Introduction
Trypanorhynch cestodes present a worldwide distribution, especially in the tropical and subtropical regions, and are among the most habitual parasite taxa of sharks and stingrays (final hosts). Larval stages of trypanorhynch cestodes parasitize numerous teleost fish and, when present in the flesh of the stock, compromise their commercial value (Overstreet, Reference Overstreet1978; Palm et al., Reference Palm, Möller and Petersen1993; Campbell & Beveridge, Reference Campbell and Beveridge1996; Palm, Reference Palm1997). In several countries there is an increased medical concern regarding human infections and allergic-related reactions due to fish parasites as a consequence of a growing worldwide consumption of raw, undercooked or poorly processed fish. Although these are frequently related to the Anisakidae family (Puente et al., Reference Puente, Anadón, Rodero, Romarís, Ubeira and Cuéllar2008; Pelayo et al., Reference Pelayo, García-Hernández, Puente, Rodero and Cuéllar2009; Broglia & Kapel, Reference Broglia and Kapel2011; Daschner et al., Reference Daschner, Cuéllar and Rodero2012), other parasites may present the same potential.
Human accidental parasitism by trypanorhynch cestodes is extremely rare and brief. There are only three reported cases and all are associated with recent crude fish ingestion. In two cases live parasites (larvae) were found in faeces (Heinz, Reference Heinz1954; Fripp & Mason, Reference Fripp and Mason1983), whereas in the third case the larva was attached to the palatine tonsil of a man (Kikuchi et al., Reference Kikuchi, Takenouchi, Kamiya and Ozaki1981). Despite the rarity of cases, Pelayo et al. (Reference Pelayo, García-Hernández, Puente, Rodero and Cuéllar2009) highlighted the hazard of human allergic reactions, even after freezing the fish. These authors reported that a Spanish population of 305 residents in Madrid presented a significant anti-trypanorhynch cestode (Gymnorhynchus gigas) seroprevalence (including IgE).
Although allergic manifestations to fish parasites are well known, there are only a few experimental models that study the allergenic potential of these antigens, and most of these involve the study of anisakis. The few models that study other fish parasites such as the trypanorhynch cestodes (G. gigas and Molicola horridus) all differ in the applied methodology. For example, the immunization protocols differ in aspects such as administration pathways, protein doses and intervals (Rodero & Cuéllar, Reference Rodero and Cuéllar1999; Vázquez-López et al., Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2001, Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2002; Gòmez-Morales et al., Reference Gòmez-Morales, Ludovisi, Giuffra, Manfredi, Piccolo and Pozio2008).
Pterobothriidae trypanorhynchs, specifically Pterobothrium spp., have been described in the mesenteric membrane, visceral serosa and flesh of marine and freshwater fish of Australia, Sri Lanka, India, Indonesia (Campbell & Beveridge, Reference Campbell and Beveridge1996; Moore et al., Reference Moore, Buckworth, Moss and Lester2003), Persian Gulf (Haseli et al., Reference Haseli, Malek, Valinasab and Palm2011), West African coast (Al-Zubaidyl & Mhaisen, Reference Al-Zubaidyl and Mhaisen2011), Gulf of Mexico (Overstreet, Reference Overstreet1977; Campbell & Beveridge, Reference Campbell and Beveridge1996) and the Atlantic coastline of South America (Fonseca et al., Reference Fonseca, São Clemente, Felizardo, Gomes and Knoff2012). Considering that Micropogonias furnieri (Desmarest, 1813) is an important commercial fish which inhabits the Atlantic coastline of South America from the Gulf of Mexico to Argentina, this fish species is frequently parasitized by Pterobothrium spp. (Overstreet, Reference Overstreet1978; Alves & Luque, Reference Alves and Luque2001). There is a scarcity of data relating to the allergenic potential of Pterobothrium heteracanthum (Diesing, 1850) and therefore the aim of the present study was to determine if the crude protein extract of P. heteracanthum, the type species for this genus, has antigenic compounds which are able to induce specific immune responses in a murine experimental model.
