Introduction
The past decade or so has seen the erection of a series of seven novel genera found parasitizing the spiral intestines of members of three orders of sharks as well as one species of stingray (Caira & Durkin, Reference Caira and Durkin2006; Ruhnke et al., Reference Ruhnke, Caira and Carpenter2006a; Reyda, Reference Reyda2008; Caira et al., Reference Caira, Malek and Ruhnke2011; Cutmore et al., Reference Cutmore, Theiss, Bennett and Cribb2011; Ruhnke & Workman, Reference Ruhnke and Workman2013), and one species of skate (Ivanov, Reference Ivanov2006), which, despite sharing a series of unique ultrastructual features and similarities in overall proglottid anatomy (see Ruhnke, Reference Ruhnke2011; Cutmore et al., Reference Cutmore, Bennett, Miller and Cribb2017), differ considerably in bothridial morphology. For example, the bothridia of Doliobothrium Caira, Malek & Ruhnke, 2011 each lack an apical sucker and possess a proximal aperture; those of Orectolobicestus Ruhnke, Caira & Carpenter, 2006 each possess an apical sucker and marginal loculi; those of Ruhnkecestus Caira & Durkin, 2006 lack an apical sucker but bear facial loculi; those of Hemipristicola Cutmore, Theiss, Bennett & Cribb, 2011 bear an apical sucker and a deep central cavity; and those of Alexandercestus Ruhnke & Workman, 2013 bear an apical sucker and are highly foliose. The bothridia of Guidus Ivanov, 2006 are highly globose (Ivanov, Reference Ivanov2006). The bothridia of Nandocestus Reyda, 2008 resemble those of Orectolobicestus in bearing marginal loculi, but this genus is unique among these genera in that it parasitizes a freshwater stingray rather than sharks. It also bears circumcortical, rather than lateral, vitelline follicles (Reyda, Reference Reyda2008). Bothridial features also serve to distinguish the above genera from three allied genera of shark tapeworms erected 25 or more years ago (see Woodland, Reference Woodland1927; Yamaguti, Reference Yamaguti1952; Ruhnke, Reference Ruhnke1994). For example, the bothridia of Scyphopyllidium Woodland, 1927 and Marsupiobothrium Yamaguti, 1952 bear apical suckers and are globose in form. The bothridia of Paraorygmatobothrium Ruhnke, 1994, which with 30 valid species is by far the most speciose of these genera, each bear an apical sucker but lack all of the modifications listed above (Ruhnke, Reference Ruhnke1994) (see table 1).
Table 1. Species of Scyphophyllidium and allied genera, including previous names (if different from current name), category designations, type host information and morphological and ultrastructural features diagnostic for subsets of taxa.

a As Carcharhinus cf. dussumieri.
b As Scoliodon laticaudus.
c As Mustelus vulgaris.
d Host of specimen sequenced.
As molecular phylogenetic analyses have expanded to include greater representation of these genera, the close affinities among these genera have been confirmed. However, these works have also served to call the monophyly of the speciose, yet morphologically uniform, Paraorygmatobothrium into question relative to at least a subset of the above genera (Cutmore et al., Reference Cutmore, Theiss, Bennett and Cribb2011, Reference Cutmore, Bennett, Miller and Cribb2017; Caira et al., Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a; Ruhnke et al., Reference Ruhnke, Daniel and Jensen2020). This suggests that a critical re-evaluation of these genera is in order. The discovery of a new species parasitizing the crocodile shark, Pseudocarcharias kamoharai (Matsubara), prompted us to formally tackle that issue here. Not only does this species exhibit a blend of the diagnostic morphological features of several of the above genera, but also phylogenetic analysis of a portion of the 28S rDNA gene suggests that it is most closely related to a subset of species of Paraorygmatobothrium. In identifying the most effective strategy for aligning the classification of these taxa with their phylogenetic relationships and morphologies, we also examined new material of the poorly known genera Marsupiobothrium and Scyphophyllidium to determine the conditions of several key morphological features.
As the only member of the family Pseudocarchariidae Compagno, P. kamoharai was also interesting from a comparative standpoint because it represents the only family of lamniform sharks that has not yet been examined for cestodes. In addition to the problematic new phyllobothriidean species referred to above, this shark species was found to host two new, relatively morphologically divergent, species of the phyllobothriidean genus Clistobothrium Dailey & Vogelbein, 1990, both of which are also described below.
Materials and methods
Specimen collection
Eight specimens of P. kamoharai, consisting of five females (97–106 cm in total length [TL]) and three males (84.5–108 cm in TL), were examined. All eight specimens were collected between May 22 and June 2 of 2014 from a fish market in Santa Elena (2°12′24.4″S, 80°56′58.1″W), Ecuador. Additional information on each host can be obtained from the Global Cestode Database (www.elasmobranchs.tapewormdb.uconn.edu) by entering the specimen numbers (i.e. EC-4, EC-5, EC-8, EC-9, EC-35, EC-36, EC-54 and EC-55). A small sample of liver tissue was taken from each animal and preserved in 95% ethanol for molecular verification of host identity. In each case, the spiral intestine was removed and opened with a mid-ventral longitudinal incision and then washed with seawater. Washings were either fixed in 10% seawater-buffered formalin (9:1) for morphological work or in 95% ethanol for molecular work. In some cases, the resulting washings were examined for cestodes under a stereomicroscope in the field prior to fixation and a subset of specimens found was fixed in 10% seawater-buffered formalin and a subset was fixed in 95% ethanol. Spiral intestines of five animals were then fixed in 10% seawater-buffered formalin and two were fixed in 95% ethanol. After one or two weeks, all formalin-fixed material was transferred to 70% ethanol for storage. Material preserved in 95% ethanol was stored in a −20°C freezer.
Morphological methods
Whole mounts of worms from P. kamoharai were prepared as follows for examination with light microscopy: specimens were hydrated in a graded ethanol series, stained in Delafield's haematoxylin, differentiated in tap water, destained in acidic 70% ethanol, neutralized in basic 70% ethanol, dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted on glass slides under coverslips in Canada balsam diluted with methyl salicylate. They were then placed in a drying oven at 55°C for one week. Measurements were taken with a Zeiss Axioskop 2 Plus compound microscope (Thornwood, New York, USA) using SPOT Diagnostic Instrument Digital Camera Systems and SPOT software, version 4.6 (SPOT Imaging Solutions, Sterling Heights, Michigan, USA). Unless otherwise stated, measurements are presented in micrometres as ranges, followed in parentheses by the mean, standard deviation, total number of specimens measured and total number of measurements taken when more than one measurement was made per worm. With the exception of testes number, all proglottid measurements come from the terminal-most mature proglottid. Line drawings were made with a camera lucida attached to the Zeiss Axioskop 2 Plus compound microscope.
Temporary whole mounts of eggs were prepared as follows: gravid proglottids were transferred to a 1:10 mixture of glycerine and 70% ethanol, teased apart with a fine needle to release the eggs and placed in an open container in a fume hood overnight. They were then mounted in the same mixture on glass slides under coverslips, the edges of which were sealed with two coats of clear nail polish. Images were taken using the camera system described above.
Museum abbreviations used are as follows: LRP, Lawrence R Penner Parasitology Collection, Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, Connecticut, USA; MEPN, Museo de Colecciones Biológicas Gustavo Orcés, Escuela Politécnica Nacional, Ladrón de Guevara E11-253, Quito, Ecuador; MPM, Meguro Parasitological Museum, Tokyo, Japan; USNM, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA. Microthrix terminology follows Chervy (Reference Chervy2009). Ruhnke et al. (Reference Ruhnke, Caira, Pickering, Caira and Jensen2017) was used as the source of valid phyllobothriidean species, except that Paraorygmatobothrium musteli (van Beneden, 1850) Ruhnke, 2011 was also included.
Specimens from P. kamoharai were prepared for scanning electron microscopy (SEM) as follows: they were hydrated in a filtered graded series of ethanols, transferred to a solution of 1% osmium tetroxide and placed in a refrigerator overnight; they were then dehydrated in a filtered graded series of ethanols, placed in hexamethyldisilazane (Ted Pella Inc., Redding, California, USA) and allowed to air-dry in a fume hood for approximately 1 h. They were then mounted on aluminium stubs using double-sided PELCO carbon tabs (Ted Pella Inc.), sputter coated with 35 nm of gold/palladium and examined with a FEI Nova NanoSEM 450 field emission scanning electron microscope (FEI, Hillsboro, Oregon, USA).
In addition, two specimens of Marsupiobothrium gobelinus Caira & Runkle, 1993, taken from the same specimen of the goblin shark (Mitsukurina owstoni Jordan) from which the type material of this species was collected, were prepared for and examined with SEM as described above. The whole mounts of the hologenophores of Marsupiobothrium sp. 1, for which 28S rDNA and 18S rDNA data (LRP nos 8336 and 8337, respectively) were generated by Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a), were examined. In addition, five specimens collected from the pelagic thresher shark (Alopias pelagicus Nakamura) in Taiwan in 2013 and 2017 that we believe are conspecific with Marsupiobothrium sp. 1 of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) were prepared for and examined with SEM and as whole mounts for characterization of scolex features and egg morphology as described above. Although we were not able to borrow the type material of Marsupiobothrium alopias Yamaguti, 1952 (MPM no. SY7149) from the MPM, Iwaki Takashi kindly provided us with a series of images taken at intervals throughout the depth of the bothridia of the type specimen to help us evaluate the nature of the feature located in the centre of the bothridia that was interpreted as a sucker by Ivanov (Reference Ivanov2006) and Ruhnke (Reference Ruhnke2011). Also examined were the whole mounts of the hologenophore (LRP no. 8346) of Scyphophyllidium cf. giganteum of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) collected from Galeorhinus galeus (L) in the Chatham Rise off New Zealand, three specimens identified as Scyphophyllidium giganteum (van Beneden, 1858) Woodland, 1927 collected from G. galeus off the coast of California by Nathan Riser (LRP nos. 2742–2744), and the specimen prepared for SEM collected by Riser from G. galeus off California included in Caira et al. (Reference Caira, Jensen, Healy, Littlewood and Bray2001).
Molecular methods
The D1–D3 region of the 28S rDNA gene were sequenced for one specimen of each of the three new species we collected from P. kamoharai. The centre portion of each worm was removed for DNA extraction; the remainder of each worm was prepared as a whole mount to serve as a hologenophore (sensu Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) for the specimens sequenced.
Total genomic DNA was extracted using a MasterPure™ DNA Purification Kit (EpiCentre Technologies, Madison, Wisconsin, USA) following manufacturer's instructions. Specimens were then incubated at 65° C for 1 h and left at room temperature overnight with gentle shaking to dissolve DNA into solution. DNA quantity and quality were assessed using a NanoDrop 2000 micro-volume spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Amplification of the D1–D3 region of the 28S rDNA gene was done in a 10 µl volume with 1 µl of DNA template, 0.1 µl of 10 M of each primer, 5 µl of GoTaq® Green Master Mix (Promega, Fitchburg, Wisconsin, USA) and 3.8 µl of water. The following primer pair was used for amplification: LSU-5 (5′-TAGGTCGACCCGCTGAAYTTA-3′) (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000) and LSU-1500R (5′-GCTATCCTGGAGGGAAACTTCG-3′) (Tkach et al., Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003). Polymerase chain reaction product was purified using ExoSAP-IT.7 (Affymetrix, Inc., Santa Clara, California, USA). Sequencing was done using the primer pair LSU-55F (5′-AACCAGGATTCCCCTAGTAACGGC-3′) (Bueno & Caira, Reference Bueno and Caira2017) and LSU-1200R (5′-GCATAGTTCACCATCTTTCGG-3′) (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000). Both strands were sequenced on an ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems, Foster City, California, USA) using ABI Big Dye™ dideoxy terminators (version 3.1). Contigs for the three specimens were assembled Geneious, v. 10.1.3 (Biomatters, Newark, New Jersey, USA).
