Introduction
The cestodes of the order Trypanorhyncha Diesing, 1863 are cosmopolitan marine fish parasites, unique in having a rhyncheal apparatus (except the genus Aporhynchus Nybelin, 1918), comprising four retractable tentacles emerging from four bulbs, armed with hooks arranged in different patterns (Campbell & Beveridge, Reference Campbell, Beveridge, Khalil, Jones and Bray1994; Palm, Reference Palm2004). At present, 345 species are known (World Register of Marine Species, 2021), with complex life-cycles involving a variety of invertebrates and teleost fishes as intermediate hosts and elasmobranchs as definitive hosts (Palm, Reference Palm2004).
Progrillotia Dollfus, 1946 is the only genus of the family Progrillotiidae Palm, 2004. It consists of three species parasitic in rays as adults: Progrillotia pastinacae Dollfus, Reference Dollfus1946 (type species); Progrillotia louiseuzeti Dollfus, Reference Dollfus1969; and Progrillotia dasyatidis Beveridge, Neifar & Euzet, Reference Beveridge, Neifar and Euzet2004 (Dollfus, Reference Dollfus1946, Reference Dollfus1969; Beveridge et al., Reference Beveridge, Neifar and Euzet2004, Reference Beveridge, Haseli, Ivanov, Menoret, Schaeffner, Caira and Jensen2017; Palm, Reference Palm2004; Marques et al., Reference Marques, Santos, Cabral and Palm2005). Larvae have been recorded in marine demersal and pelagic teleosts from coastal Atlantic waters as well as from the Black Sea, Marmara Sea and the Persian Gulf (Palm, Reference Palm2004; Marques et al., Reference Marques, Santos, Cabral and Palm2005; Oguz & Bray, Reference Oguz and Bray2008; Al-Niaeem et al., Reference Al-Niaeem, Al-Azizz, Al-Ataby and Majeed2014; Polyakova et al., Reference Polyakova, Kornyushin and Maslennikova2014, Reference Polyakova, Gaevskaya, Kornyushin and Biserova2017; Özer & Öztürk, Reference Özer, Öztürk, Sezgin, Bat, Ürkmez, Arıcı and Öztürk2017; Çelik & Oğuz, Reference Çelik and Oğuz2021). Another species, Progrillotia dollfusi Carvajal & Rego, Reference Carvajal and Rego1983, originally reported as plerocerci from sciaenid fishes from South America (Carvajal & Rego, Reference Carvajal and Rego1983; Pereira & Boeger, Reference Pereira and Boeger2005), has been supposed (Beveridge et al., Reference Beveridge, Neifar and Euzet2004; Palm, Reference Palm2004) and proved (Menoret & Ivanov, Reference Menoret and Ivanov2009) as a member of the genus Grillotia Guiart, 1927 (Lacistorhynchidae), currently known as Grillotia (Christianella) carvajalregorum Menoret & Ivanov, Reference Menoret and Ivanov2009 (see also Beveridge & Campbell, Reference Beveridge and Campbell2010).
The three-spined stickleback Gasterosteus aculeatus L. is a small-sized euryhaline fish widespread in temperate and subarctic shallow coastal and inland waters throughout the Holarctic Region (Wootton, Reference Wootton1976, Reference Wootton1984; Mattern, Reference Mattern, Östlund-Nilsson, Mayer and Huntingford2007). It has been recorded as an intermediate, paratenic or definitive host of more than 20 cestode species (Wootton, Reference Wootton1976; Ermolenko, Reference Ermolenko1992; Hoffman, Reference Hoffman1999; Palm et al., Reference Palm, Klimpel and Bucher1999; Pugachev, Reference Pugachev2002; Barber, Reference Barber, Östlund-Nilsson, Mayer and Huntingford2007; Kirjušina & Vismanis, Reference Kirjušina and Vismanis2007; Poulin et al., Reference Poulin, Blanar, Thieltges and Marcogliese2011). Data on the occurrence of trypanorhynch cestodes from G. aculeatus are scarce (Linton, Reference Linton1924; Palm, Reference Palm2004; Pospekhov et al., Reference Pospekhov, Atrashkevich and Orlovskaya2010).
