Introduction
Water may play a role at several crucial stages in the life cycles of trichostrongyloids, especially the development, and probably hatching, of eggs, prevention of desiccation of eggs and hatched larvae, and the migration of infective larvae out of the faeces and on to herbage (as reviewed by O'Connor et al., Reference O'Connor, Walkden-Brown and Kahn2006). For Nematodirus species, in which eggs develop in the soil, water is also likely to play a role in the liberation of eggs from dung. Furthermore, rehydration of larvated eggs has been suggested to accelerate hatching, such that peaks in larval abundance on herbage, and risk of infection and disease, increase when rain follows a dry period (Graham et al., Reference Graham, Harris and Ollerenshaw1984; Haskell, Reference Haskell2008). However, this hypothesis has not been thoroughly examined. Sound understanding of the effects of rainfall on larval dynamics is needed if predictions of the transmission and epidemiology of Nematodirus spp., especially the pathogenic species N. battus, are to be appropriately configured and useful to managers. This is especially important as climate change alters the epidemiology of trichostrongyloid species (van Dijk et al., Reference Van Dijk, David, Baird and Morgan2008, Reference Van Dijk, Sargison, Kenyon and Skuce2010) while anthelmintic resistance limits the options for dealing with their effects in livestock.
As shown by Gibson & Everett (Reference Gibson and Everett1981), Nematodirus eggs do not develop in dung and may die if captured in dung for prolonged periods. Rainfall is likely to influence the disintegration of dung and the release of eggs into the soil. Onward development in the soil also depends on water availability. Laboratory experiments carried out by Parkin (Reference Parkin1976) clearly showed that, regardless of high or low relative air humidity, N. battus eggs which were completely deprived of free water did not manage to develop. However, at pasture, soil particles are surrounded by a layer of water, which is retained by forces of capillarity, osmotic pressure and gravity (Parkin, Reference Parkin1975a). At osmotic pressures reflecting soil saturations between full water capacity and wilting point, N. battus eggs are still able to take up water (Parkin, Reference Parkin1975b). Only eggs incubated in molar solutions (1 m) are not able to develop beyond the second larval stage, while those incubated in salt solutions of strengths between 10− 4 and 0.1 m manage to develop just as well as aqueous controls (Parkin, Reference Parkin1976). In temperate regions, access to water is therefore unlikely to be limiting to the development of Nematodirus species in soil.
Embryonated eggs and larvae of Nematodirus spp. are remarkably resistant to desiccated environments. Parkin (Reference Parkin1976) reported no appreciable mortality after keeping air-dried embryonated eggs at a relative humidity of 33% for 15 weeks. Only approximately one-quarter of hatched infective larvae died as the result of this treatment when applied for 10 weeks (Parkin, Reference Parkin1972). Zurliiski (Reference Zurliiski1978, as quoted by Anderson, Reference Anderson and Anderson2000), who dried the L3-stage larvae of N. spathiger at 15–20°C for 166 days, confirmed that such desiccated larvae were still infective to lambs.
Anecdotal evidence suggests that when a period of rain follows a prolonged period of drought, the incidence of clinical nematodirosis may increase. Therefore, amongst UK veterinarians, it is a popular belief that rain can induce hatching. For example, during the spring of 2003, a year in which a relatively high disease incidence was recorded, the temperature rose above the lower threshold for hatching in April but hardly any disease was witnessed during this very dry month (Veterinary Investigation Diagnosis Analysis (VIDA) surveillance data, van Dijk et al., Reference Van Dijk, David, Baird and Morgan2008). The peak incidence of disease was measured towards the end of May, just after rains ended a prolonged period of drought. Similar circumstances occurred during the very dry spring of 2007, in which no peak of larval emergence was measured at pasture until the start of the very wet month of July (van Dijk, unpublished data). If the hatching of eggs when water becomes available after a period of desiccation indeed represents an alternative mechanism to the described temperature-induced hatching (van Dijk & Morgan, Reference Van Dijk and Morgan2008), this phenomenon will have to be included in mathematical models of the epidemiology of nematodirosis. As a result of climate change, periods of drought, followed by a limited number of days with heavy rainfall, have been predicted to become more frequent (Hennessy et al., Reference Hennessy, Gregory and Mitchell1997; Tapiador et al., Reference Tapiador, Sanchez and Gaertner2007).
With regards to the mechanism behind a role for water in the hatching process, a first working hypothesis would be that, during droughts, water becomes limiting for the hatching of eggs that have been temperature-primed to hatch. However, Parkin (Reference Parkin1975b), reproducing soil moisture stresses at a level observed at wilting point during droughts, not only observed the hatching of eggs submitted to this treatment but also suggested that, after heavy rains, aeration of the soil becomes the limiting factor for hatching. In further experiments, Parkin (Reference Parkin1976) observed the hatching of cold-sensitized embryonated eggs in 0.1–10− 4 m salt solutions. This suggests that soil moisture is unlikely to be limiting for the hatching process and also that the presence of water is no guarantee of hatching.