Molecular phylogenetic analysis
For comparative purposes, sequence data for a portion of the 28S rDNA gene were obtained from GenBank for a total of 33 species of Alexandercestus, Doliobothrium, Guidus, Hemipristicola, Marsupiobothrium, Nandocestus, Orectolobicestus, Paraorygmatobothrium, Ruhnkecestus, Scyphophyllidium and Thysanocephalum Linton, 1890. Also included were 14 species belonging to eight other genera of phyllobothriideans (see table 2), including Clistobothrium. Based on the phylogenetic relationships indicated in the tree resulting from the analyses of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a), Disculiceps sp. 1 of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) and Cathetocephalus thatcheri Dailey & Overstreet, 1973 were included as outgroups, again using data obtained from GenBank. These sequences ranged in length from 726 bp to 1214 bp. Details of the specimens included in the molecular phylogenetic analysis are given in table 2.
Table 2. Taxa used in the phylogenetic analysis, with their revised names, host species, GenBank numbers and source of data.
a Taxa for which only D2 data are available.
Sequences were originally aligned and trimmed in Geneious, version 10.1.3. They were then aligned using PRANK (Löytynoja & Goldman, Reference Löytynoja and Goldman2010) on the webPRANK Server using the default settings, but with the ‘+F flag’ removed. The best-fitting model of evolution was determined using jModelTest, v. 2.1.10 (Guindon & Gascuel, Reference Guindon and Gascuel2003; Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) based on the evaluation of 88 models on the CIPRES Science Gateway (Miller et al., Reference Miller, Pfeiffer and Schwartz2010). Sample-size corrected Akaike Information Criterion values were used to evaluate goodness of fit. A maximum likelihood (ML) analysis was conducted using Garli, v. 2.01 (Zwickl, Reference Zwickl2006), also on the CIPRES Science Gateway (Miller et al., Reference Miller, Pfeiffer and Schwartz2010). Default Garli configuration settings were used with the following exceptions: the starting tree topology was set to ‘random’, the number of attachment branches evaluated per terminal was set to 84 (i.e. twice the number of terminals in the matrix) and the number of independent search replicates was set to 100. Based on the results of the jModelTest analysis, TVM + I + G was employed as the model of evolution. Bootstrap (BS) values resulting from 1000 BS replicates were also generated with Garli v. 2.01 using the configuration settings specified above. BS values were displayed on the best tree using SumTrees v. 4.0.0 in DendroPy v. 4.0.3 (Sukumaran & Holder, Reference Sukumaran and Holder2010).
Results
Morphology and ultrastructure of poorly known genera
Marsupiobothrium alopias Yamaguti, 1952 (fig. 1a)
When he erected Marsupiobothrium in 1952, Yamaguti established M. alopias, from a host identified as the common thresher shark (Alopias vulpinus Bonnaterre), in Japan, as the type species. The bothridia were described as pear-shaped sacs with sphincter-like muscles and a submarginal apical sucker. We are unaware of any additional material of this species having been collected since that time. Despite the global distribution of A. vulpinus (see Compagno, Reference Compagno1984), we have not encountered this tapeworm in any of the over 50 specimens of common thresher sharks we have examined for cestodes at shark tournaments off New England, USA, or in fish markets in Taiwan. As a consequence, the type material remains the only available material of this species and this species has yet to be included in a molecular phylogenetic analysis or examined using SEM.
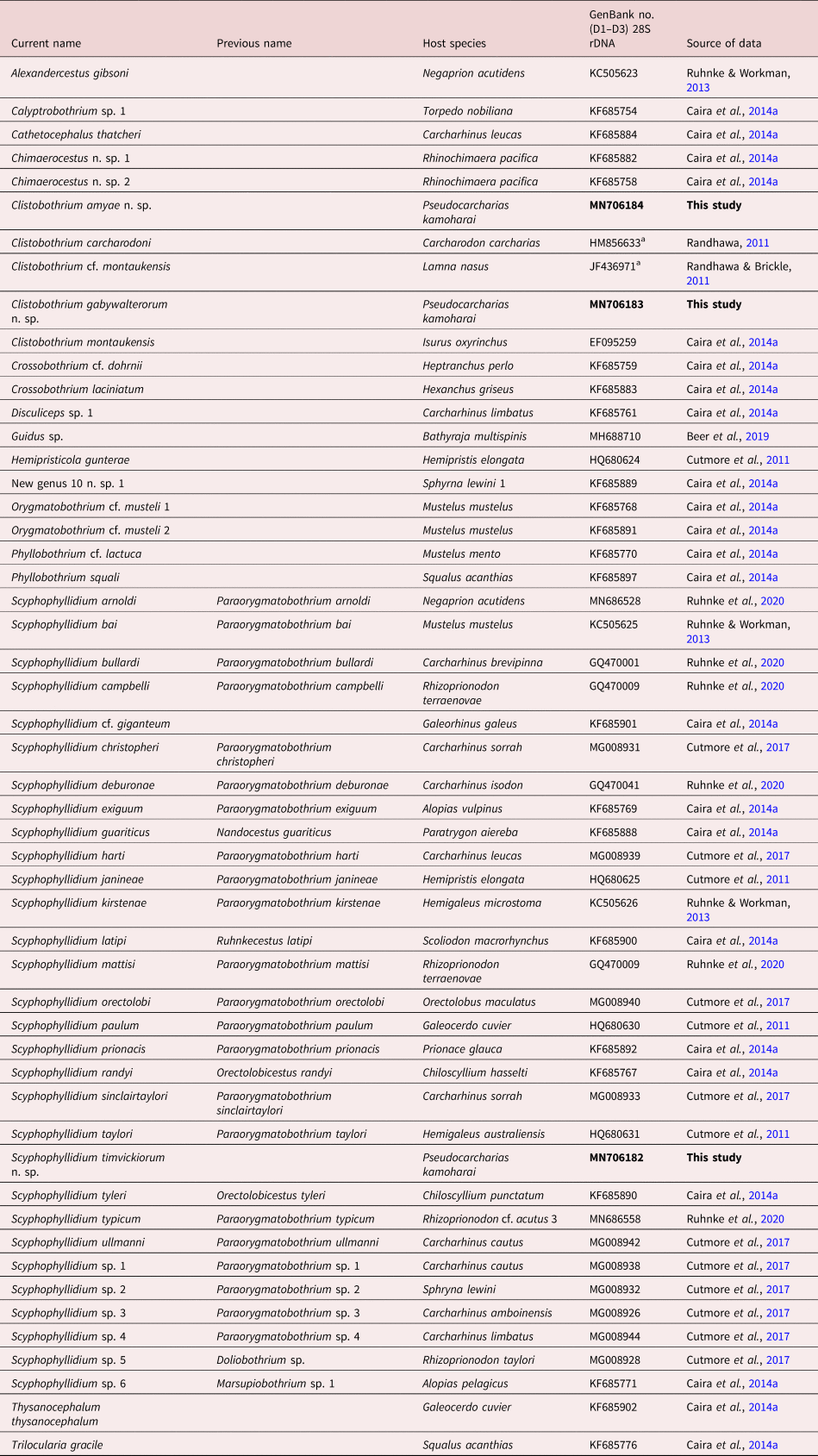
Marsupiobothrium alopias was re-described by both Ivanov (Reference Ivanov2006) and Ruhnke (Reference Ruhnke2011) based on their examination of the type material. Their work raises an interesting question regarding the nature of a feature found on the centre of the proximal surface of the globose bothridia of this species. No mention of such a feature was made by Yamaguti (Reference Yamaguti1952). However, both Ivanov (Reference Ivanov2006) and Ruhnke (Reference Ruhnke2011) reported the presence of a sucker on the centre of each bothridium. Our examination of the images of the bothridia of the type specimen provided to us by the MPM (fig. 1a) indicates that this feature is actually a proximal aperture, rather than a sucker. Unfortunately, beyond scutes on the strobila, the microtriches on the scolex of M. alopias have not yet been characterized.
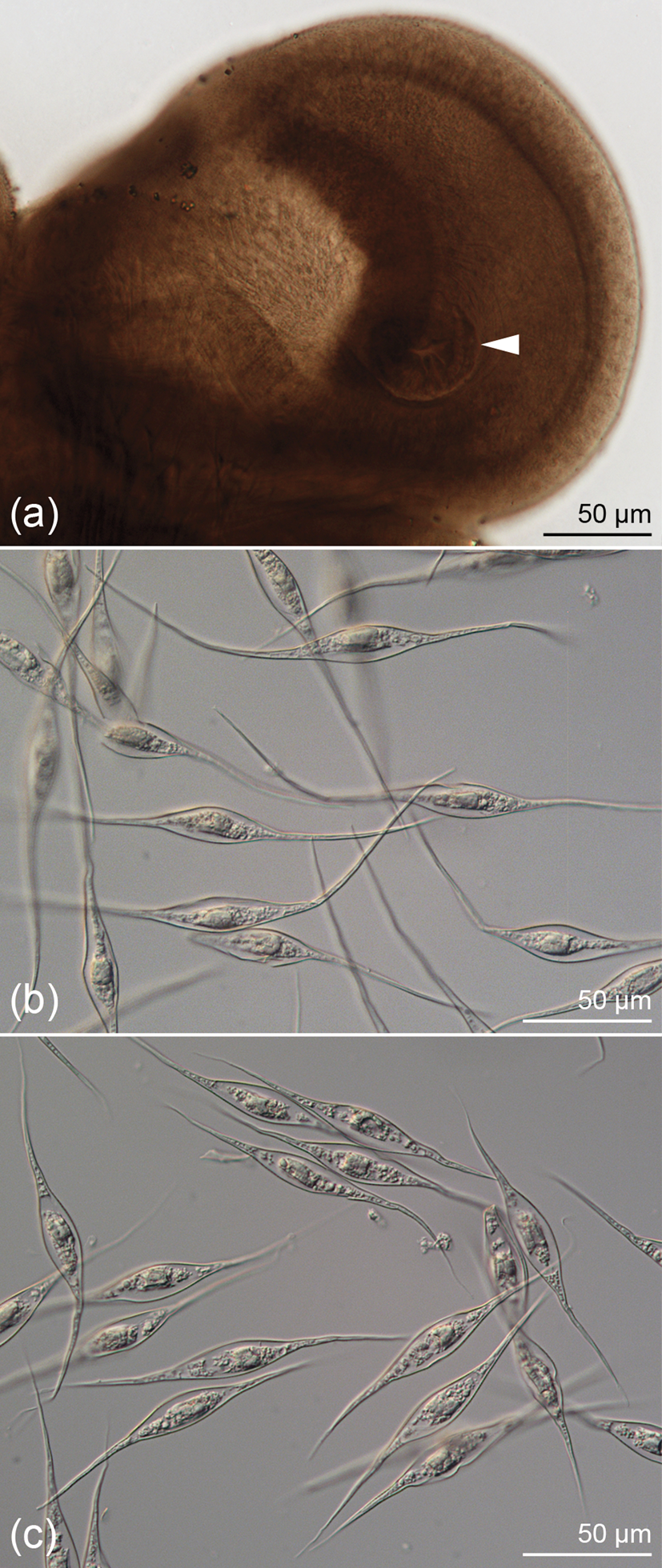
Fig. 1. Photomicrographs of species of Scyphophyllidium. (a) Bothridium of the holotype of Marsupiobothrium alopias (now Scyphophyllidium alopias) (MPM no. SY7149) from Alopias vulpinus in Japan; proximal aperture indicated by arrowhead. (b) Eggs of Marsupiobothrium sp. 1 of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) (now Scyphophyllidium sp. 6) from Alopias pelagicus in Taiwan. (c) Eggs of Scyphophyllidium timvickiorum Caira, Hayes & Jensen n. sp. from Pseudocarcharias kamoharai in Ecuador.