Materials and methods
Collection of larval cestodes and preparation of crude protein extracts
Pterobothrium heteracanthum plerocerci and blastocysts were collected manually with the aid of scissors and forceps from naturally infected whitemouth croakers, M. furnieri (Desmarest, 1823), purchased in fish markets of Niterói municipality, Rio de Janeiro State, Brazil. Crude P. heteracanthum protein extract (PH-CPE) was obtained after extensive washing of plerocerci and blastocysts with sterile 0.1 m phosphate-buffered saline (PBS), pH 7.3, supplemented with 5% penicillin and 5% streptomycin. Larvae were homogenized in a Potter–Elvehjem homogenizer (Thomas Scientific, Swedesboro, New Jersey, USA) after a final wash with non-supplemented, sterile PBS. The homogenate was then submitted to six 30-s cycles of the Tissue Ruptor (Qiagen Instruments AG, Zurich, Switzerland) and the final suspension was centrifuged at 30,000 g at 4°C for 30 min. The supernatant was filtered using a 0.22 μm filter (MillexGV, Millipore Corporation, Billerica, Massachusetts, USA). The same protocol was used to prepare a crude fish (M. furnieri) protein extract (MF-CPE), which was used as a control antigen for the serological assays. Protein concentrations of PH-CPE and MF-CPE were estimated according to Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951). To determine the molecular weight range of the PH-CPE, 0.03 mg of the extract was submitted to SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) using a 12%, 100 × 100 mm gel (Vertical System, Bio-Rad, Hercules, California, USA) for 2 h at 140 V, as described by Laemmli (Reference Laemmli1970).
Immunological procedures
Ten-week-old female BALB/c mice were separated into three experimental groups (n= 6) and one control group (n= 5). Each experimental group was immunized intraperitoneally (i.p.) with a suspension containing either 10, 50 or 100 μg/mouse of PH-CPE and 2.0 mg of alum (Al(OH)3) in a final volume of 200 μl on days 0 and 35. Controls were sham immunized with sterile saline and alum.
Blood samples were collected from each animal from the retro-orbital plexus on days 0 (pre-immunization for paired controls), 14, 21, 35, 42, 49 and 56 (post-immunization). Samples were centrifuged to obtain sera, which were stored at − 20°C until used.
Specific IgG and IgE serum levels were measured by enzyme-linked immunosorbent assay (ELISA) as described by Antunes et al. (Reference Antunes, Costa, Campos, Paschoal, Garrido, Siqueira, Teixeira and Cardoso2009). Briefly, 96-well microtitre plates (Nunc-Imuno™ Plate Maxi Sorp™ surface; Nalge Nunc International, Rochester, New York, USA) were coated with 20 μg/ml (1 μg/well) of PH-CPE. Serum samples (diluted 1:100 in PBS v/v) were submitted to a threefold serial dilution for IgG and a twofold serial dilution for IgE titration. After extensive washing, plates were incubated with peroxidase-conjugated (HRP) rabbit anti-mouse IgG (H+L, Sigma-Aldrich Israel, Rehovot, Israel) or HRP rat anti-mouse IgE ɛ (Invitrogen, Camarillo, California, USA) antibodies (50 μl/well), as recommended by the manufacturers. Reactions were developed with 50 μl/well of OPD substrate (0.04% O-phenylene-diamine (Sigma-Aldrich); 0.04% hydrogen peroxide in phosphate-citrate buffer (pH 5.0)). The chromogenic reaction was stopped with 50 μl/well of 3 n sulphuric acid. The optical density (OD) was determined by spectrophotometry (Anthos 2010, Krefeld, Germany) at 492 nm. ELISA scores were computed by running sums of ODs between 1:100 and 1:2700 (IgG) or 1:100 and 1:800 (IgE) of the serum dilutions (an approximate calculus of the area under the dilution curve). Each score represents the mean ± standard error (SEM) for each experimental group.
Cross reactivity to fish proteins was assessed with an IgG ELISA essentially as described above using MF-CPE as the coating antigen.
Immunoblotting was used to determine the reactivity profile of specific IgG and IgE. Initially 0.03 mg of PH-CPE was submitted to the same SDS-PAGE conditions, followed by the transfer of the protein bands from the separating gel to the nitrocellulose membrane using a Semi-dry blotter (Bio-Rad). Subsequently, the membranes were blocked with 5% fat-free milk (Nestle) in PBS solution overnight, washed with 0.05% PBS-Tween, dried at room temperature (RT) and cut in strips. Two strips were incubated overnight at RT with each serum sample diluted 1:100 v/v in blocking buffer, with constant rocking. After washing with TBS (Tris-buffered saline)–Tween, one membrane strip for each serum was incubated with peroxidase-labelled goat anti-mouse IgG (Bio-Rad) for 2 h and the other was exposed to rat anti-mouse IgE (Invitrogen) for 3 h, followed by HRP-goat anti-rat IgG (H+L, Invitrogen) for 2 h at RT with constant rocking. After the final wash, the peroxidase substrate (3.3′-diaminobenzidine; Sigma-Aldrich) was added to develop the Ag/IgG or Ag/IgE interactions. All antibodies were used according to the manufacturer's recommendation.
