypes identified as A. braziliense. The human experimental sample sequences did not cluster together to form a different clade on their own but formed a clade with the dog hookworm A. caninum GenBank haplotypes (table 3).
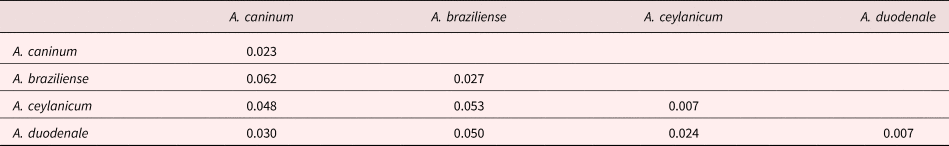
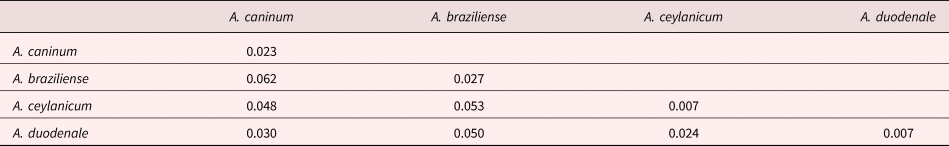
Various Ancylostoma clades are separated by shallow genetic distances, and mean genetic p-distances within species clades ranged from 0.007 to 0.023 (supplementary table S3 and table 4) and were smaller than those between species clades, which ranged from 0.024 to 0.062 substitutions per site, as might be expected according to the genetic species concept (Baker and Bradley, Reference Baker and Bradley2006). Overall, the mean within-Ancylostoma-species genetic distance was 0.016, whereas the between-species distance was 0.040.
Table 4. Mean genetic p-distances within (diagonal) and between (below diagonal) Ancylostoma species isolated from our study and from GenBank.
Discussion
Through coproscopical examination of faecal samples, the overall prevalence of hookworm-like eggs in both KwaZulu-Natal and Mpumalanga provinces was found to be 23.03% (82/356); of those positive on coproscopy, 21.07% (75/356) were also positive on coproculture. The decrease in sensitivity of coproculture was not expected, as this method is considered to be more reliable than coproscopy (Ziem et al., Reference Ziem2006). This may have been due to differences in intensity of infection in each sample; for instance, if the host was not heavily infected and the sample had a small amount of faeces with low numbers of hookworm eggs resulting in the portion of faeces cultured having few or no eggs.
Fifty-five of the 75 samples that yielded hookworm-like larvae were successfully PCR-amplified using primers designed to amplify the nuclear ribosomal ITS1-5.8S rDNA region. The failure of the other 20 samples to amplify may have been due to low hookworm DNA concentrations, as the intensity of infection across the samples may not have been the same. The BsuRI enzyme, which was expected to distinguish U. stenocephala from A. caninum, A. ceylanicum and A. braziliense, did not digest any of the samples, indicating the absence of U. stenocephala from the sample set. Forty of the samples were not digested by either BsuRI or EcoRII and produced products consistent with a size of 404 bp, and these were deemed to be A. caninum (Liu et al., Reference Liu2015). Fifteen samples were digested by EcoRII: these digestion patterns did not correspond with the banding patterns reported by Liu et al. (Reference Liu2015) for pure samples of either A. ceylanicum or A. braziliense, as they presented more bands (up to 5) than could be explained by the presence of either of these species alone (2 or 3 bands, respectively). For seven of the samples, there was also an extremely small band, which probably represents unincorporated primers (e.g. samples C3, C17 and C26). The combination of bands was, however, consistent with the presence of probably a mixture of A. caninum, A. ceylanicum and A. braziliense, or a mixed infection of A. caninum and A. braziliense. This came as a surprise, as A. ceylanicum has not been documented in Africa previously but has been reported to be endemic in Asia (Bowman et al., Reference Bowman2010; Liu et al., Reference Liu2015; Shepherd et al., Reference Shepherd, Wangchuk and Loukas2018), Australia (Bowman et al., Reference Bowman2010; Shepherd et al., Reference Shepherd, Wangchuk and Loukas2018) and South America (Bowman et al., Reference Bowman2010).
Sequences of three of the samples (samples C3, C17 and C26) appeared to be A. braziliense, whereas the other 12 were consistent with A. caninum. These sequencing results were more consistent with what was expected or predicted, as there have been reports of A. caninum and A. braziliense infections in South African dogs (Minnaar and Krecek, Reference Minnaar and Krecek2001; Lamb et al., Reference Lamb, Napier and Mukaratirwa2012). However, in the case of mixed infections it is likely that if a sequence can be obtained it will be that of the numerically dominant species present in the mixed infection, i.e. either A. braziliense or A. caninum, as mentioned above. One would not expect to be able to identify more than one species from a single sequence electropherogram, as mixtures of equal amounts of more than one species of hookworm are likely to lead to confusing patterns. This may explain why, in these cases, the sequencing results identify one species only, whereas the PCR-RFLP results identify mixtures of more than one Ancylostoma species. Further, this may explain why the PCR-RFLP patterns for some samples are consistent with the presence of A. ceylanicum in a mixture, although it was not detected during sequencing. Examination of the lanes of the PCR-RFLP gels where multiple bands were observed shows that they were not all of equal intensity, consistent with the idea that some of the species were present in the mixture at higher concentrations than others.