Marsupiobothrium gobelinus Caira & Runkle, 1993 (fig. 2a–c)
This species has also not been reported since the time of its original description from the goblin shark (M. owstoni Jordan) by Caira & Runkle (Reference Caira and Runkle1993). Its bothridia were characterized as globose, each with an apical sucker; no mention was made of a proximal aperture. Details of the surfaces of the scolex given in the original description were limited to mention of the fact that all surfaces of the bothridia and stalks (referred to as peduncles) were covered with ‘slender, blade-like microtriches’ (Caira & Runkle, Reference Caira and Runkle1993: 85); no SEM images were provided. Our examination of two additional specimens with SEM here confirmed the absence of apertures on the proximal surfaces of the bothridia (fig. 2a), and also that the capilliform filitriches on the anterior regions of the strobila are not arranged in scutes. The distal bothridial surfaces were found to bear a relatively unique form of spinithrix (fig. 2b). These spinitriches most closely resemble the trifurcate form of Chervy (Reference Chervy2009). However, only their tips are trifid and, rather than bearing three extensions of similar length, these spinitriches bear one long central extension flanked on each side by a much shorter extension. In addition, the distal tips of all three extensions are rounded, rather than pointed (inset fig. 2b). Filitriches were not seen on this surface. The proximal bothridial surfaces were found to be covered with densely arranged narrow gladiate spinitriches and capilliform filitriches (fig. 2c). Inclusion of this species in molecular phylogenetic analyses, and, thus, confirmation of its phylogenetic position, awaits the collection of material preserved in ethanol for molecular work.
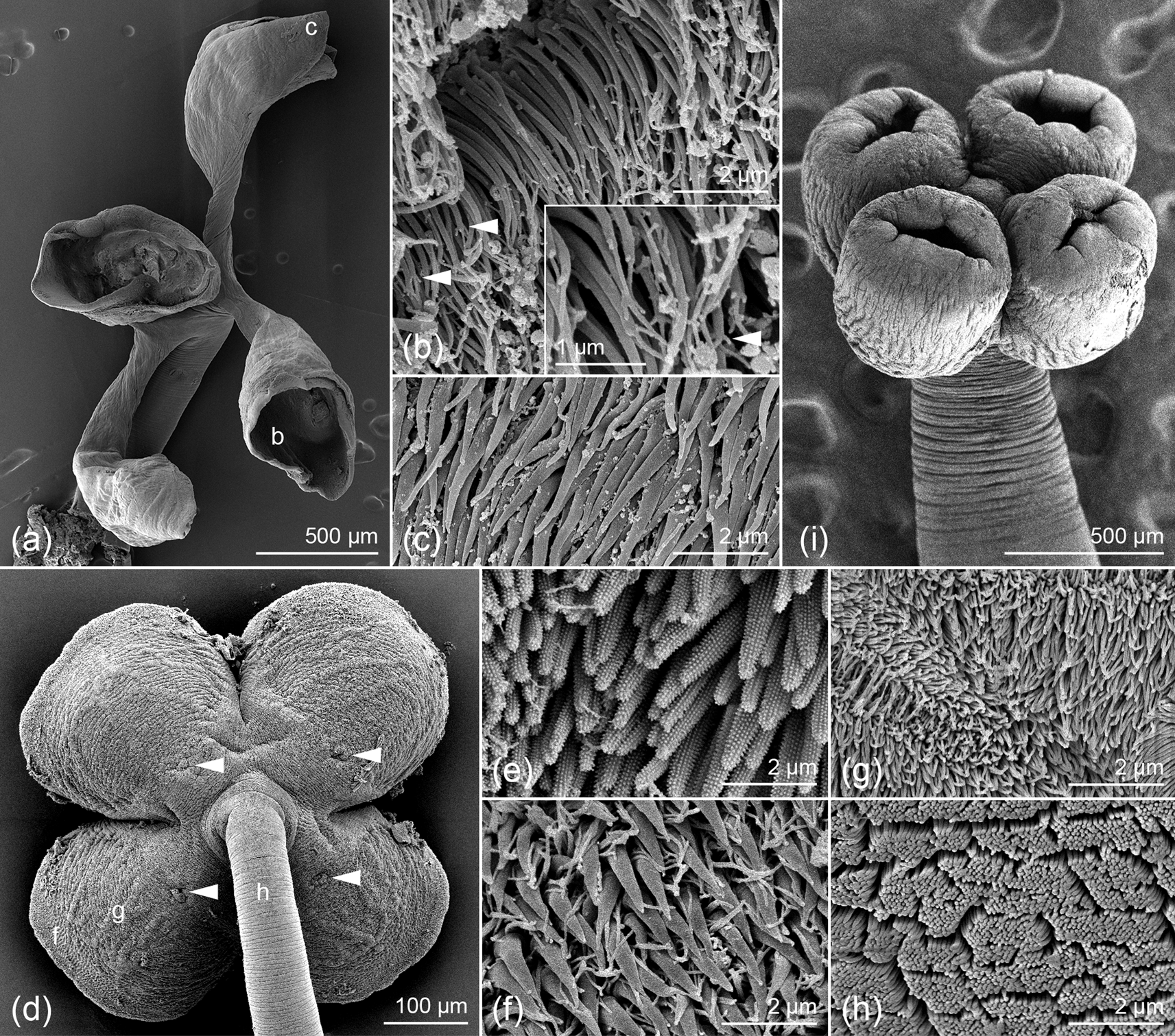
Fig. 2. Scanning electron micrographs of Marsupiobothrium gobelinus (now Scyphophyllidium gobelinus) from Mitsukurina owstoni in Australia. (a) Scolex; small letters indicate locations of details in (b) and (c). (b) Distal bothridial surface (with close-up inset); arrowheads indicate trifid tips of gladiate spinitriches. (c) Proximal bothridial surface. Scanning electron micrographs of Marsupiobothrium sp. 1 of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) (now Scyphophyllidium sp. 6) from Alopias pelagicus in Taiwan. (d) Scolex, proximal view; small letters indicate locations of details in (f–h); arrowheads indicate proximal apertures on each bothridium. (e) Distal bothridial surface. (f) Proximal bothridial surface adjacent to rim. (g) Proximal bothridial surface away from rim. (h) Surface of strobila. (i) Scanning electron micrograph of Scyphophyllidium cf. giganteum from Galeorhinus galeus in New Zealand (modified from Caira et al., Reference Caira, Jensen, Healy, Littlewood and Bray2001).
Marsupiobothrium sp. 1 (figs 1b and 2d–h)
Previously characterized, based solely on molecular data, the new material allowed us to characterize some of the basic morphological features of this species for the first time. This species was found to conspicuously differ from M. gobelinus in that it bears apertures on its proximal bothridial surfaces (fig. 2d). The distal bothridial surfaces were found to bear gongylate columnar spinitriches (fig. 2e). The proximal bothridial surfaces near the bothridial rims were found to bear a band of densely arranged simple gladiate spinitriches interspersed with capilliform filitriches (fig. 2f); the proximal surfaces away from the rim were seen to bear only capilliform filitriches (fig. 2g). In terms of its utrastructural features, the capilliform filitriches on the anterior regions of the strobila were not arranged as scutes (fig. 2h); in this respect, this species also differs conspicuously from M. alopias. The availability of gravid proglottids allowed us to characterize the eggs of this species as being spindle-shaped with bipolar filaments that are uneven in length (fig. 1b).
Scyphophyllidium giganteum (van Beneden, 1858) Woodland, 1927 (fig. 2i) and S. cf. giganteum
When Woodland (Reference Woodland1927) erected Scyphyophyllidium, he did so in a somewhat cursory fashion. His knowledge of the species was based on a single specimen, 95 mm in length, collected from the spiral intestine of a triakid shark identified as Galeus vulgaris Fleming (synonym of Galeorhinus galeus) collected off Plymouth, UK. He considered this specimen to be conspecific with the species identified by van Beneden (Reference van Beneden1858) as Anthobothrium giganteum van Beneden, 1858 collected off Belgium from a shark he identified as ‘milandre’ (also considered to be G. galeus). Woodland provided no formal generic diagnosis. Instead, he described the details of his specimen and included the designation ‘gen. n.’ after the name of the species in the heading of that treatment. Following examination of specimens from sharks identified as Eugaleus galeus (L) (also a synonym of G. galeus) from Sète, France, Euzet (Reference Euzet1959: 59) provided the following brief diagnosis of the genus: ‘Scolex à 4 bothridies ovoides globuleuses, à ouverture antérieure, ne pouvant s’étaler. Pas de ventouse accessoire. Ovaire tétralobé. Vagin débouchant antérieurement à la poche du cirre. Vitellogènes latéraux. Pores génitaux alternant irrégulièrement’, along with illustrations of a scolex and proglottid. (“Scolex with 4 ovoid globose bothridia, an anterior aperture, unable to spread. Accessory sucker lacking. Ovary tetralobed. Vagina opening anterior to the cirrus pouch. Vitellaria lateral.”) Euzet (Reference Euzet, Khalil, Jones and Bray1994) subsequently added several additional details to the diagnosis, including the fact that the strobila was acraspedote and apolytic, the testes were numerous and post-vaginal testes were present on the poral side.
Based on the examination of three specimens from Nathan Riser, we were able to confirm the interpretation by Caira et al. (Reference Caira, Jensen, Healy, Littlewood and Bray2001) of the bothridia as highly globose (fig. 2i); there is no evidence of the presence of proximal bothridial apertures. This work also confirmed the observation of Ruhnke (Reference Ruhnke2011) that the bothridia each bear a small apical sucker. Furthermore, these specimens clearly bear capilliform filitriches on the strobila that are arranged as scutes. The proglottids of these specimens are consistent with the illustrations of van Beneden (Reference van Beneden1858) and Euzet (Reference Euzet1959) in being longer than wide.
The only representative of Scyphophyllidium included in molecular phylogenetic work to date is the species identified as S. cf. giganteum by Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) from G. galeus off the Chatham Rise in New Zealand. The morphology of the hologenophore is consistent with S. giganteum in most respects. Its bothridia are globose, bear apical suckers and lack proximal apertures. It also clearly bears scutes on its strobila. However, unlike the mature proglottids of S. giganteum, which are longer than wide, those of this specimen are substantially wider than long. Thus, we concur with Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) that this specimen should continue to be provisionally referred to as S. cf. giganteum.