A second hypothesis would be that, after rain, a rapid influx of water into desiccated eggs forms a hatching stimulus. In this scenario, the desiccated eggs may hatch as a result of the suddenly increasing hydrostatic pressure. It has been proposed that such an uptake of water is a vital step in the hatching process (Parkin, Reference Parkin1975a). Parkin (Reference Parkin1976) started investigating this hypothesis. ‘Chilled’, air-dried eggs were incubated above salt solutions representing certain lowered relative humidities (RH). On return to water, a somewhat earlier and more extensive hatch of the eggs that had been exposed to lowered RH, compared with aqueous controls, was recorded. However, a 100% RH treatment (air-dried eggs kept above distilled water), on return to water, hatched more rapidly than any of the other treatments, casting doubt on Parkin's conclusion that exposure to lowered humidity significantly increased hatching magnitude and rate. Eggs in the soil are likely to experience high osmotic pressures applied by surrounding soil particles and salts, and thus the moisture stresses experienced in the soil may be far greater than that represented by lowered air humidity alone (Parkin, Reference Parkin1975b). Moreover, Parkin worked with ‘chilled’ eggs only. It has been observed that the nematode Aphelenchus avenae rapidly changes its lipid reserves into carbohydrate reserves when undergoing desiccation (Madin & Crowe, Reference Madin and Crowe1975), suggesting that increased osmotic pressure caused by an increase in carbohydrates may play a role in protection from desiccation. Nematodirus spp. change lipid reserves into trehalose when eggs are kept below 10°C (Ash & Atkinson, Reference Ash and Atkinson1983; van Dijk & Morgan, Reference Van Dijk and Morgan2008) and therefore chilled eggs may be more resistant to desiccation. Dehydration–rehydration cycles would then have different influences on the hatching of chilled and non-chilled eggs. The hatching of non-chilled eggs appears to play an important role in the adaptability of N. battus to variable climates, and potentially to its adaptation to new geographic areas and to climate change (van Dijk & Morgan, Reference Van Dijk and Morgan2010), and interactions between chilling and water requirements are worthy of further investigation.
The aims of the work described here are to establish whether: (1) rehydration of desiccated N. battus eggs leads to temporally altered hatching patterns; (2) hatching induced by rehydration would be likely to free enough larvae to cause clinical disease; (3) chilled eggs are better protected from desiccation compared to non-chilled eggs.
Materials and methods
Treatment of eggs
Eggs from lambs naturally infected with N. battus were isolated as described in van Dijk & Morgan (Reference Van Dijk and Morgan2008), and incubated to the infective L3 stage at 20°C for 7 weeks. After thorough mixing, half of these eggs were chilled at 4°C for 4 weeks while the other half remained at 20°C. Eggs from both treatments were then transferred to, and mixed with, saturated salt solutions (containing an excess of solids). At 20°C, these solutions represent relative humidities of 95% (potassium nitrate, KNO3), 70% (ammonium nitrate, NH4NO3), 55% (magnesium nitrate, Mg(NO3)26H2O) and 33% (magnesium chloride, MgCl26H2O) (Winston & Bates, Reference Winston and Bates1960). The salts were chosen for their lack of toxic effects (Winston & Bates, Reference Winston and Bates1960; Parkin, Reference Parkin1976).
The egg-containing salt sludges were kept in the inner part of 50 ml filter tubes with pores 0.45 μm in size (Maxi-Spin®, Alltech Inc., Deerfield, Illinois, USA) (fig. 1). The area within the Falcon tubes, outside the filter, was also filled with salt sludge, and the lid was closed tightly and covered with a layer of Parafilm® (Pechiney Plastic Packaging, Chicago, Illinois, USA). One tube was set up for each of three replicates within one treatment.

Fig. 1 Schematic diagram of the filter tubes used in desiccation experiments.
As Parkin (Reference Parkin1976) showed that varying the length of exposure to different RH, between 7 and 98 days, does not significantly influence the hatching process, an exposure time of 7 days was used in these experiments. After 1 week, during which the tubes were kept in an incubator at 20°C, the inner, filter, part of the filter tubes was filled to the top with filtered water and the salts mixed with this water. The tubes were centrifuged at 2000 rpm until no water remained in the filters. This process was repeated four times, which removed all visible salt from the filter, and then twice more to wash any remaining salt off the eggs. Eggs were then transferred to a larger amount of filtered water and three replicates of approximately 500 eggs were pipetted into wells, as described in van Dijk & Morgan (Reference Van Dijk and Morgan2008).