The presence of multiple bands in this study in the EcoRII digests of certain samples, in contrast to the observations of Liu et al. (Reference Liu2015), may also be explained on the basis that their study was carried out in China, which is geographically distant and climatically different from South Africa, and may therefore have different patterns of prevalence of Ancylostoma species. It is also possible that the samples that produced multiple banding patterns were contaminated with nematode larvae of a different family. Furthermore, Liu et al. (Reference Liu2015) noted that A. caninum isolates from their study (China) were phylogenetically distinct from A. caninum isolates from the USA and Brazil. This was apparent on the neighbour-joining tree based on ITS1 and 5.8S rRNA sequences, in which A. caninum isolates from Brazil and the USA clustered to form a clade that was distinct from that formed by A. caninum isolated from China (Liu et al., Reference Liu2015). Such within-species diversity is also likely to be found in hookworm species other than A. caninum. Lamb et al. (Reference Lamb, Napier and Mukaratirwa2012) reported unique southern African ITS haplotypes of hookworm species in dogs from Durban, South Africa, which may also explain the differences in band patterns observed in our study.
All dogs (three in total) from the Siyathuthuka community in Mpumalanga province were coproscopy-positive for hookworm-like eggs, although isolates from only one of these was successfully amplified and identified with A. caninum. By comparison, dogs from KwaZulu-Natal province had a prevalence of hookworm-like eggs of 25.6% (75/293). Thus there appears to be a higher hookworm prevalence in the Siyathuthuka community than in KwaZulu-Natal. This may be attributable to socio-economic differences, as Siyathuthuka is a rural area where there is poor veterinary care of animals, whereas dogs from Durban metropole, although deemed stray, were mostly lost and belonged to owners who took care of them, including regular deworming. Other studies have also found that rural areas or resource-poor communities tend to have higher hookworm prevalence when compared to urban areas where owners are most often capable of looking after their dogs (Minnaar and Krecek, Reference Minnaar and Krecek2001; Liu et al., Reference Liu2015). Furthermore, prevalence of dog hookworms in the Durban metropole would be expected to be higher, as human hookworms were reported to be more common in the KwaZulu-Natal province than in any other region of the country (Hotez and Kamath, Reference Hotez and Kamath2009) due to the conducive environmental conditions (favourable environment and soil types for hookworm survival and transmission). This is particularly true along the coastal plain of KwaZulu-Natal where Durban is situated (Mabaso et al., Reference Mabaso2003). The prevalence of hookworm-like eggs in dogs from Siyathuthuka community in Mpumalanga province should be interpreted with caution, as the sample size was too small to reach a conclusion on the actual prevalence of hookworms in this locality. For better comparison and validation of these results, however, more samples should be collected from dogs and humans in resource-poor and high-income areas of Mpumalanga and KwaZulu-Natal provinces.
Although there have been reports on the prevalence of dog hookworms in South Africa (Minnaar et al., Reference Minnaar, Krecek and Rajput1999, Minnaar and Krecek, Reference Minnaar and Krecek2001; Mukaratirwa and Singh, Reference Mukaratirwa and Singh2010) and on their molecular identification (Lamb et al., Reference Lamb, Napier and Mukaratirwa2012), this study is the first to report of A. caninum infection in humans (school-aged children in Ingwavuma area, KwaZulu-Natal province). All three human sample sequences were identified as A. caninum, and neither of the two species known to infect humans (N. americanus and A. duodenale) (Hawdon, Reference Hawdon1996; Zhan et al., Reference Zhan2001; Mabaso et al., Reference Mabaso2003; Phosuk et al., Reference Phosuk2013; Hasegawa et al., Reference Hasegawa2014, Reference Hasegawa2017; George et al., Reference George2016; Smout et al., Reference Smout2017) were detected, even though both species have been reported to be co-endemic in sub-Saharan Africa (Mabaso et al., Reference Mabaso2003; Hotez and Kamath, Reference Hotez and Kamath2009). Ancylostoma caninum larvae have been reported to infect humans, manifesting as cutaneous larva migrans (Bowman et al., Reference Bowman2010). There have also been reports of A. caninum readily developing into adults in the human gut and causing eosinophilic enteritis (Brown and Copeman, Reference Brown and Copeman2003; Landmann and Prociv, Reference Landmann and Prociv2003; Bowman et al., Reference Bowman2010; Mackenstedt et al., Reference Mackenstedt, Jenkins and Romig2015; Xie et al., Reference Xie2017; Shepherd et al., Reference Shepherd, Wangchuk and Loukas2018), even though Landmann and Prociv (Reference Landmann and Prociv2003) reported that eggs had never been detected in the faeces of patients. Reports on human infection by A. caninum have mainly come from Australia (Landmann and Prociv, Reference Landmann and Prociv2003; Traub et al., Reference Traub2004; Palmer et al., Reference Palmer2007; Ngui et al., Reference Ngui2012), and reports on hookworm-derived eosinophilic enteritis in humans in southern Africa are non-existent. The detection of eggs of A. caninum in humans in this study was a surprise, as this indicates that A. caninum is able to complete its cycle in humans and this constitutes the first report of a patent A. caninum infection in humans with evidence of eggs in faeces. No human hookworm species apart from A. caninum were detected in Ingwavuma area of KwaZulu-Natal. The prevalence of presumed A. caninum through coproscopy was 6% (3/50), consistent with the report by Mabaso et al. (Reference Mabaso2003) that human hookworm prevalence in KwaZulu-Natal decreases with distance inland above 150 m a.s.l. westwards due to changes in topography and temperature.