Molecular phylogenetic analysis
The tree resulting from our ML analysis is shown in fig. 3. Two of the three new species discovered in P. kamoharai grouped as members of a clade that also included the three other species of Clistobothrium for which data were available in GenBank. The third new species grouped most closely with the species identified as Marsupiobothrium sp. 1 by Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) (referred to as Scyphophyllidium sp. 6 in fig. 3); this clade was sister to two of the 22 species included in the analysis that were originally assigned to Paraorygmatobothrium. The clade consisting of these four species grouped as sister taxon (with a BS value of 98%) to a larger clade that included the 20 remaining species originally assigned to Paraorygmatobothrium included in our analysis, as well as the species included in our analysis that were originally assigned to Doliobothrium, Nandocestus, Orectolobicestus, Ruhnkecestus and Scyphyophyllidium. The specimen of Hemipristicola gunterae grouped as the sister taxon to this larger clade, with Alexandercestus gibsoni as the sister taxon to this group. In contrast, the specimen of Guidus sp. grouped well outside of the above clade, as the sister taxon of a clade consisting of Phyllobothrium squali Yamaguti, 1952 and Calyptrobothrium sp. 1.
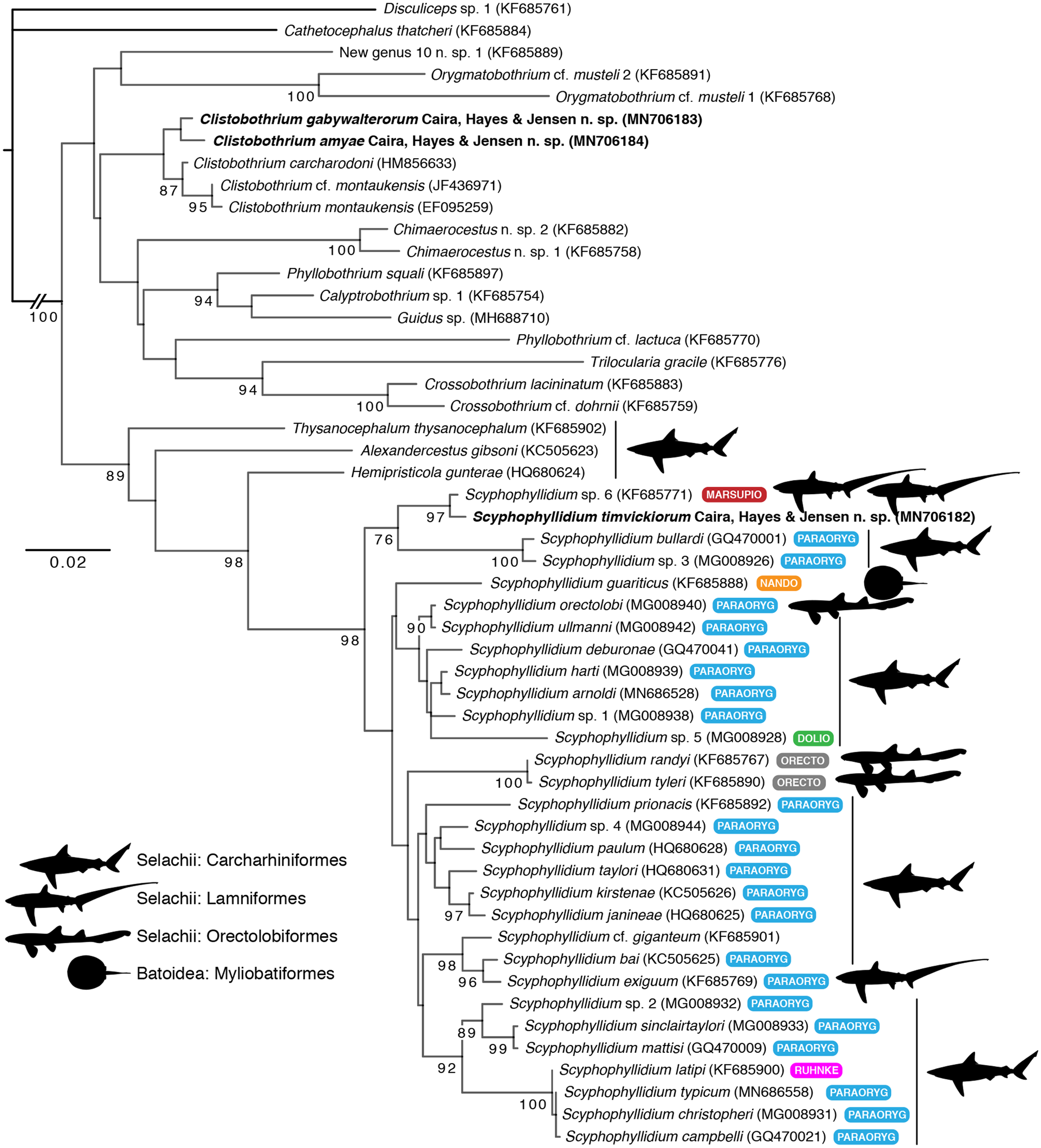
Fig. 3. Phylogenetic tree resulting from maximum likelihood (ML) analysis of a portion of the 28S rDNA gene for select phyllobothriideans, with focus on species previously assigned to Paraorygmatobothrium and allied genera, showing placement of three new species (in bold); Cathetocephalus thatcheri and Disculiceps sp. 1 were used as outgroups. Taxon labels include new generic assignments, GenBank numbers and abbreviations of previous generic assignments. Nodal support values presented as ML bootstrap values; only values greater than or equal to 70 are shown). Scale bar indicates nucleotide substitutions per site. Abbreviations: DOLIO, Doliobothrium; MARSUPIO, Marsupiobothrium; NANDO, Nandocestus; ORECTO, Orectolobicestus; PARAORYG, Paraorygmatobothrium; RUHNKE, Ruhnkecestus.
Revision of classification of seven genera in question
Our examination of material of Marsupiobothrium and Scyphyophyllidium expanded our understanding of the key features of both genera, and also served to confirm the latter as a valid genus. To help inform the development of an optimal solution for resolving the non-monophyly of Paraorygmatobothrium relative to six of the above genera (i.e. Doliobothrium, Marsupiobothrium, Nandocestus, Orectolobicestus, Ruhnkecestus and Scyphophyllidium), we also examined the key morphological features exhibited by their 44 described species. As can be seen from table 1, the majority of these features are present in a variety of non-unique combinations across the members of these seven genera. In light of these data and the results of our molecular phylogenetic analysis, synonymization of six of these genera is in order. As the oldest valid name, Scyphophyllidium is hereby designated as the valid name for the larger, more inclusive genus; Doliobothrium, Marsupiobothrium, Nandocestus, Orectolobicestus, Paraorygmatobothrium and Ruhnkecestus are designated as its junior synonyms. The 42 described species and six undescribed species currently assigned to the latter six genera are hereby transferred to Scyphophyllidium. The new combinations and their appropriate authority citations are given in table 1. A revised, expanded diagnosis of the genus that accommodates all of these species is provided below.
Scyphophyllidium Woodland, 1927 revised
Synonyms. Doliobothrium Caira, Malek & Ruhnke 2011, Marsupiobothrium Yamaguti, 1952, Nandocestus Reyda, 2008, Orectolobicestus Ruhnke, Caira & Carpenter, 2006, Paraorygmatobothrium Runke, 1994 and Ruhnkecestus Caira & Durkin, 2006.
Diagnosis. Phyllobothriidea. Worms euapolytic or apolytic. Scolex with four bothridia, with or without cephalic peduncle. Bothridia globose or flat, often with band of muscle fibres around perimeter, without or occasionally with two facial semi-circular muscle bands, with or without marginal loculi, without or occasionally with facial loculi, without or occasionally with proximal aperture. Proximal bothridial surfaces with serrate gladiate or occasionally gladiate spinitriches. Distal bothridial surfaces with serrate gladiate, gongylate columnar or gongylate gladiate spinitriches. With or without neck. Capilliform filitriches on strobila usually arranged in scutes. Immature proglottids wider than long; usually becoming longer than wide with maturity. Genital pores usually alternating irregularly, located in anterior half of proglottid. Testes numerous, one to two layers deep in cross section; post-vaginal testes present. Cirrus sac containing coiled, armed cirrus. Ovary near posterior end of proglottid, H-shaped in dorsoventral view, tetralobed in cross section. Uterus ventral to vagina, median, extending anteriorly from anterior margin of ovary to well past cirrus sac. Vitellarium follicular; follicles usually arranged in two lateral bands, rarely circumcortical; bands extending almost entire length of proglottid, usually reduced or interrupted by ovary and cirrus sac. Excretory ducts in two lateral pairs. Eggs generally spindle-shaped, occasionally spherical.
Type species. Scyphophyllidium giganteum (van Beneden, 1858) Woodland, 1927.
Additional species. Forty-three listed in table 1. Parasites of the spiral intestines of sharks of the orders Carcharhiniformes, Lamniformes and Orectolobiformes, and occasionally batoids of the order Myliobatiformes. Cosmopolitan.
Remarks
As a result of these generic synonymizations, 44 species are now recognized as valid members of Scyphophyllidium. In order to expedite the future description of new species in this genus, the implementation of a strategy to help simplify comparisons, following Ghoshroy & Caira (Reference Ghoshroy and Caira2001) for the speciose onchoproteocephalidean genus Acanthobothrium Blanchard, 1848, is in order. To this end, we have circumscribed eight categories of Scyphophyllidium based on the most conspicuous and informative scolex features. Each of the 44 described species and seven undescribed species has been assigned to one of these categories (see table 1). This strategy facilitates future descriptions by limiting the comparisons required to establish novelty to only those species belonging to the same category as each new species. The inclusion of ultrastructural features in this categorization strategy is appropriate because the characterization of microtriches has become essentially routine in the description of new species in these phyllobothriideans. We note that these categories do not reflect the phylogenetic relationships of their members; they were designated solely to help expedite and shorten future descriptions.
The eight categories are as follows. Category 1: species with globose bothridia, each with a proximal aperture; the species given this category designation are the three species formerly assigned to Doliobothrium (now S. haselii, S. musculosum and Scyphophyllium sp. 5) and the species formerly known as M. alopias (now S. alopias) and Marsupiobothrium sp. 1 (now Scyphophyllidium sp. 6). Category 2: species with bothridia bearing marginal loculi; species given this category designation are the six formerly assigned to Orectolobicestus (now S. chiloscyllii, S. kelleyae, S. lorettae, S. mukahensis, S. randyi and S. tyleri), the single species formerly assigned to Nandocestus (i.e. S. guariticus) and, based on re-interpretation of SEMs, also S. orectolobi (of Cutmore et al., Reference Cutmore, Bennett, Miller and Cribb2017) and S. janineae (of Ruhnke et al., Reference Ruhnke, Healy and Shapero2006b). Category 3: species with facial loculi or facial semi-circular muscle bands; taxa given this category designation are the two species with facial semi-circular muscle bands formerly assigned to Paraorygmatobothrium (now S. barberi and S. taylori) as well as the single species with facial loculi formally assigned to Ruhnkecestus (now S. latipi). Category 4: species with globose bothridia that lack proximal apertures; species given this category designation are the two described and one undescribed original members of Scyphyophyllidium (i.e. S. giganteum, S. cf. giganteum and S. uruguayense) and the species formerly referred to as M. gobelinus (now S. gobelinus). Category 5: species with bothridia that are essentially flat, lack proximal apertures, marginal loculi, facial loci and semi-circular muscle bands, and bear serrate gladiate spinitriches on their distal bothridial surfaces; species currently given this category designation are the following 11 species formerly assigned to Paraorygmatobothrium: S. angustum, S. arnoldi, S. bullardi, S. campbelli, S. harti, S. kirstenae, S. paulum, S. prionacis, S. roberti, S. typicum and S. ullmanni. Category 6: species with bothridia that are essentially flat, lack proximal apertures, marginal loculi, facial loculi and semi-circular muscle bands, and bear gongylate columnar spinitriches on their distal bothridial surfaces; species currently given this category designation are the following eight species formerly assigned to Paraorygmatobothrium: S. bai, S. christopheri, S. exiguum, S. floraformis, S. mobedii, S. rodmani, S. sinclairtaylori and S. sinuspersicense. Category 7: species with bothridia that are essentially flat, lack proximal apertures, marginal loculi, facial loculi and semi-circular muscle bands, and bear gongylate gladiate spinitriches on their distal bothridial surfaces; species currently given this category designation are two species formerly assigned to Paraorygmatobothrium, now S. deburonae and S. mattisi. Category 8: this is a temporary category designation that currently includes species with flat, unmodified bothridia (all previously assigned to Paraorygmatobothrium), the surfaces of which have yet to be characterized using SEM. SEM characterization of species in this category will allow them to be transferred to category 5, 6 or 7, depending on whether their distal bothridial surfaces are found to bear serrate gladiate, gongylate columnar or gongylate gladiate spinitriches, respectively. Species given this category designation are the following five species, all formerly assigned to Paraorygmatobothrium: S. filiforme, S. leuci, S. musteli, S. nicaraguensis and S. triacis, as well as four of the six undescribed species formerly assigned to Paraorygmatobothrium (now Scyphophyllidium sp. 1 through 4, respectively).