A salt-toxicity control was set up for the 33% RH treatment as follows. Batches of embryonated chilled and non-chilled eggs were air dried at 20°C in a small concave glass dish, which was then placed on top of the salt, within Falcon tubes. Care was taken to avoid any contact of the eggs with salt and the tubes were sealed in the same manner. After 1 week at 20°C, eggs were removed from the glass dish by pouring filtered water over the dishes and collecting them in a 500 ml glass bowl. The bowl was filled to the top and the suspension stirred. After the eggs had sedimented out, the supernatant water was siphoned off and fresh filtered water added to the bowl, then the eggs were pipetted into wells.
Control eggs were kept in filtered water at 20°C for 1 week, subsequently pipetted into wells, as above, and put at 15°C. The hatching process of all treatments was started simultaneously, in the same incubator. In all treatments, eggs were hatched at 15°C. The numbers of eggs and larvae present were counted 2 h after returning the eggs to water, and the number of live and dead larvae counted on day 1 and then every other day until day 31. Larvae were counted as dead when immotile and in the characteristic stretched-arched position. Eggs that are severely damaged by either desiccation or salt toxicity may, as a result of rapid water influx and sudden egg expansion, rupture and release their larvae on return to water. The number of hatched larvae present in wells on day zero, the day that eggs were placed at their hatching temperature, may therefore give an indication of the proportion of eggs damaged by the salt treatments.
Data analysis
The influence of humidity treatment, and chilling, on the proportions of eggs hatching on day zero, within 2 h of returning them to water, was tested in a two-way ANOVA. It was assumed that hatching induced by the influx of water, if this phenomenon existed, would commence rapidly after return to water. The influence of chill and humidity treatment on the total proportions of eggs hatching on hatching-day 1, and the proportions of eggs producing live larvae at this time, were analysed in a three-way ANOVA. This process was repeated for the total proportions hatching, which, for all treatments, had been completed on or before day 27. Proportions were arcsine transformed for analysis. The time taken for 50% of the eggs to hatch was analysed in a two-way ANOVA with factors ‘chill experience’ and ‘humidity treatment’. In all analyses, Tukey's pairwise comparison was applied for the humidity treatments. The proportions of eggs producing live larvae at the time of maximum hatching of the salt-toxicity controls were compared with their in-salt counterparts in a two-way ANOVA with factors ‘salt contact’ and ‘chill experience’.