In the course of a survey of helminth parasites of fishes from the Lake Atanasovsko Wetlands, Bulgaria (Stoyanov et al., Reference Stoyanov, Neov, Pankov, Radoslavov, Hristov and Georgiev2015, Reference Stoyanov, Huyse, Pankov and Georgiev2016, Reference Stoyanov, Georgieva, Pankov, Kudlai, Kostadinova and Georgiev2017a, Reference Stoyanov, Mutafchiev, Pankov and Georgievb, Reference Stoyanov, Mutafchiev, Pankov and Georgiev2018), the trypanorhynch cestode P. dasyatidis has been found as a parasite of the three-spined stickleback. In this article, we present the first record of P. dasyatidis in G. aculeatus and provide data on the morphology of its plerocerci as well as comments on its life-cycle.
Materials and methods
The present study was carried out in the northern part of the Lake Atanasovsko Wetlands, declared as a Ramsar site (in 1984) and a managed nature reserve (in 1999). These wetlands represent a complex of shallow coastal habitats highly variable in their salinity and hydrological regime, that is, a hyperhaline lagoon partially used in salt production, a system of brackish and freshwater canals and ponds as well as freshwater marshes surrounding the lagoon (Ivanov et al., Reference Ivanov, Sotirov, Rozhdestvenski and Vodenicharov1964; Vassilev et al., Reference Vassilev, Vassilev and Yankov2013). A total of 134 individuals of the three-spined stickleback G. aculeatus (42 individuals from brackish water and 92 individuals from freshwater) were examined for helminth parasites in May, July and September 2012–2013. These were 33 mature individuals with total length 5.5–8.1 cm (av. ± standard deviation (SD) 6.91 ± 0.67 cm) and 101 immature individuals with total length 1.2–3.6 cm (av. ± SD 2.44 ± 0.59 cm). No fish of this species were caught in September 2012. The fish was caught by seines and traps. The five fish specimens infected with P. dasyatidis were sampled on 12–13 May 2013 from a freshwater pond (42°34′45″N, 27°28′29″E) adjacent to the canal connecting the sea and salt ponds.
Fish specimens were kept alive in containers with aerated water taken from the habitat. In the laboratory, each fish was dissected under a stereomicroscope within the next 24 h. The recovered 12 cestode specimens were fixed in hot saline and transferred to 70% ethanol. Subsequently, six of them were stained in iron acetocarmine (Georgiev et al., Reference Georgiev, Biserkov and Genov1986), dehydrated in an ascending ethanol series, cleared in dimethyl phthalate and mounted in Canada balsam. In addition, for better visualization of the tentacular armature, four specimens were mounted in Berlese's medium (Swan, Reference Swan1936). Two specimens were unsuccessfully used for DNA extraction.
The specimens (four slides in Canada balsam and four slides in Berlese's medium) are preserved in the Helminthological Collection of the Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Accession Nos. IBER-BAS C0161.3.1–8. Two slides of specimens mounted in Canada balsam are in the collection of Professor Harry Palm, University of Rostock, Germany.
The measurements taken and the related terminology are as proposed by Palm (Reference Palm2004). The characters measured were: BLL, blastocyst length; BLMW, blastocyst maximum width; BUL, bulb length; BUW, bulb width; BTL, body total length; HIRB, hooks of intercalary rows base length; HIRL, hooks of intercalary rows total length; HPRB, hooks of principal rows base length; HPRL, hooks of principal rows total length; PBOL, pars bothrialis length; PBOW, pars bothrialis width; PPBL, pars postbulbosa length; PPBW, pars postbulbosa width; PVL, pars vaginalis length; PVW, pars vaginalis width; and SL, scolex length. The metrical data are presented as the range, followed by the mean and the number of measurements taken (n) (table 1). The standard deviation is given only when n ≥ 30. All measurements are presented in micrometres.
Table 1. Metrical data of Progrillotia dasyatidis Beveridge, Neifar & Euzet, Reference Beveridge, Neifar and Euzet2004 (plerocerci) from different fish species and localities in Europe. For abbreviations, see Materials and Methods section.