Scyphophyllidium timvickiorum Caira, Hayes & Jensen n. sp. (figs 1c, 4 and 5)
ZooBank number for species: urn:lsid:zoobank.org:act:9A4F7760-BF71-490A-A6E2-66133921276D.
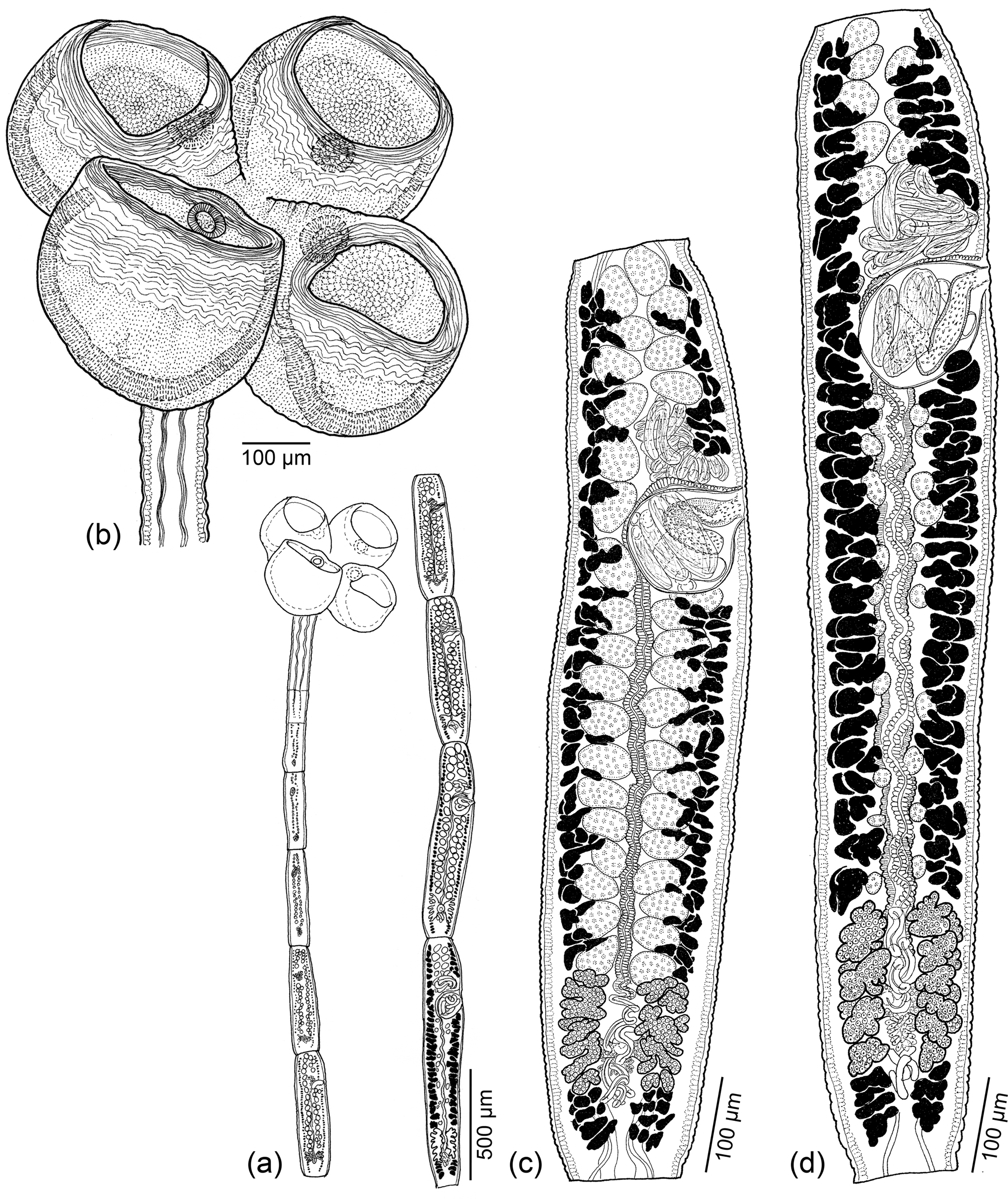
Fig. 4. Line drawings of Scyphophyllidium timvickiorum Caira, Hayes & Jensen n. sp. (a) Whole worm (holotype: MEPN no. 49443). (b) Scolex (holotype: MEPN no. 49443). (c) Subterminal mature proglottid showing testes (holotype: MEPN no. 49443). (d) Terminal mature proglottid (holotype: MEPN no. 49443).
Based on four gravid, eight mature and one immature worm, two scoleces examined with SEM and whole mounts of its strobila, and four preparations of eggs from detached gravid proglottids. Worms euapolytic, slightly craspedote, 4.2–9.8 (6.4 ± 1.7; 13) mm long; 7–21 (13 ± 4; 13) total proglottids per worm; maximum width 646–872 (743 ± 69; 13) near middle of scolex. Scolex 594–855 (723 ± 71; 11) long, with two dorsal and two ventral bothridia; cephalic peduncle lacking. Bothridia globose, highly muscular, with proximal apertures (fig. 5b) and single loculus, 267–451 (352 ± 34; 13; 39) long by 308–453 (376 ± 29; 12; 41) wide, bearing apical sucker; apical sucker 46–87 (67 ± 10; 12; 38) long by 57–99 (78 ± 10; 12; 40) wide; rims of bothridia with conspicuous band of circular muscle fibres.

Fig. 5. Scanning electron micrographs of Scyphophyllidium timvickiorum Caira, Hayes & Jensen n. sp. (a) Scolex, lateral view; small letters indicate locations of details in (d–g). (b) Scolex, proximal view; arrowheads indicate proximal apertures on each bothridium. (c) Close-up of proximal aperture of one bothridium. (d) Distal bothridial surface. (e) Proximal bothridial surface near rim. (f) Proximal bothridial surface of gladiate spinithrix band adjacent to rim. (g) Proximal bothridial surface away from rim.
Distal bothridial surfaces covered with gongylate columnar spinitriches and acicular filitriches (fig. 5d). Proximal bothridial surfaces with gladiate spinitriches and capilliform filitriches on rim (fig. 5e), with band of densely arranged gladiate spinitriches adjacent to rim (fig. 5f), with capilliform filitriches only on remainder of surfaces (fig. 5g). Capilliform filitriches on strobila not arranged in scutes.
Immature proglottids 6–19 (11 ± 4; 12) in number, approximately rectangular, becoming conspicuously longer than wide with maturity. Mature proglottids 1–4 (1 ± 1; 12) in number; terminal mature proglottid 688–1438 (1036 ± 198; 12) long by 192–267 (229 ± 23; 12) wide; length:width ratio 2.8–6.5:1 (4.6 ± 1.1; 12). Gravid proglottids 0–1 (n = 4). Testes 35–51 (42 ± 5; 13; 31) in total number, 13–19 (16 ± 2; 13; 31) in postporal field, 22–49 (36 ± 6; 10, 30) long by 23–70 (41 ± 9; 10, 30) wide, length:width ratio 0.49–1.63:1 (0.9 ± 0.25; 8; 30), arranged in 2–4 irregular columns anterior to cirrus sac, 1–2 columns in poral or aporal fields. Cirrus sac pyriform, 90–175 (143 ± 26; 12) long by 79–108 (99 ± 9; 12) wide, containing coiled cirrus; cirrus covered with spinitriches (fig. 4c, d). Vas deferens minimal, coiled at anteriomedial margin of cirrus sac. Genital pores unilateral (n = 2) or irregularly alternating (n = 11), 74–86% (79 ± 3; 12) of proglottid length from posterior end. Vagina weakly sinuous, extending from ootype, along midline of proglottid to anterior margin of cirrus sac then laterally along anterior margin of cirrus sac to open into common genital atrium. Ovary near posterior end of proglottid, H-shaped in frontal view, 94–235 (166 ± 36; 12) long by 108–178 (129 ± 20; 11) wide, tetralobed in cross section, weakly lobulated. Mehlis’ gland posterior to ovary. Vitellarium follicular; follicles irregular in shape, arranged in two lateral bands; each band consisting of 2–3 columns of follicles, extending throughout length of proglottid interrupted by cirrus sac and ovary. Uterus ventral, extending from ovarian bridge to anterior to cirrus sac. Four excretory ducts, in one dorsal and one ventral pair. Eggs spindle-shaped with bipolar filaments; filaments unequal in length (fig. 1c).
Taxonomic summary
Type and only known host. Pseudocarcharias kamoharai (Matsubara), crocodile shark (Lamniformes: Pseudocarchariidae).
Site of infection. Spiral intestine.
Type locality. Santa Elena (2°12′24.4″S, 80°56′58.1″W), Santa Rosa de Salinas, eastern Pacific Ocean, Ecuador.
Additional localities. None.
Etymology. This species honours CH's parents, Tim and Vicki Hayes, for their unwavering support of her academic pursuits.
Specimens deposited. Holotype (MEPN no. 49443); two paratypes consisting of one immature and one gravid worm (MEPN nos 49444–49445); five paratypes consisting of four mature and one gravid worm (LRP nos 10138–10142) and five slides with glycerine mounts of eggs (LRP nos 10144–10148); SEM voucher (LRP no. 10143); five paratypes consisting of one gravid and four mature worms (USNM nos 1608084–1608088). Specimen examined with SEM retained in JNC's personal collection.
Molecular sequence data. 28S rDNA hologenophore (LRP no. 9311 [EC-5-P1V]) for GenBank no. MN706182.
Remarks
Scyphophyllidium timvickiorum n. sp. is a category 1 species in that its bothridia bear proximal apertures. It differs from the three other species assigned to this category in its possession of fewer testes (i.e. 35–51 vs 69–74, 74–92 and 155–187, in S. musculosum, S. alopias and S. haselii, respectively). It further differs from S. haselii and S. musculosum in that its bothridia bear, rather than lack, apical suckers. In addition, it is a much smaller worm than S. alopias (4.2–9.8 vs 25.4–26.2 mm TL). Unlike S. alopias and S. haselii, it also lacks scutes on its strobila.