Results
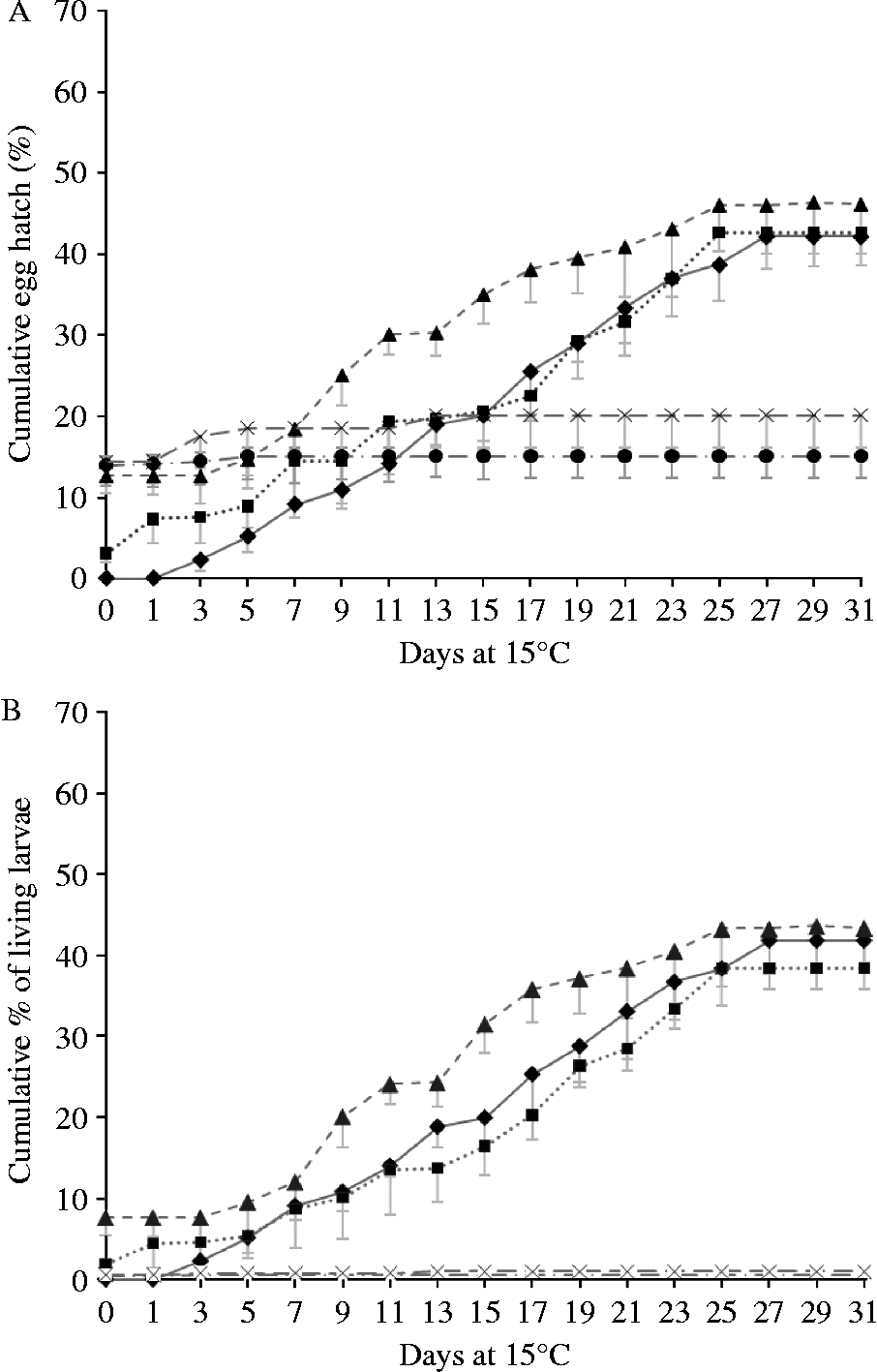
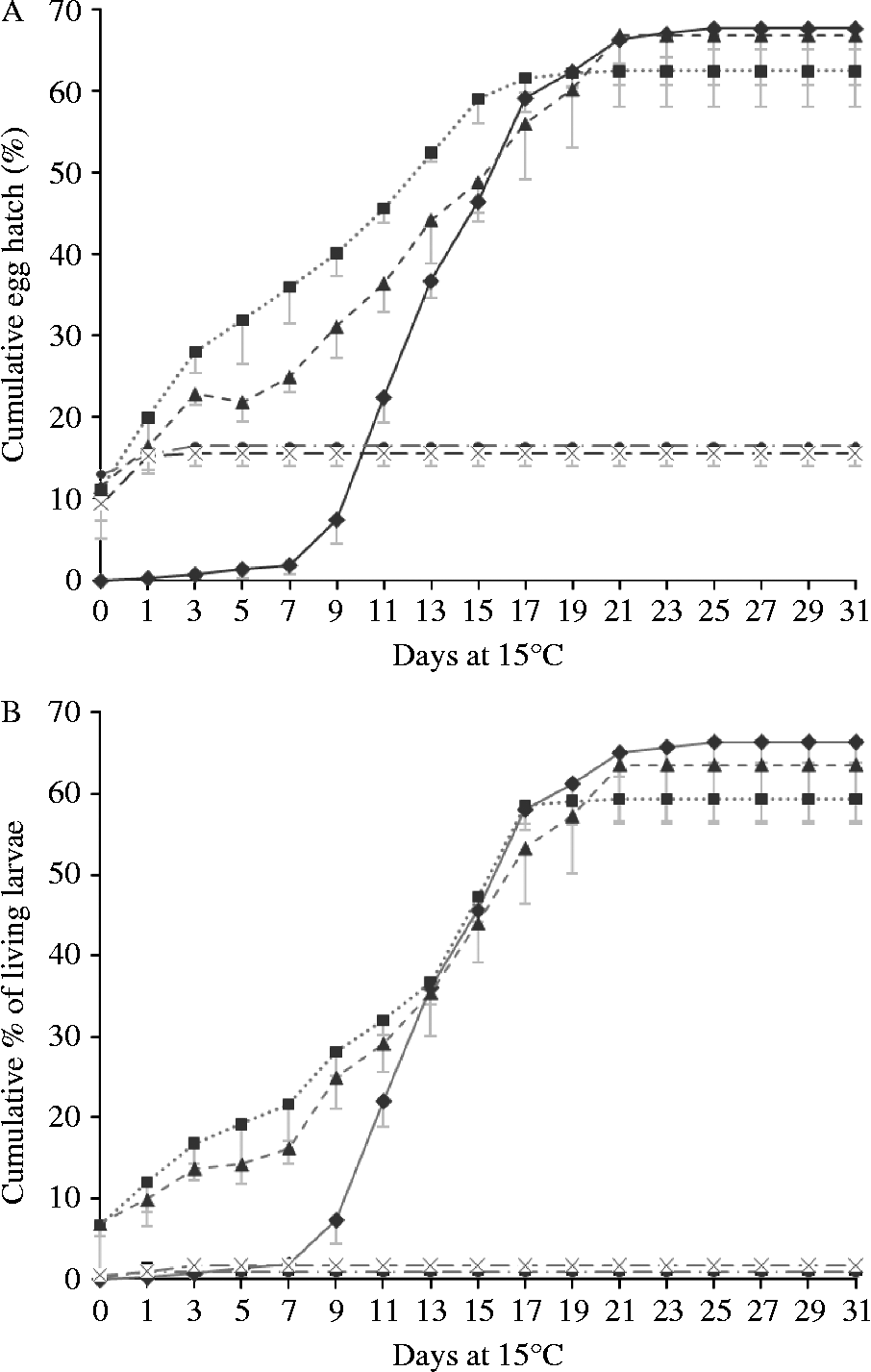
The hatching of all eggs (figs 2A and 3A), and of the eggs yielding live larvae (figs 2B and 3B), are illustrated in fig. 2 (non-chill treatments) and fig. 3 (chill treatments). Differences between chilled and non-chilled treatments in total proportions of eggs hatching closely followed patterns described by van Dijk & Morgan (Reference Van Dijk and Morgan2008). In all RH treatments, a proportion of the eggs hatched on being returned to water. In the 95% and 70% RH treatments a small proportion of these yielded dead larvae, but in the 55% and 33% RH treatments virtually all larvae released from eggs on day zero were dead. None of these larvae showed signs of desiccation. All filled their cuticle tightly while showing the slightly arched, stretched-out position characteristic of larvae that have been dead for at least several hours, suggesting that they had either been dead for some time or were showing signs of overhydration. The proportions of larvae hatching within hours of being returned to water was significantly higher in the 70, 55 and 33% RH treatments than at 95% or 100% RH (F 4,29 = 40.434, P < 0.001) but did not significantly differ from each other (P ≥ 0.608). In the 95% RH treatment more eggs hatched than in the control (P < 0.001). Chilling had no influence on the number of eggs hatching on day zero (F 1,29 = 1.067, P = 0.314).