Data analysis
Tukey's test was performed for statistical analyses using GraphPad InStat software (www.graphpad.com). Differences were considered statistically significant at a P value < 0.05.
Results and discussion
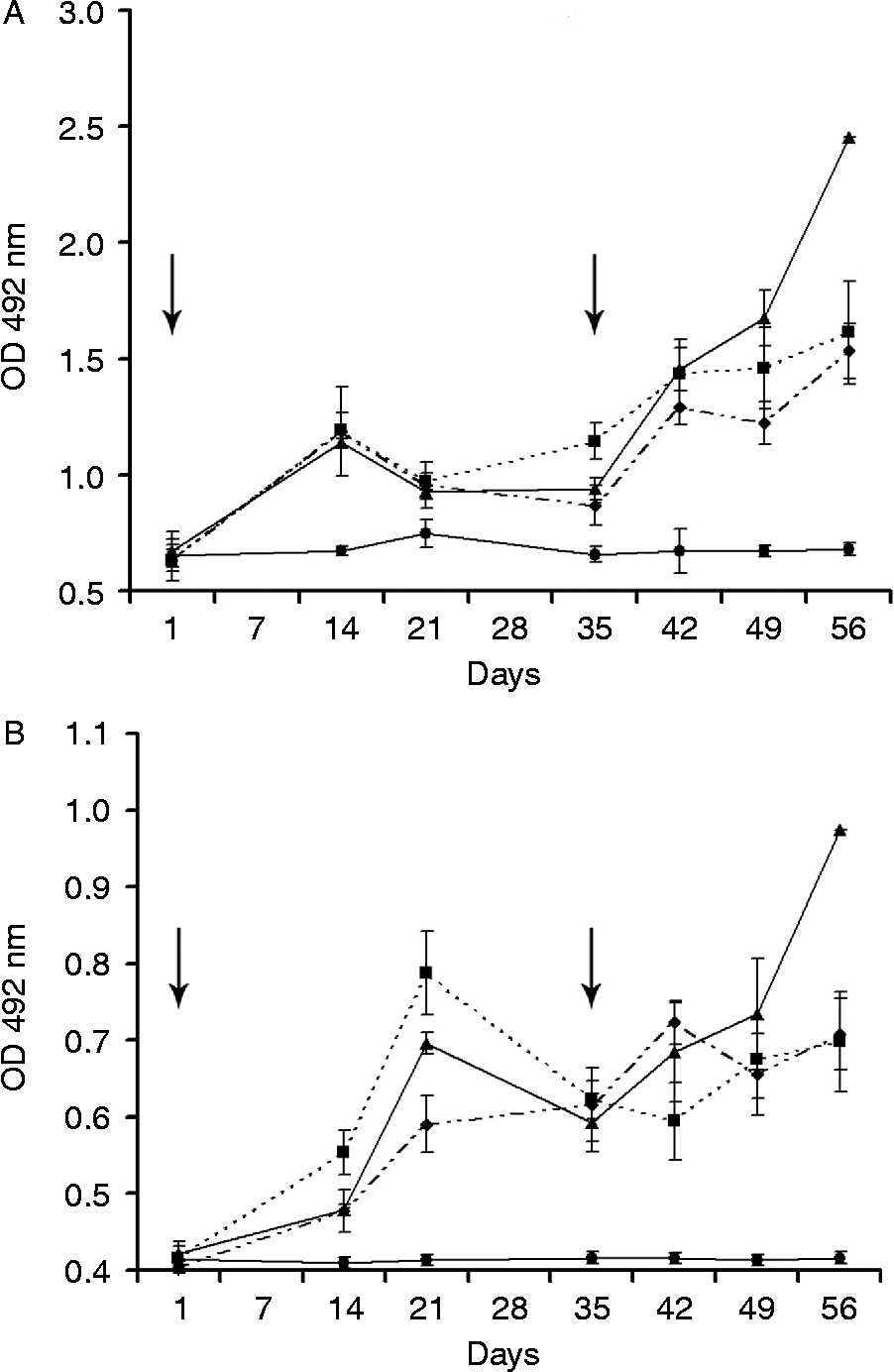
After the primary immunization, all experimental groups presented a significant increase (P < 0.001) of specific IgG and IgE levels on day 14 when compared with controls, and there was no significant difference between PH-CPE doses for IgG. On day 42 (7 days after booster immunization), both IgG and IgE levels of all experimental groups increased significantly (P < 0.001) when compared with controls. On day 49, a significant difference was observed within the experimental groups due to the protein concentration for IgG levels. The group that received 10 μg of PH-CPE presented significantly lower antibody titres when compared to the groups that received 50 μg, (P < 0.05) and 100 μg (P < 0.05). For the group that was immunized with 50 μg of PH-CPE, a significant increase of IgG and IgE titres was observed on day 56 (21 days after booster immunization) when compared to groups immunized with 10 μg (P < 0.001) or 100 μg (P < 0.001) of PH-CPE (fig. 1).