Clistobothrium amyae Caira, Hayes & Jensen n. sp. (figs 6 and 7a–e)
ZooBank number for species: urn:lsid:zoobank.org:act:42A65147-09A3-4950-8DC8-EFD58A495651.
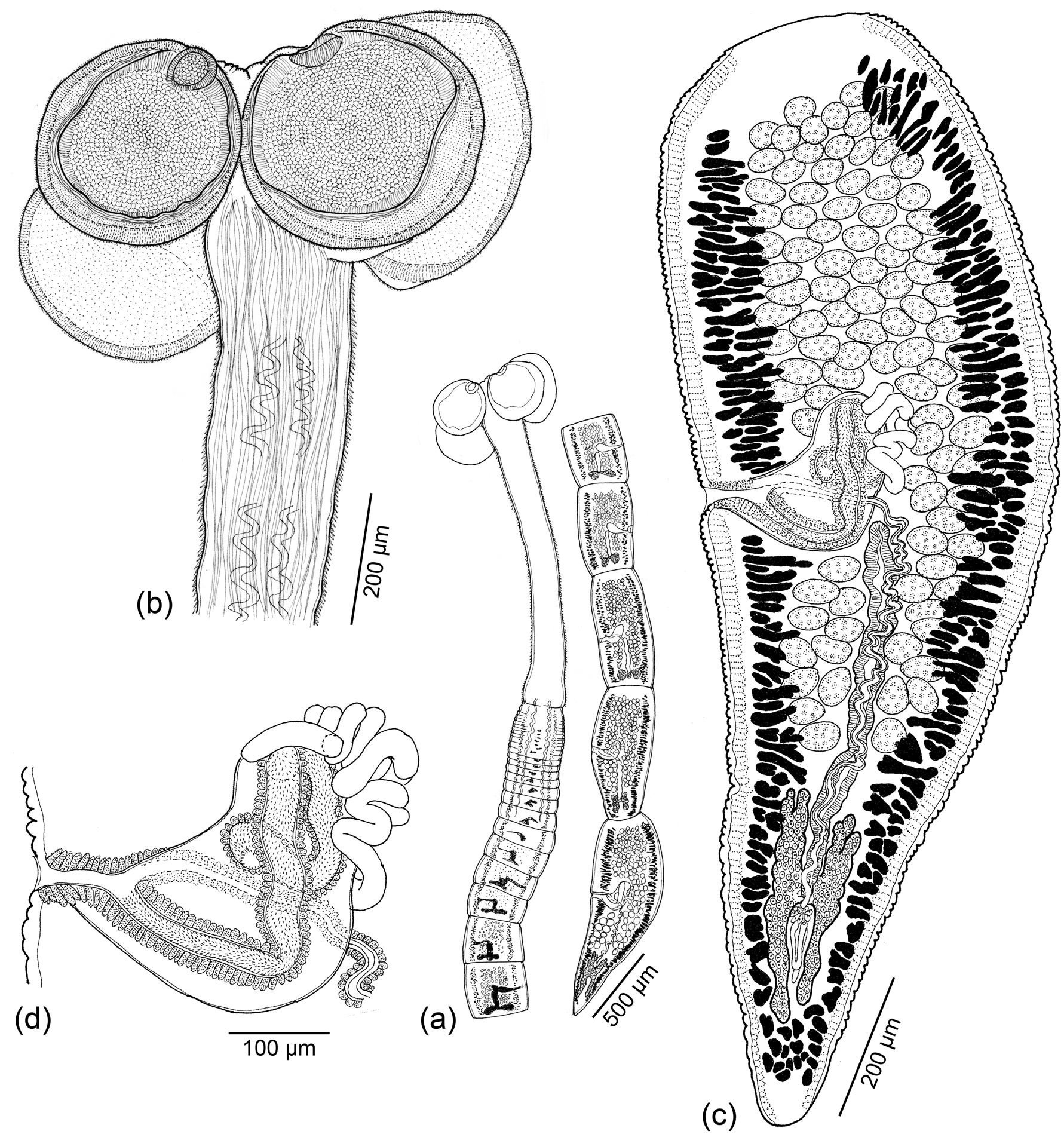
Fig. 6. Line drawings of Clistobothrium amyae Caira, Hayes & Jensen n. sp. (a) Whole worm (holotype: MEPN no. 49441). (b) Scolex (holotype: MEPN no. 49441). (c) Terminal mature proglottid (holotype: MEPN no. 49441). (d) Detail of terminal genitalia (holotype: MEPN no. 49441).

Fig. 7. Scanning electron micrographs of Clistobothrium amyae Caira, Hayes & Jensen n. sp. (a) Scolex; small letters indicate locations of details in (b–e). (b) Distal bothridial surface. (c) Proximal bothridial surface. (d) Surface of cruciform apical region. (e) Surface of cephalic peduncle. Scanning electron micrographs of Clistobothrium gabywalterorum Caira, Hayes & Jensen n. sp. (f) Scolex; small letters indicate locations of details in (g–j). (g) Distal bothridial surface. (h) Distal surface of apex of bothridia anterior to apical sucker. (i) Proximal bothridial surface. (j) Cephalic peduncle surface.
Based on whole mounts of seven mature worms, three scoleces examined with SEM and whole mounts of their strobila. Worms euapolytic, acraspedote, 7.7–16.5 (11.4 ± 3.6; 7) mm long; 25–40 (32 ± 6; 7) total proglottids per worm; maximum width at level of scolex. Strobila without distinct dorsomedian muscle band. Scolex with cruciform apical region, two dorsal and two ventral round, stalked bothridia, and cephalic peduncle. Scolex proper 281–759 (547 ± 158; 6) long by 499–970 (821 ± 154; 7) wide; bothridia 203–428 (374 ± 64; 6; 11) long by 200–464 (367 ± 79; 6; 14) wide; each bothridium with one circular, apical sucker 68–125 (89 ± 18; 4; 18) long by 70–123 (95 ± 18; 6; 16) wide; stalks 141–469 (351 ± 182; 2; 3) long by 80–224 (146 ± 55; 4; 6) wide. Cephalic peduncle conspicuous, 1020–3840 (2226 ± 1015; 7) long.
Distal bothridial surfaces covered with slender, aristate gladiate spinitriches and capilliform filitriches (fig. 7b). Proximal bothridial surfaces densely covered with slender gladiate spinitriches and capilliform filitriches (fig. 7c). Apex of cruciform region covered with sparsely arranged capilliform filitriches (fig. 7d). Cephalic peduncle densely covered with moderately sized, slender gladiate spinitriches and sparsely arranged capilliform filitriches (fig. 7e).
Immature proglottids 24–39 (31 ± 6; 7) in number, wider than long, becoming longer than wide with maturity. Mature proglottids one in number, longer than wide; terminal proglottid 423–2283 (1434 ± 589; 7) long by 207–453 (378 ± 84; 7) wide; length-to-width ratio 1.1–3.7:1 (2.3 ± 1.1; 7). Testes 73–106 (87 ± 11; 7; 13) in total number, 7–15 (11 ± 3; 7; 11) in postporal field, distributed in 4–6 (4.7 ± 0.9; 10) columns anterior to cirrus sac, round to oblong, 24–54 (35 ± 8; 6; 24) long by 24–58 (45 ± 7; 6; 24) wide. Cirrus sac J-shaped, 181–343 (227 ± 59; 6) long by 56–128 (87 ± 24; 7) wide, containing coiled cirrus; cirrus covered with minute spinitriches. Vas deferens minimal, coiled at anteriomedial and medial margins of cirrus sac. Genital pores lateral, irregularly alternating, 44–61% (51 ± 6; 7) of proglottid length from posterior end. Vagina sinuous, extending from ootype, along midline of proglottid mid-level of cirrus sac, crossing cirrus sac ventrally then extending along anterior margin of cirrus sac to enter genital atrium anterior to cirrus. Ovary near posterior end of proglottid, H-shaped in frontal view, 162–341 (234 ± 72; 6) long by 78–109 (95 ± 13; 6) wide, bilobed in cross section, weakly lobulated. Mehlis’ gland posterior to ovarian bridge. Vitellarium follicular; follicles irregular in shape, arranged in two lateral bands; each band consisting of 3–5 columns of follicles, extending throughout length of proglottid, interrupted by cirrus sac. Uterus ventral, extending from ovarian bridge to posterior margin of cirrus sac. Four excretory ducts, in one dorsal and one ventral pair.
Taxonomic summary
Type and only known host. Pseudocarcharias kamoharai (Matsubara), crocodile shark (Lamniformes: Pseudocarchariidae).
Site of infection. Spiral intestine.
Type locality. Santa Elena (2°12′24.4″S, 80°56′58.1″W), Santa Rosa de Salinas, eastern Pacific Ocean, Ecuador.
Additional localities. None.
Etymology. This species honours Dr Amy Donahue for her enthusiastic and innovative support of outreach science activities in her role as Vice Provost for Academic Operations at the University of Connecticut.
Specimens deposited. Holotype (MEPN no. 49441); one paratype (MEPN no. 49442); three paratypes (LRP nos 10132–10134); three SEM vouchers (LRP nos 10135–10137); two paratypes (USNM nos 1608082–1608083). Specimens examined with SEM retained in the JNC's personal collection.
Molecular sequence data. 28S rDNA hologenophore (LRP no. 10109 [EC-54-100 V]) for GenBank no. MN706184.
Remarks
Clistobothrium amyae n. sp. differs conspicuously from all three of its described congeners in its possession of an extremely elongate cephalic peduncle with gladiate spinitriches, rather than a cephalic peduncle that is extremely short as in C. carcharodoni Dailey & Vogelbein, 1990 and lacks spinitriches or is essentially lacking as in both Clistobothrium montaukensis Ruhnke, 1993 and Clistobothrium tumidum (Linton, 1922) Ruhnke, Reference Ruhnke1993. The apical suckers of the bothridia of this new species are also substantially smaller than those of its three congeners (50–115 vs 280–360 and 310–500 in diameter, respectively in C. tumidum and C. montaukensis, and 417–461 long by 333–398 wide in C. carcharodoni). This new species further differs from C. tumidum and C. montaukensis in that its bothridia are flat rather than foliose. In addition, C. amyae n. sp. is a much shorter worm than both C. carcharodoni and C. montaukensis (5.6–15.8 vs 24–40 and 38.5–119.5 mm TL, respectively). It also bears many fewer proglottids than C. montaukensis and C. tumidum (30–66 vs more than 100 in both of the latter species).
Across the D2 region of the 28S rDNA alignment, this new species differs from the undescribed species reported from the porbeagle shark by Randhawa & Brickle (Reference Randhawa and Brickle2011) by 22 bp, and, thus, likely represents a distinct species.
Clistobothrium gabywalterorum Caira, Hayes & Jensen n. sp. (figs 7f–j and 8)
ZooBank number for species: urn:lsid:zoobank.org:act:F6B8EDF1-D078-45A0-B185-091BA120FA5E.