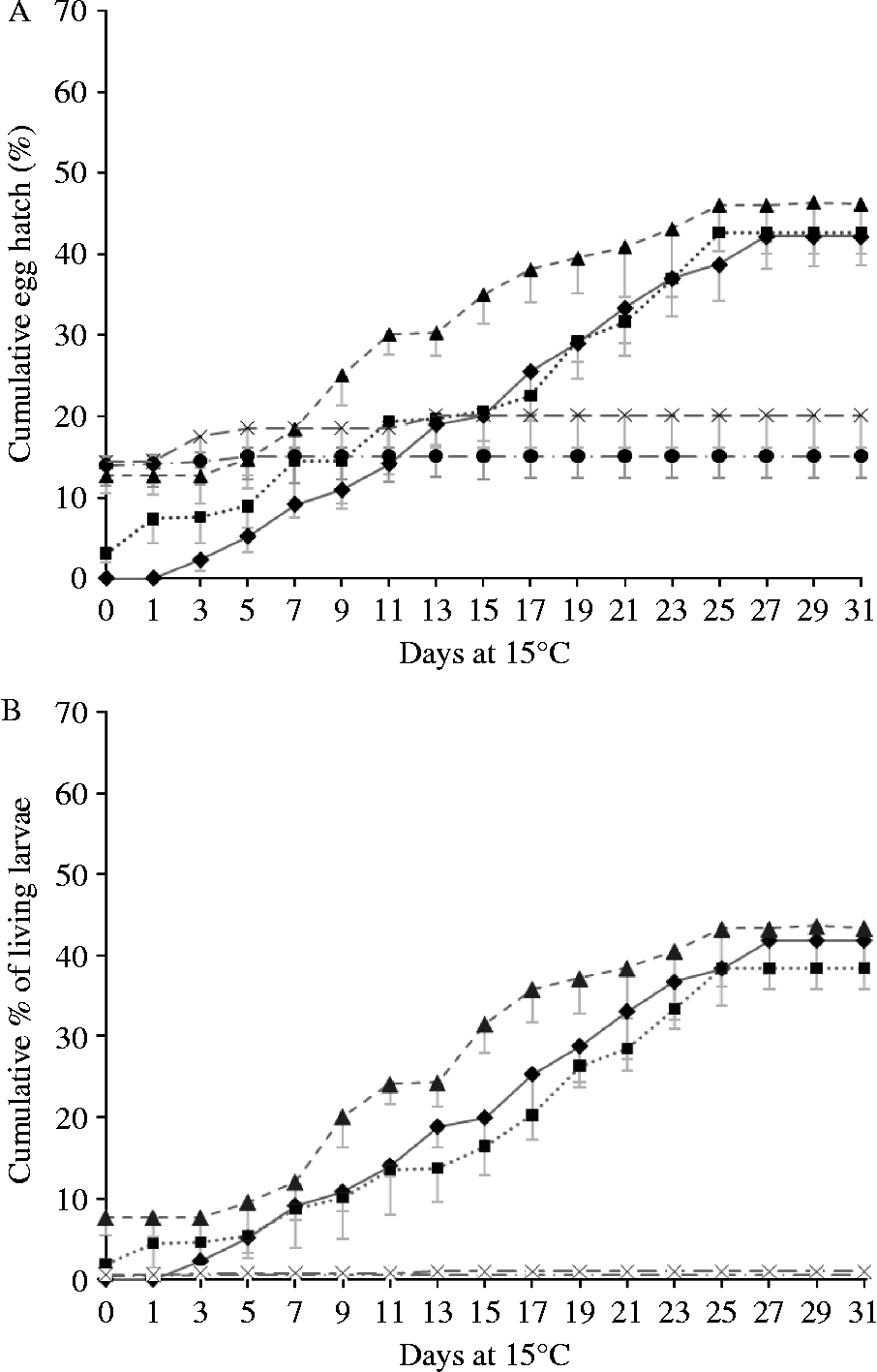
Fig. 2 Hatching of desiccated, non-chilled eggs after rehydration and placement at 15°C: (A) cumulative total proportion of eggs hatching; and (B) the percentage hatching of live larvae. The non-interrupted line/diamond data points represent the control; the dotted line/square data points, the 95% relative humidity (RH) treatment; the dashed line/triangle data points, the 70% RH treatment; the interrupted line/circle data points, the 55% RH treatment; and the dashed line/cross data points the 33% treatment. Error bars represent standard deviations. Data points of 33 and 55% RH treatments overlap.
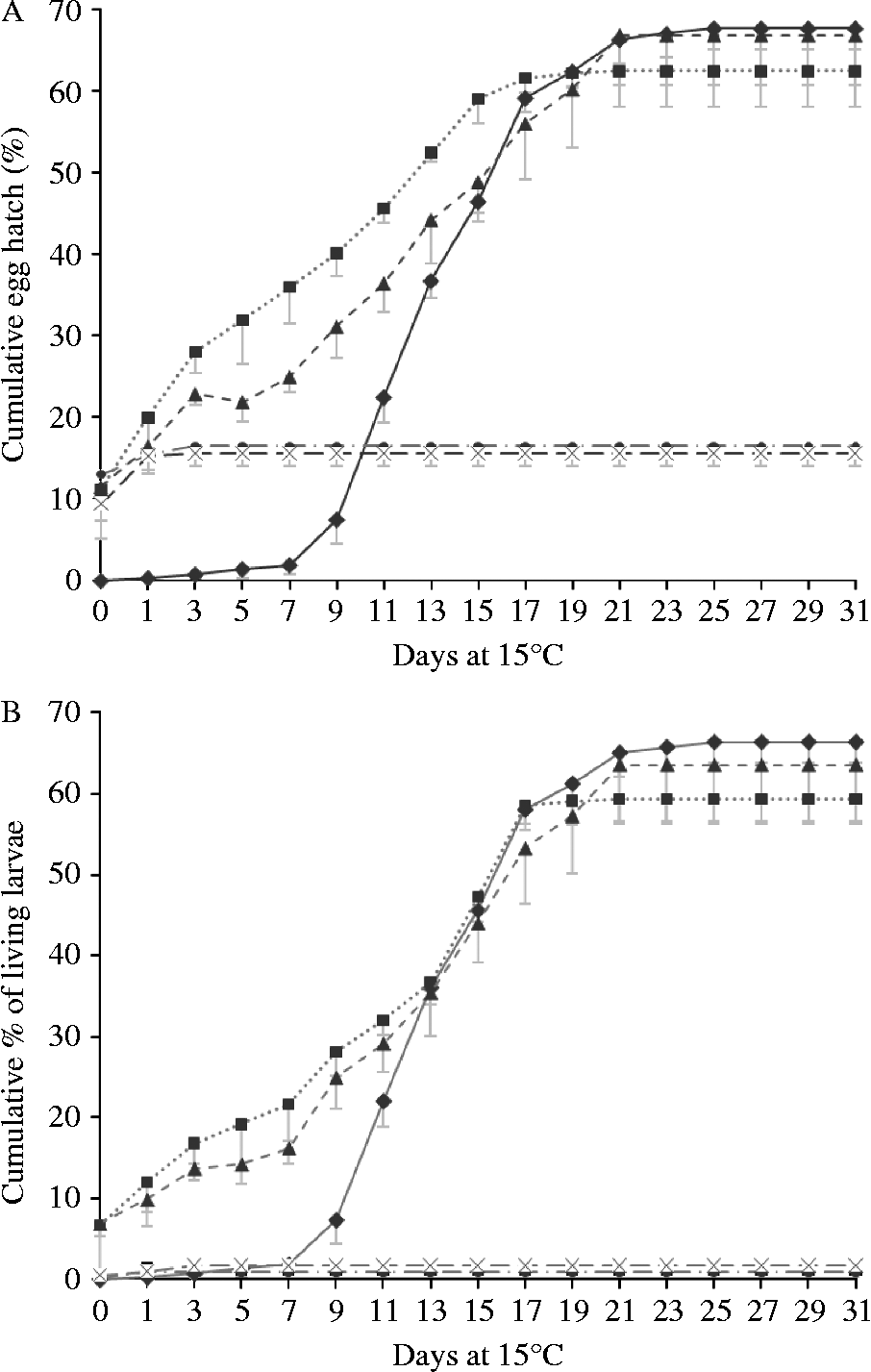
Fig. 3 Hatching of desiccated, chilled eggs after rehydration and placement at 15°C: (A) cumulative total proportion of eggs hatching; and (B) the percentage hatching of living larvae. The non-interrupted line/diamond data points represent the control; the dotted line/square data points, the 95% relative humidity (RH) treatment; the dashed line/triangle data points, the 70% RH treatment; the interrupted line/circle data points, the 55% RH treatment; and the dashed line/cross data points, the 33% treatment. Error bars represent standard deviations. Data points of 33 and 55% RH treatments overlap.