The infection parameters prevalence (%), intensity (range, mean ± standard error) and abundance (mean ± standard error) follow the definitions by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Results
Progrillotiidae Palm, 2004.
Progrillotia dasyatidis Beveridge, Neifar & Euzet, Reference Beveridge, Neifar and Euzet2004.
Site of infection: Gallbladder.
Infection characteristics: prevalence 3.73%; intensity 1–7; mean intensity 2.40 ± 1.17; and mean abundance (0.09 ± 0.06).
Description (based on 8 plerocerci with evaginated scolex; figs 1 and 2; for metrical data, see table 1): scolex acraspedote, with maximum width at pars bothrialis, with broadly-rounded anterior margin (fig. 1). Pars bothrialis short, provided with two large, oval bothria, with free non-thickened margins and without posterior notch, opposite one another, one dorsal and one ventral (fig. 1b). Pars vaginalis longer than pars bothrialis (table 1), comprising sinuous tentacular sheaths (fig. 1b). Prebulbar organ present, prominent, at level of tentacle–bulbar junction (fig. 2d). Pars bulbosa containing four bulbs; bulb muscular, thick-walled, sausage-like, comprising retractor muscle originating at basis of bulb, surrounded by aggregations of glandular cells (fig. 2d). Pars postbulbosa very short, lacking in one specimen. Tentacles elongate, protruding beyond anterior margin of scolex (fig. 1); provided with heteroacanthous atypical armature of solid hooks. Metabasal armature consisting of repetitive principal half-rows of six hooks extending from internal to external surface and a single intercalary half-row consisting of tiny hooks, three hooks on antibothridial surface (fig. 2a) and five to seven hooks arranged in arc on external surface of tentacle (fig. 2b). Hooks 1(1′) of principal rows uncinate, with anterior extension of base. Hooks 2(2′) uncinate but with shorter base and more elongate blade than hooks 1(1′). Remaining four hooks falciform (fig. 2c). Hooks of intercalary rows uncinate, with relatively long base; blade fine, slightly curved (fig. 2c). Blastocyst strongly elongated, with maximum width approximately at mid-length; posterior margin broadly-rounded (fig. 1a).
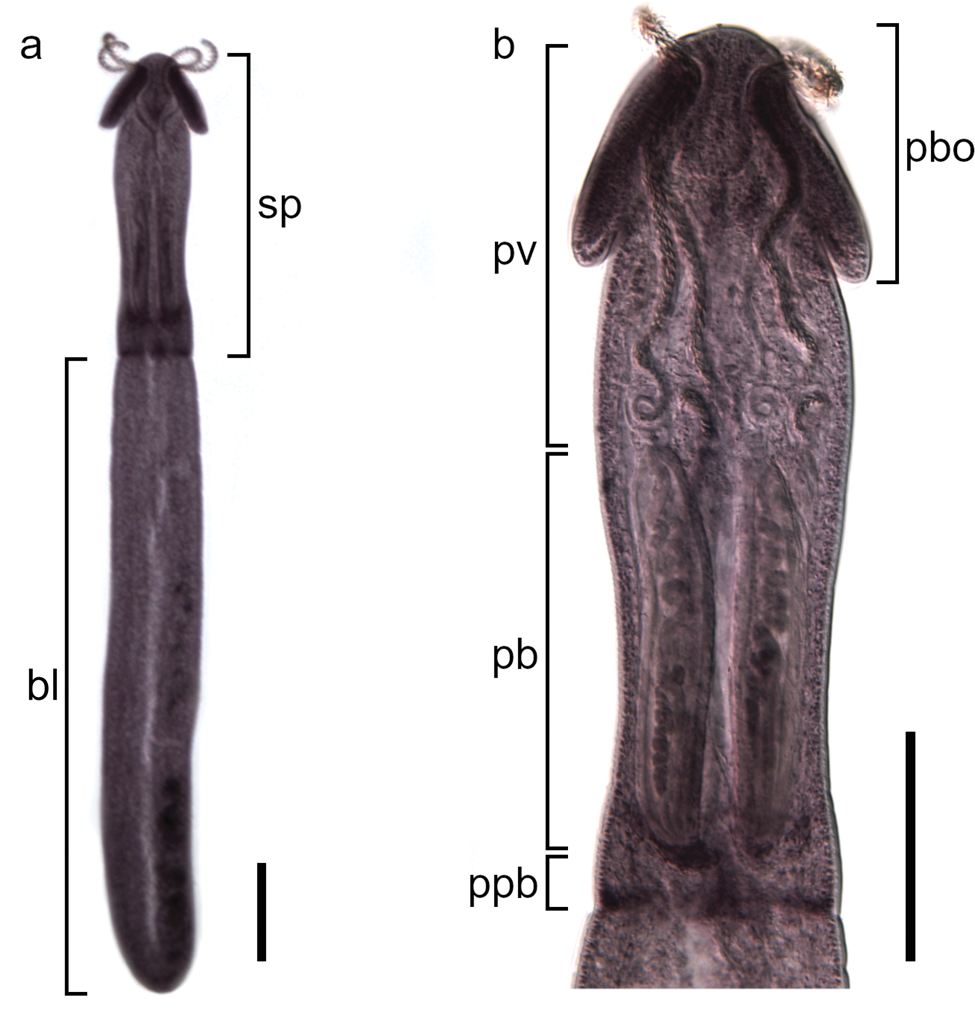
Fig. 1. Progrillotia dasyatidis Beveridge, Neifar & Euzet, Reference Beveridge, Neifar and Euzet2004, plerocerci from Gasterosteus aculeatus, Bulgaria: (a) general view (sp, scolex peduncle; bl, blastocyst); and (b) scolex (pbo, pars bothrialis; pv, pars vaginalis; pb, pars bulbosa; ppb, pars postbulbosa). Scale-bars: (a) 250 μm; (b) 200 μm.