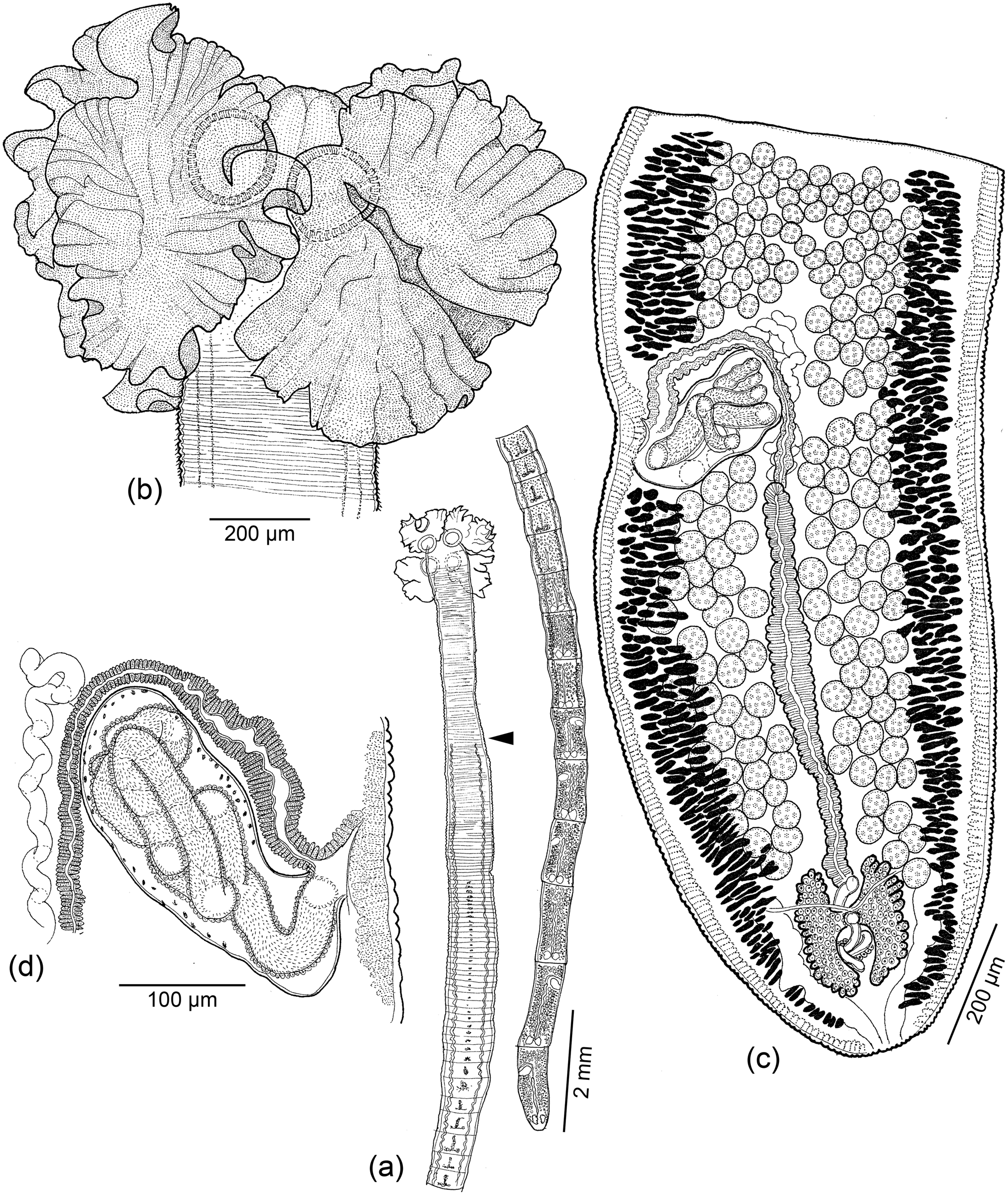
Fig. 8. Line drawings of Clistobothrium gabywalterorum Caira, Hayes & Jensen n. sp. (a) Whole worm; arrowhead indicates posterior extent of cephalic peduncle (paratype: USNM no. 1608081). (b) Scolex (holotype: MEPN no. 49440). (c) Terminal mature proglottid (paratype: USNM no. 1608081). (d) Detail of terminal genitalia of terminal mature proglottid (paratype: USNM no. 1608081).
Based on one mature worm, two immature worms, one scolex observed with SEM and the whole mount of its strobila. Worms euapolytic, acraspedote, 23.8 (n = 1) mm long; maximum width 1850–2163 (2007 ± 221; 3) at level of scolex; 127 (n = 1) total proglottids per worm. Strobila without distinct dorsomedian muscle band. Scolex consisting of four bothridia and cephalic peduncle; cruciform apical region lacking. Scolex proper 1456–1581 (1512 ± 64; 3) long by 1850–2163 (2003 ± 157; 3) wide. Bothridia foliose, 697–1227 (915 ± 222; 3; 8) long by 907–1518 (1277 ± 247; 3; 7) wide; each bothridium with single apical sucker; apical suckers 203–356 (292 ± 48; 3; 10) long by 284–373 (325 ± 35; 3; 11) wide. Cephalic peduncle 2579–3016 (2798 ± 309; 2) long.
Distal surfaces of apex of bothridia anterior to apical sucker with slender gladiate spinitriches and capilliform filitriches (fig. 7h). Distal surfaces of bothridia and apical suckers densely covered with capilliform filitriches (fig. 7g). Proximal bothridial surfaces covered with slender, aristate gladiate spinitriches and capilliform filitriches (fig. 7i). Cephalic peduncle densely covered with gladiate spinitriches (fig. 7j); filitriches not seen in this region.
Immature proglottids 123 (n = 1) in number, wider than long; mature proglottids four (n = 1) in number; terminal mature proglottid 1401 (n = 1) long by 591 (n = 1) wide; length:width ratio 2.4:1 (n = 1). Testes 164–185 (175 ± 15; 2) in total number, 44–51 (48 ± 5; 2) in postporal field, spherical, 36–54 (47 ± 5.8; 1; 6) in diameter, arranged in 7–10 irregular columns anterior to cirrus sac, 3–4 irregular columns in poral and aporal fields. Vas deferens minimal, coiled, medial, extending slightly anterior to and posterior to cirrus sac. Cirrus sac J-shaped, 138 (n = 1) long by 203 (n = 1) wide, containing coiled, armed cirrus. Genital pores lateral, irregularly alternating, 68% (n = 1) of proglottid length from posterior end of terminal proglottid. Vagina weakly sinuous, extending from ootype along midline of proglottid to anterior margin of cirrus sac, then laterally along anterior margin of cirrus sac to open into common genital atrium anterior to cirrus. Ovary posterior, H-shaped in frontal view, 210 (n = 1) long by 68 (n = 1) wide, weakly lobate, bilobed in cross section. Mehlis’ gland posterior to ovarian bridge. Vitellarium follicular; follicles irregular in shape, arranged in two lateral bands; each band consisting of six to eight columns of follicles, interrupted by the cirrus sac. Uterus ventral, extending from level of ovary to posterior margin of cirrus sac. Four excretory ducts, in one dorsal and one ventral pair.
Taxonomic summary
Type and only known host. Pseudocarcharias kamoharai (Matsubara), crocodile shark (Lamniformes: Pseudocarchariidae).
Site of infection. Spiral intestine.
Type locality, Santa Elena (2°12′24.4″S, 80°56′58.1″W), Santa Rosa de Salinas, eastern Pacific Ocean, Ecuador.
Additional localities. None.
Etymology. This species honours Gabriela del Pilar Flores Rivera and Walter Gilberto Tigrero González of the Ministerio de Producción, Comercio Exterior, Inversiones y Pesca, Ecuador, for sharing their extensive knowledge of local elasmobranch catches and assistance with all aspects of the fieldwork and permitting process that made our collections in Ecuador possible.
Specimens deposited. Holotype (MEPN no. 49440); one immature paratype (LRP no. 10130); SEM voucher (LRP no. 10131); one paratype (USNM no. 1608081). Scolex examined with SEM retained in JNC's personal collection.
Molecular sequence data. 28S rDNA hologenophore (LRP no. 8673 [EC-54-1V]) for GenBank no. MN706183.
Remarks
The description of a new species based on the limited amount of material available here is typically not advisable. However, this new species exhibits clear morphological and molecular differences from its four described congeners. Clistobothrium gabywalterorum n. sp. differs conspicuously from C. carcharodoni and C. amyae in that its bothridia are foliose, rather than flat. In addition, it is a much smaller worm than C. montaukensis (23.8 vs 38.5–119.5 mm) and a much larger worm than C. amyae (23.8 vs 5.6–15.8 mm). It exhibits a greater number of testes than C. amyae and C. carcharodoni (164–185 vs 77–127 and 91–123, respectively) and fewer testes than C. montaukensis and C. tumidum (165–185 vs 198–263 and 234–307, respectively). Unlike all species except C. amyae, this new species also possesses a long cephalic peduncle that bears gladiate spinitriches.
Across the 728 bp in the D2 region of the 28S rDNA alignment, which includes data for all five species of Clistobothrium for which sequence data are now available, this species differs from C. carcharodoni by 11 bp, from C. montaukensis by 24 bp, from C. amyae by 12 bp and from the undescribed species reported from the porbeagle shark by Randhawa & Brickle (Reference Randhawa and Brickle2011) identified as Clistobothrium cf. montaukensis by 16 bp.
The most recent diagnosis of Clistobothrium, which is that of Ruhnke (Reference Ruhnke2011), is revised below to accommodate both of the above new species.
Clistobothrium Dailey & Vogelbein, Reference Dailey and Vogelbein1990 revised
Diagnosis. Phyllobothriidea. Worms apolytic or euapolytic. Strobila with or without distinct longitudinal dorsomedian band of muscles. Scolex with two dorsal and two ventral bothridia, usually with dome-shaped or cruciform apical region. Each bothridium with apical sucker and one flat or foliose loculus, with or without conspicuous stalk. Cephalic peduncle short or elongate. Immature proglottids wider than long; mature proglottids at least twice as long as wide. Testes numerous; postporal testes present. Cirrus sac containing coiled cirrus; cirrus armed with small spinitriches. Genital atrium present. Vagina crossing or extending anterior to cirrus sac, opening anterior to cirrus sac. Ovary posterior, H-shaped in dorsoventral view, bilobed in cross section. Uterus ventral, extending from ovary to posterior margin of cirrus sac in mature proglottids, extending to anterior margin of cirrus sac in gravid proglottids. Eggs spherical; surface mamillated or spinose.
Type species. Clistobothrium carcharodoni Dailey & Vogelbein, 1990.
Additional species: C. amyae Caira, Hayes & Jensen n. sp., C. montaukensis Ruhnke, 1994, C. tumidum (Linton, 1922) Ruhnke, 1994, C. gabywalterorum Caira, Hayes & Jensen n. sp. Parasites of the spiral intestine of sharks of the order Lamniformes. Cosmopolitan.
Discussion
As of this writing, a total of 45 described species are considered to belong to Scyphophyllidium. An additional seven undescribed species that have been included in molecular phylogenetic analyses from previously unexplored host species, should also now be considered to belong to the genus. To avoid future confusion, six of these seven species are formally assigned the following unique numerical designations (see table 1): Paraorygmatobothrium sp. 1 through 4 of Cutmore et al. (Reference Cutmore, Bennett, Miller and Cribb2017) are assigned the designations Scyphophyllidium sp. 1 through 4, respectively. Doliobothrium sp. of Cutmore et al. (Reference Cutmore, Bennett, Miller and Cribb2017) is assigned the designation Scyphophyllidium sp. 5. Marsupiobothrium sp. 1 of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) is assigned the designation Scyphophyllidium sp. 6. Scyphophyllidium cf. giganteum of Caira et al. (Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a) is considered to represent a distinct, seventh species for the reasons described above.