After day zero many live larvae hatched from the 95% and 70% RH treatments, but only up to 1% of eggs from the 55% or 33% treatments. The 55% and 33% treatments were therefore omitted from further analysis. On day 1, more eggs had hatched in the 70% and 95% treatments than in the control (F 2,35 = 65,370, P < 0.001), while the 95% and 70% treatments could not be separated (P = 0.516). Differences between treatments in the total proportion of eggs hatching and eggs yielding live larvae were significant (F 1,35 = 30.868, P < 0.001), and significantly more live larvae were present in the lowered humidity treatments than in the control (F 2,35 = 7.834, P = 0.002). In the chill treatments, more eggs hatched than in the non-chill treatments (F 1,35 = 36.370, P < 0.001) and dehydration significantly increased the proportion hatching (F 2,35 = 8.547, P = 0.002). However, chilling had no influence on the proportions of eggs producing dead larvae (F 1,35 = 0.766, P = 0.390).
At the time of maximum hatching, as expected, significantly more eggs hatched in the chill treatments than in the non-chill treatments (F 1,35 = 151.997, P < 0.001), but the proportion hatching was not influenced by humidity treatment (F 2,35 = 1.962, P = 0.162). Significantly fewer eggs than those that hatched produced live larvae (F 1,35 = 5.843, P = 0.024) but there was no significant interaction with either humidity treatment (F 2,35 = 0.492, P = 0.617) or chill treatment (F 2,35 = 1.322, P = 0.285). Again, the chilling of eggs had no influence on the proportion of eggs producing dead, or live, larvae (F 1,35 = 0.217, P = 0.646).
Regarding the timing of the hatch, even though differences between treatments spanned only a couple of days, dehydrated eggs reached the point of 50% hatch faster than eggs of the control treatment (F 2,17 = 21.700, P < 0.001). Chilled eggs also reached the time taken to 50% hatch significantly sooner (F 1,17 = 40.000, P < 0.001), and dehydrated, chilled eggs hatched the earliest (F 2,17 = 34.300, P < 0.001).
The 33% RH salt toxicity controls hatched in a manner very similar to those of the 33% RH in-salt treatments: both in the chilled and non-chilled treatments similar proportions hatched on day zero (mean 0.14 (range 0.10–0.15) and 0.16 (0.15–0.18), respectively) and very few of these larvae were alive. In the following days a very small proportion of the eggs hatched. The proportion of eggs producing live larvae on the day of maximum hatch was significantly higher in the salt-toxicity controls (F 1,11 = 191.726, P < 0.001) than in the in-salt 33% RH treatment. This proportion was also significantly higher in the non-chill salt control treatment than in the chill salt control treatment (F 1,11 = 72.487, P < 0.001). However, the mean proportion of larvae alive at the time of maximum hatch was only 0.07 (range 0.04–0.11), indicating that differences caused by salt toxicity were small.
Discussion
Within days of returning desiccated eggs to water, some significant hatching was recorded. A significant proportion of these eggs produced dead larvae. However, the number of live larvae present was, in the 95% and 70% RH treatments, still higher than in the control. The osmotic pressure applied by the salts producing RH of 55% and 33% appeared to have damaged nearly all eggs irreversibly. Initially, a higher proportion of eggs hatched at lower RH, but these were dead, and in any case quickly overtaken by the proportion hatching from higher RH treatments. From day 12 onwards the hatching pattern of the 95% and 70% RH treatments was very similar to that of the control. The number of days taken for 50% of the eggs to hatch was slightly reduced, but the overall cumulative proportions of larvae hatching, and the proportions of eggs producing live larvae, were not affected by 95% or 70% humidity treatment. It appears that severe desiccation kills eggs, while moderate desiccation followed by rehydration induces some mildly accelerated hatching, but not a mass hatch.