Fig. 2. Progrillotia dasyatidis Beveridge, Neifar & Euzet, Reference Beveridge, Neifar and Euzet2004, plerocerci from Gasterosteus aculeatus, Bulgaria: (a) metabasal armature on antibothridial surface of tentacle; (b) basal and metabasal armature on external surface of tentacle; (c) types of hooks: principal rows (1/1′, uncinate hooks; 2/2′, uncinate but with shorter base hooks; 3/3′ and remaining hooks falciform); intercalary rows (ih, intercalary hook, uncinate); and (d) bulb (agc, aggregation of glandular cells; po, prebulbar organ; rm, retractor muscle; te, tentacle). Scale-bars: (a, b) 20 μm; (c) 10 μm; (d) 100 μm.
Discussion
The morphology of the present larvae is compatible with the generic characteristics of Progrillotia (Campbell & Beveridge, Reference Campbell, Beveridge, Khalil, Jones and Bray1994; Beveridge et al., Reference Beveridge, Neifar and Euzet2004; Palm, Reference Palm2004). The position of tentacles in the available specimens does not allow observations of the internal surface, thus the characteristic empty field between hooks 1(1′) of the principal rows has not been observed in the present study. The plerocerci from the Lake Atanasovsko Wetlands are identified as P. dasyatidis based on the armature comprising a single intercalary half-row of tiny hooks between principal rows. Each half-row consists of three hooks on the antibothridial surface and five to seven hooks on the external surface of the tentacle (fig. 2a, b). The presence of single intercalary rows is the main character distinguishing P. dasyatidis from the other two congeners possessing two intercalary rows between principal rows (Beveridge et al., Reference Beveridge, Neifar and Euzet2004; Palm, Reference Palm2004; Marques et al., Reference Marques, Santos, Cabral and Palm2005). This species identification is also supported by the presence of overlapping ranges of the main metrical characters with those reported by Marques et al. (Reference Marques, Santos, Cabral and Palm2005) for plerocerci of P. dasyatidis from Atlantic waters (table 1). The only exceptions are the wider pars bothrialis, the longer pars vaginalis and the longer hooks 5(5′) and 6(6′) of the principal rows than those in the previous description of plerocerci (table 1). However, these metrical differences are minor and can be considered as intraspecific variations. In addition, Marques et al. (Reference Marques, Santos, Cabral and Palm2005) reported maximum width of the scolex at the pars bulbosa vs. maximum width at the pars bothrialis in the present description. It could be attributed to differences in the condition of the specimens studied. The present specimens were recovered from fish hosts kept alive prior to examination and tapeworms were fixed after being relaxed in saline while Marques et al. (Reference Marques, Santos, Cabral and Palm2005) studied plerocerci sampled from commercially-captured fish.