Ancylostoma caninum was also detected in the lioness sample from Timbavati Private Nature and Game Reserve. Results obtained for the prevalence of hookworm infection in the wildlife sampled are not a true reflection of the actual prevalence due to the small sample size in this study. The small sample size was due to difficulties related to safety of staff in these premises while attempting to obtain faecal samples from the animals. Xie et al. (Reference Xie2017) have reported similar challenges in obtaining wildlife samples from game reserves despite hookworm infections in wildlife (especially canids) posing a threat to human health.
Phylogenetic analysis based on the nuclear ribosomal ITS (ITS1) region and 5.8S sequences indicated that all except four samples formed a clade with A. caninum. Results of this study are consistent with the finding from other studies that A. caninum is the most commonly recorded hookworm species in dogs globally (Mukaratirwa and Busayi, Reference Mukaratirwa and Busayi1995; Minnaar et al., Reference Minnaar, Krecek and Rajput1999; Minnaar and Krecek, Reference Minnaar and Krecek2001; Traub et al., Reference Traub2004; Palmer et al., Reference Palmer2007; Lamb et al., Reference Lamb, Napier and Mukaratirwa2012; Liu et al., Reference Liu2015; Ayinmode et al., Reference Ayinmode, Obebe and Olayemi2016), although the prevalence is not generally high. Three experimental samples formed a group with A. braziliense, while one (Hap 8) was an outlier, sister to the other Ancylostoma species (Clade B). Haplotype 8 had a p-distance ranging from 8.7% A. braziliense to 14% A. caninum. Experimental haplotype 8, therefore, was shown to be a unique haplotype, different from the unique southern African haplotypes recorded by Lamb et al. (Reference Lamb, Napier and Mukaratirwa2012). The experimental haplotype 8 (C3) was sampled from a location different from the SPCA location of Lamb et al. (Reference Lamb, Napier and Mukaratirwa2012).
Mixed infections were detected in our study; however, the procedure we followed is not the best to detect mixed infections, and therefore there may have been more samples with mixed infections that were overlooked, as both A. caninum and A. braziliense have been reported in some of the same areas of study (Lamb et al., Reference Lamb, Napier and Mukaratirwa2012).
Future work could focus on the development of multiplex PCR assays with primers specific for the hookworm species of southern Africa to improve the detection of mixed infections. Studies based on the mitochondrial cytochrome oxidase subunit I gene (COI) might also give valuable complementary information on enabling identification of hookworm species. There is also a need for future studies on hookworm infections focusing on all provinces of South Africa, with larger sample sizes, especially wildlife and human samples, to add to the scarce information.
Supplementary material
To view supplementary material for this article, please visit https://dx.doi.org/10.1017/S0022149X19000130
Author ORCIDs
P. I. Ngcamphalala, 0000-0002-1086-9522; S. Mukaratirwa, 0000-0001-6843-1497
Acknowledgements
We would like to thank the managers and cleaning staff at the SPCAs (Amanzimtoti, Springfield, Kloof highway and Ballito) and Westville veterinary clinic for their help with dog faecal sample collection, Dr Louis La Grange who helped in the collection of wildlife and dog samples in Mpumalanga province, and Dr Muhubiri Kabuyaya for human sample collection in Ingwavuma, KwaZulu-Natal province.
Financial support
This study was supported by the South African Medical Research Council for the field work under the “Self-Initiated Research Grant” awarded to SM and the National Research Fund (NRF) bursary awarded to PN.
Conflict of interest
None.
Ethical standards
Ethical clearance for the collection of faecal samples was granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (AREC/103/015 and BREC no: BE449/15).