It is interesting to consider the host associations of these 52 species of Scyphophyllidium. By far the majority of the described species (i.e. 32 of 45) parasitize carcharhiniform sharks (i.e. ground sharks). In terms of the remaining 13 described species, seven parasitize orectolobiform sharks (i.e. carpet sharks), five parasitize lamniform sharks (i.e. mackerel sharks) and one parasitizes a freshwater stingray. The majority of the known undescribed species (i.e. six of seven) also parasitize carcharhiniform sharks; the remaining one species parasitizes a lamniform shark.
The topology of the tree resulting from our phylogenetic analysis suggests that instances of associations with hosts other than carcharhiniform sharks represent host-switching events from carcharhiniform sharks in either an immediate or slightly more distant ancestor. At a minimum, there appear to have been two switches to lamniform sharks, two switches to orectolobiform sharks and one switch to a stingray. Given the trophic nature of cestode transmission, it is tempting to suggest these host switches may reflect similarities in diet. Jensen & Bullard (Reference Jensen and Bullard2010), who conducted the most extensive work to date identifying the final intermediate hosts of Scyphophyllidum species formerly assigned to Paraorygmatobothrium, determined that bony fish play this role in the life cycles of all of the species they examined, several of which are among the carcharhiniform-hosted species represented in our study. Indeed, the diets of Orectolobus maculatus (Bonnaterre), Chiloscyllium punctatum Müller & Henle, A. vulpinus (Bonnaterre), A. pelagicus Nakamura and even that of Paratrygon aiereba (Müller & Henle) all include bony fish to a greater or lesser extent (Compagno, Reference Compagno1984; Last & Stevens, Reference Last and Stevens2009; de Carvalho et al., Reference de Carvalho, Lovejoy, Rosa, Reis, Kullander and Ferraris2003, respectively). However, this does not explain the presence of S. randyi in Chiloscyllium hasselti (Bleeker), which does not appear to consume bony fish (Compagno & Neim, Reference Compagno, Niem, Carpenter and Niem1998). Nor does it explain the lack of reports of this genus from the many other species of Orectolobiformes, Lamniformes and stingrays, which are too numerous to list here, the diets of which also include bony fish.
Table 1 summarizes what is known about the distribution of some of the key morphological and ultrastructural features in the 52 (described and undescribed) species of Scyphyophyllidium, subsets of which were used to establish the six genera now considered to be junior synonyms of the latter genus. The topology of our phylogenetic tree indicates that many of these characters are either homoplasious or unique to single species (i.e. autapomorphies). Examples of homoplasious characters include: the presence of marginal loculi (S. orectolobi, S. janineae, S. randyi and S. tyleri, and S. guariticus); globose rather than flat bothridia (S. cf. giganteum and S. timvickiorum and Scyphophyllidium sp. 6); the presence of proximal bothridial apertures (Scyphophyllidium sp. 5 and S. timvickiorum). An example of an autapomorphic feature is the presence of facial loculi in S. latipi. Also intriguing is the fact that the majority (i.e. ten) of the 13 species of Scyphophyllidium, for which gravid proglottids have been observed, exhibit spindle-shaped eggs. The exceptions are S. barberi and S. guariticus, both of which have spherical eggs and S. leuci with eggs that Watson & Thorson (Reference Watson, Thorson and Thorson1976) reported were either with or without knobs. It would be interesting to determine the full extent of spindle-shaped eggs across the other species of Scyphophyllidium.
Evidence supporting the close affinities among at least subsets of the genera synonymized here with Scyphophyllidium also comes from SEM. The majority of these species exhibit the somewhat unusual conditions of capilliform filitriches on the strobila that are arranged in scutes, serrate gladiate spinitriches on the proximal bothridial surfaces and serrate gladiate, gongylate columnar or gongylate gladiate spinitriches on their distal bothridial surfaces. To our knowledge, the only other cestode taxa that possess one or more of these ultrastructural features for which sequence data are also available, are species of Alexandercestus, Guidus, Hemipristicola, Orygmatobothrium Diesing, 1863 and, possibly also in modified form (see below), Thysanocephalum Linton, 1890. The topology of our molecular phylogenetic tree suggests that Thysanocephalum is the sister taxon of the clade consisting of Scyphophyllidium + Hemipristicola + Alexandercestus, in which case, all three ultrastructural features may have originated in the shared common ancestor of these four genera. In contrast, the presence of these features in the more distantly related Orygmatobothrium appears to be homoplasious.
We have taken a relatively conservative approach here with respect to the genera we have synonymized with Scyphophyllidium. However, in the future, serious consideration should be given to whether Hemipristicola and possibly also Alexandercestus should also be synonymized with Scyphophyllidium. Beyond sharing subsets of the above unique ultrastructural features with Scyphophyllidium, Cutmore et al. (Reference Cutmore, Bennett, Miller and Cribb2017) found the monotypic Hemipristicola to nest deeply among species now assigned to Scyphyophyllidium in the trees resulting from both their Bayesian and ML phylogenetic analyses of NADH1 amino acid data. Morphologically, H. gunterae differs from species of Scyphophyllidium in its possession of a deep central cavity in each of its bothridia. But, it is possible this feature will ultimately also be found to represent an autapomorphy for this species. Both species of Alexandercestus can be distinguished from existing members of Scyphophyllidium in their possession of foliose bothridia, but the bothridia of Alexandercestus manteri Ruhnke & Workman, 2013 are only weakly foliose. It will be interesting to see the results of future phylogenetic work that includes A. manteri. Fortunately, even if both genera are ultimately determined to be synonyms of Scyphophyllidium, the latter remains the oldest, and thus valid, name for the genus. Although Guidus shares highly muscular, globose bothridia, and filitriches arranged in scutes with subsets of species of Scyphophyllidium, its placement well outside of all of these taxa in the tree resulting from our phylogenetic analysis indicates that these features are homoplasious in this skate-hosted taxon.
In contrast, the bothridia of Thysanocephalum are distinctive in consisting of ‘a small specialized anterior loculus followed by an extensive posterior loculus that is narrow at its connection to the anterior loculus, but expands almost immediately into a large, extensively folded, bifid structure’ (Caira et al., Reference Caira, Jensen and Healy1999: 103). Furthermore, rather than scutes, the surfaces of the strobila of T. thysanocephalum bear ‘leaf-like’ structures (Caira et al., Reference Caira, Jensen and Healy1999: 125). In combination, these distinctive morphological features and the topology of our molecular phylogenetic tree justify retaining this as a valid genus.
The placement of six phyllobothriidean genera into synonymy with Scyphophyllidium was a major action that necessitated substantial revision of the classification of the order. While the molecular phylogenetic analyses (Cutmore et al., Reference Cutmore, Theiss, Bennett and Cribb2011, Reference Cutmore, Bennett, Miller and Cribb2017; Caira, et al., Reference Caira, Jensen, Waeschenbach, Olson and Littlewood2014a; Ruhnke et al., Reference Ruhnke, Daniel and Jensen2020; our analyses here) supporting this action have all been based on data from a limited selection of genes (i.e. D1–D3 of 28S rDNA, 18S rDNA and/or NADH1), the taxon sampling has been relatively robust. In terms of described species, our analysis included the only species originally assigned to Hemipristicola, one of two species originally assigned to Marsupiobothrium, the only species originally assigned to Nandocestus, two of five species originally assigned to Orectolobicestus, the only species originally assigned to Ruhnkecestus, one of two species originally assigned to Scyphophyllidium. In addition, our analysis included 22 of the 34 species previously assigned to Paraorygmatobothrium, with representation from all three orders of sharks known to host species previously assigned to this genus. With respect to undescribed species, our taxon sampling included one species originally assigned to Doliobothrium, one originally assigned to Marsupiobothrium, one originally assigned to Scyphophyllidium and four species originally assigned to Paraorygmatobothrium. Although we believe this work has paved the way for the development of a more thorough understanding of the evolution and host associations of these cestodes, a larger sampling of molecular markers is necessary for the elucidation of robust clades within the genus.
The shark order Lamniformes is unusual among elasmobranchs in its extremely high ratio of families to genera – the 15 extant species of lamniforms are currently assigned to nine genera in seven families, four of which are monotypic. Work to date on the cestodes of lamniforms indicates that the cestode faunas of each family are highly divergent relative to one another (Linton, Reference Linton1889, Reference Linton1922; Yamaguti, Reference Yamaguti1935, Reference Yamaguti1952; Dailey, Reference Dailey1969, Reference Dailey1971; Kurochkin & Slankis, Reference Kurochkin and Slankis1973; Beveridge & Campbell, Reference Beveridge and Campbell1988; Dailey & Vogelbein, Reference Dailey and Vogelbein1990; Caira & Runkle, Reference Caira and Runkle1993; Ruhnke, Reference Ruhnke1993, Reference Ruhnke2011; Palm, Reference Palm2004; Caira et al., Reference Caira, Jensen, Yamane, Isobe, Nagasawa, Yano, Morrissey, Yabumoto and Nakaya1997, Reference Caira, Jensen, Waeschenbach and Littlewood2014b; Olson & Caira, Reference Olson and Caira2001). Our interest in examining the cestodes of the monotypic Pseudocarchariidae was motivated largely by the fact that this family had not been examined for cestodes. This host species has eluded examination previously in large part because, unlike many of the other lamnid species, its flesh generally has little appeal for human consumption (Compagno, Reference Compagno1984) and, thus, this shark is infrequently landed in fish markets around the world. Our arrival in Ecuador during what is locally considered to be ‘crocodile shark season’ (i.e. May and early June) when this species is landed, at least in the region of Santa Elena, was thus, extremely fortuitous.
The two new species of Clistobothrium reported here bring the total number of described species to five; Randhawa & Brickle's (Reference Randhawa and Brickle2011) report of the undescribed species C. cf. montaukensis expands the total to six. While the two species described here parasitize the monotypic Pseudocarchariidae, the remaining four species parasitize members of the Lamnidae – specifically, Carcharodon carcharias L., Isurus oxyrinchus Rafinesque and Lamna nasus Bonnaterre. Thus, it would be extremely interesting to examine the two remaining species of lamnids (i.e. Isurus paucus Guitart and Lamna ditropis Hubbs & Follett), neither of which has been examined for Clistobothrium. We believe both are highly likely to host additional members of the genus.
Acknowledgements
We thank Francisco Concha and Fernando Marques for their assistance with fieldwork. Collections in Ecuador were conducted under permit no. 006-IC-FA-DPSE-MA-2014 issued by the Director Provincial del Miniserio de Ambiente. We are grateful to Óscar Daniel Carreño Maldonado, Responsible de Vida Silvestre, Santa Elena, for assistance with obtaining permission for us to collect in Ecuador, and Daniel Castillo Rodríguez for expediting the permitting process. We thank Iwaki Takashi of the MPM for providing us with a sizeable series of detailed images of the bothridia of the type specimen of M. alopias. We are grateful to Maria Pickering and Kaitlin Gallagher for generating the D1–D3 28S rDNA sequence data for the three specimens of the new cestode species included in the analysis. We also thank David Andres Donoso Vargas of the MEPN and Anna Phillips of the USNM for assistance with the deposit of specimens.
Financial support
This work was supported by the National Science Foundation (grant numbers 1457762, 1457776, 1921404, 1921411). Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and international guides on the care and use of animals.