The plerocerci of P. dasyatidis were found in the lumen of the gallbladder of large-sized mature fish individuals from a small freshwater marsh. Most probably, these are anadromous sticklebacks entering coastal wetlands from the nearby sea where definitive hosts (rays) occur. The anadromous mature sticklebacks undertake breeding migrations (March–July) to inland waters (Wootton, Reference Wootton1976, Reference Wootton1984). No cestode infection has been found in any of the small-sized immature fish individuals examined in the same wetlands in the course of the present study.
Progrillotia dasyatidis was originally described from the spiral valve of the Tortonese's stingray Dasyatis tortonesei Capapé (Dasyatidae) off the Mediterranean coast of Tunisia (type-host and type-locality) and from Dasyatis pastinaca (L.) near Arcachon on the Atlantic coast of France (Beveridge et al., Reference Beveridge, Neifar and Euzet2004). Marques et al. (Reference Marques, Santos, Cabral and Palm2005) recorded plerocerci of this species in several pleuronectiform and batrachoidiform hosts off the Atlantic coast of Portugal. As with the vast majority of trypanorhynchs, however, the life-cycles of Progrillotia spp. are still insufficiently studied (Palm, Reference Palm2004; Marques et al., Reference Marques, Santos, Cabral and Palm2005). Due to the close phylogenetic relationships of Progrillotia and the genera of the family Eutetrarhynchidae, it is assumed that they have similar three-host life-cycles (Palm, Reference Palm2004; Marques et al., Reference Marques, Santos, Cabral and Palm2005). Moreover, Progrillotia spp. and eutetrarhynchids of the genus Oncomegas Dollfus, 1929 develop plerocerci with evaginated scolex in teleost hosts (Palm, Reference Palm2004; Marques et al., Reference Marques, Santos, Cabral and Palm2005). It is believed that their life-cycle includes copepods as first intermediate hosts, higher crustaceans and other marine invertebrates as second intermediate hosts and elasmobranchs as definitive hosts (Palm, Reference Palm2004). The teleosts have been suggested to be either paratenic hosts or obligatory further intermediate hosts in their life-cycles (Marques et al., Reference Marques, Santos, Cabral and Palm2005). Polyakova et al. (Reference Polyakova, Kornyushin and Maslennikova2014) considered that the life-cycle of P. dasyatidis is successfully completed in higher crustaceans as second intermediate hosts while teleosts are paratenic hosts only. In our study, except plerocerci with evaginated scoleces, no other developmental stages have been observed. This is in agreement with the suggestion that teleosts are paratenic hosts for P. dasyatidis, consistent with the developmental model considering teleosts as paratenic hosts (Polyakova et al., Reference Polyakova, Kornyushin and Maslennikova2014). As juveniles, the three-spined sticklebacks feed basically on copepods but, as they grow, the proportion of copepods decreases and the share of higher crustaceans, molluscs and other invertebrates increases in their diet (Wootton, Reference Wootton1976). These invertebrates are believed to be second intermediate hosts of eutetrarhynchids and related groups (Palm, Reference Palm2004). A similar infection pattern for P. dasyatidis has also been observed in the red mullet Mullus barbatus L. (Mullidae) off the Anatolian Black Sea coast, which also shift their diet from copepods to bigger invertebrates when growing (Öztürk & Yesil, Reference Öztürk and Yesil2018).