The osmotic pressures applied in these experiments are likely to be higher than those experienced at pasture (Parkin, Reference Parkin1975b). At osmotic pressures naturally found in the soil, desiccation of eggs may not occur at all. However, even if the osmotic pressures applied in the present experiments are found in the field, rehydration-induced hatching of approximately 10% of the population as live larvae is unlikely to account for increased clinical disease following rainfall. Increased death rates of eggs may also decrease larval abundance when desiccation becomes too severe. Interestingly, chilling did not influence either the total proportion of eggs hatching or the proportion releasing live larvae at any time. On the contrary, in the chill treatments more eggs hatched on the first day after return to water than in the non-chill treatments. There does not appear to be an easy explanation for this. From these results it appears that the hypothesis that trehalose protects the eggs of N. battus from desiccation has to be refuted. Waller (Reference Waller1971) compared the eggshell morphology of Trichostrongylus colubriformis with that of Haemonchus contortus and proposed that an inner layer of a lipid-like substance present in the eggs of the former, but not of the latter, was involved in its greater resistance to desiccation. Parkin (Reference Parkin1972) detected a similar, amorphous layer in the eggs of N. battus and reached similar conclusions. If such a layer indeed forms the main defence barrier against the loss of water, it could explain why, in the present study, chilled and non-chilled eggs were affected in a similar way. Also, it could explain why the hatching of humidity-treated eggs appeared to be a threshold phenomenon: at 95% RH treatment fewer eggs hatched on return to water than at 70%. Thus, at the hygroscopic pressures applied by the 70% RH treatment, more eggs were affected but not irreversibly damaged. However, in the 55% and 33% RH treatments virtually all eggs had died. This is consistent with a hypothesis of a layer preventing loss of water up to a certain threshold but not above. Also, on day zero very similar proportions of eggs hatched in the 70, 55 and 33% RH treatments, suggesting that the egg shell may have been damaged in the same manner by all treatments.
The presence of a protective layer within the egg could also explain why salt toxicity apparently had little effect. In the experiment, eggs were incubated in, rather than above, the salt solutions of increasing hygroscopic strengths, the osmotic pressure of which quite likely exceeds those experienced by the eggs in the field. As a result, a lowered proportion of eggs releasing live larvae during the hatching process could be the result of severe desiccation of the egg, salt toxicity or a combination of both. However, it is unlikely that the toxicity of three different salts would cause identical hatching effects on day zero. Dead larvae present on day zero did not have a distorted appearance but looked plumper than normal, suggesting that it was overhydration that killed them, presumably because salt had leaked into the egg. Also, the 95% and 70% RH treatments produced the same proportion of live larvae as the control at the time of maximum hatch, precluding a significant effect of salt toxicity. The 33% RH salt toxicity control, in the most severe humidity treatment, showed a significantly higher proportion hatch than the in-salt treatments. Unfortunately, it cannot be estimated whether the lower proportion of live larvae produced in the in-salt treatments is due to salt toxicity or increased osmotic pressure. However, it can be estimated that salt toxicity, in this treatment, contributed at most approximately 5% (range 2.1–9.9%) to larval death.
It therefore appears that eggs exposed to high osmotic pressures at an RH of 55% or below die. A proportion of eggs exposed to lower osmotic pressures may hatch on rehydration but this proportion is very small compared with that of eggs hatching normally after exposure to temperatures within the hatching range (van Dijk & Morgan, Reference Van Dijk and Morgan2008, Reference Van Dijk and Morgan2009). The extent of this type of hatching is unlikely to be of epidemiological importance and cannot account for elevated disease observed after rainfall.
In conclusion, sudden increased availability of water did not appear to induce mass hatching of N. battus eggs. Moreover, water is not a limiting factor for the migration of larvae which have escaped dung (van Dijk & Morgan, Reference Van Dijk and Morgan2011). Therefore, the emergence of N. battus larvae, which at the time of hatching are already incorporated into the soil, after a period of rainfall is more likely to be temperature related. In the field, effects of temperature and rainfall are confounded. On cloudy days, lower maximum temperatures may fall below the upper threshold for hatching (van Dijk & Morgan, Reference Van Dijk and Morgan2008), resulting in increased hatching opportunities, and giving the appearance of rain-induced hatching. In terms of modelling N. battus epidemiology, it can be concluded that rainfall may only have to be included to model potential egg losses and prolonged egg development phases resulting from delays in the incorporation of desiccated dung into the soil.