In the Black Sea basin, two congeners, P. louiseuzeti and P. dasyatidis, have been recorded as adults and plerocerci (Naydenova & Solonchenko, Reference Naydenova, Solonchenko, Sokolov and Korneeva1989; Polyakova, Reference Polyakova2009, Reference Polyakova2020; Popjuk, Reference Popjuk2009; Gaevskaya, Reference Gaevskaya2012; Polyakova et al., Reference Polyakova, Kornyushin and Maslennikova2014, Reference Polyakova, Gaevskaya, Kornyushin and Biserova2017; Tepe et al., Reference Tepe, Oğuz and Heckmann2014; Kvach & Drobiniak, Reference Kvach and Drobiniak2017; Özer & Öztürk, Reference Özer, Öztürk, Sezgin, Bat, Ürkmez, Arıcı and Öztürk2017; Çelik & Oğuz, Reference Çelik and Oğuz2021). In addition, Polyakova & Biserova (Reference Polyakova and Biserova2016) recorded adults of Progrillotia sp. from the thornback ray Raja clavata L. (Rajidae) and plerocerci in the gallbladder of the knout goby, Mesogobius batrachocephalus (Pallas) (Gobiidae), as a possible fourth representative (not described) of this genus. In the Black Sea basin, P. dasyatidis has been recorded from the rays D. pastinaca and R. clavata (Polyakova et al., Reference Polyakova, Gaevskaya, Kornyushin and Biserova2017) as well as from several demersal and pelagic teleost fishes (Polyakova et al., Reference Polyakova, Kornyushin and Maslennikova2014; Tepe et al., Reference Tepe, Oğuz and Heckmann2014; Kornyychuk et al., Reference Kornyychuk, Dmitrieva, Yurakhno, Polyakova, Pronkina, Popjuk, Tarina and Rudenko2016a, Reference Kornyychuk, Pronkina, Polyakova, Galaktionov and Gaevskayab, Reference Kornyychuk, Polyakova and Pronkina2022; Öztürk & Yesil, Reference Öztürk and Yesil2018; Polyakova, Reference Polyakova2020; Çelik & Oğuz, Reference Çelik and Oğuz2021). Oguz & Bray (Reference Oguz and Bray2008) recorded P. dasyatidis (plerocerci) from the gallbladder of the black goby, Gobius niger L. (Gobiidae), from the adjacent Sea of Marmara, Turkey. Until now, no records of Progrillotia spp. from Bulgarian waters have been published. In addition, there were no records of this genus from G. aculeatus.
Only two trypanorhynch species, that is, Lacistorhynchus tenuis (van Beneden, 1858) (syn. Rhynchobothrium bulbifer Linton, 1889) (plerocerci) (Lacistorhynchidae) from the North Atlantic Ocean off Woods Hole, Massachusetts (USA) (Linton, Reference Linton1924; Palm, Reference Palm2004) and Nybelinia surmenicola Okada in Dollfus, 1929 (plerocerci) (Tentaculariidae) from the Gizhiga River, Magadan Oblast, Russia (Pospekhov et al., Reference Pospekhov, Atrashkevich and Orlovskaya2010) have been recorded as parasites of G. aculeatus. Therefore, the present study expands the knowledge about the geographical distribution and the host range of the fish parasites of the genus Progrillotia.
Acknowledgements
We are grateful to the Ministry of Environment and Waters of the Republic of Bulgaria for licenses (NCZP-151/11.05.2012 and NCZP-168/29.04.2013) to carry out studies in the Lake Atanasovsko Reserve. The field study was based at the Lake Atanasovsko Field Station of the Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences. The assistance of the staff of this station as well as of Konstantin Popov, Dr Plamen Pankov and Dr Pavel Nikolov is acknowledged. We thank Professor Harry W. Palm, University of Rostock, Germany, for kindly consulting us in taxonomy of the parasite group, and Dr Gergana Vasileva for reading the manuscript.
Financial support
Facilities developed in the frameworks of the projects WETLANET (FP7 CAPACITIES Grant 229802) and CEBDER (Bulgarian National Science Fund, Grant DO 02-15/2009) were used. Field trips and the laboratory work were partly funded by the Bulgarian National Science Fund (Grant YS DO 02-271/18.12.2008).
Conflicts of interest
None.
Ethical standards
The Scientific Council of the Institute of Biodiversity and Ecosystem Research–Bulgarian Academy of Sciences (IBER-BAS), acting as an ethics board, reviewed and approved the ethical standards applied in this study (Decision 2012/02/14/6).