Fig. 1 Kinetics of specific IgG (A) and IgE (B) serum levels of BALB/c mice immunized intraperitoneally on days 0 and 35 (arrows) with 10 μg (♦), 50 μg (▲) or 100 μg (■) of crude Pterobothrium heteracanthum extract and control (●); mean values ( ± SEM) of optical densities (OD) (approximation of the area under the dilution curves) of individual mouse sera.
No specific humoral response to either PH-CPE or MF-CPE was detectable in the serum of any mouse before the priming immunization, or of any animal of the control group during the whole experiment. No cross-reactions were observed between PH-CPE and MF-CPE antigens.
In accordance with the literature (Rodero & Cuéllar, Reference Rodero and Cuéllar1999; Vázquez-López et al., Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2001; Martínez de Velasco et al., Reference Martínez de Velasco, Rodero, Zapatero and Cuéllar2002), in which high IgE and IgG (mainly IgG1) levels are known to be related to the regulation of hypersensitivity reactions, our results indicate the allergenic potential of PH-CPE. Previous studies evaluating the immunogenicity of trypanorhynch extracts in murine models used protein concentrations that were at least 50 μg/mouse (Rodero & Cuéllar, Reference Rodero and Cuéllar1999; Vázquez-López et al., Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2001; Gòmez-Morales et al., Reference Gòmez-Morales, Ludovisi, Giuffra, Manfredi, Piccolo and Pozio2008). Our results show that doses as low as 10 μg/mouse of PH-CPE are capable of inducing a specific response in BALB/c mice. The present results corroborate previous data indicating that the BALB/c mouse is a potential murine model for identifying and characterizing allergens of a protein nature after antigenic challenging by the i.p. route (Dearman & Kimber, Reference Dearman and Kimber2001; Gòmez-Morales et al., Reference Gòmez-Morales, Ludovisi, Giuffra, Manfredi, Piccolo and Pozio2008; Van der Ventel et al., Reference Van der Ventel, Nieuwenhuizen, Kirstein, Hikuam, Jeebhay, Swoboda, Brombacher and Lopata2011). Oral administration could better mimic the actual human exposure to fish parasites by feeding. However, due to the mechanism of oral tolerance, the capacity of the IgE response in murine models by this same route may not be sensitive or reliable enough, with conflicting results as already observed (Dearman & Kimber, Reference Dearman and Kimber2001; Vázquez-López et al., Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2001, Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2002; Gòmez-Morales et al., Reference Gòmez-Morales, Ludovisi, Giuffra, Manfredi, Piccolo and Pozio2008).
The oral route implies that allergens will be subjected to digestion, so in order to be able to elicit an IgE response, they have to be resistant to digestion. The two cases of human transitory infection by trypanorhynch cestodes showed that their larvae can survive the passage through the human digestive tract, being still alive when shed in the faeces of the host (Heinz, Reference Heinz1954; Fripp & Mason, Reference Fripp and Mason1983). These reports indicated the possibility of larval resistance to human digestion. In addition, the local environment of the intestine could influence the passage of molecules through intestinal mucosa to the gut-associated immune system (GALT). Recent experimental study showed that induction of oral tolerance or systemic immunization with a new protein depends on the local environment of the intestine (Paschoal et al., 2009). Thus, oral exposure of a new protein in an inflamed intestine could lead to systemic immunization. In the clinical scenario, these results would suggest that people with inflammatory bowel disease, when exposed to new proteins, can develop multiple food allergies.
There are divergent opinions about the trigger of allergic manifestations involving fish parasites. Some consider that it only happens after ingestion followed by infection with live parasites, such as the Anisakis simplex larvae (Daschner et al., Reference Daschner, Cuéllar and Rodero2012). However, there are records showing allergic conditions associated with the ingestion of dead larvae, and therefore without occurrence of an infection, just with an exposure to antigens (Fernández de Corres et al., Reference Fernández de Corres, Audicana, Del Pozo, Muñoz, Fernández, Navarro, García and Diez1996; Audicana et al., Reference Audicana, Ansotegui, Fernández de Corres and Kennedy2002; Audicana & Kennedy, Reference Audicana and Kennedy2008). The results of Pelayo et al. (Reference Pelayo, García-Hernández, Puente, Rodero and Cuéllar2009) showed that even without report of human infection with G. gigas, there was an induction of a specific immune response (including IgE) against this cestode species in a Spanish population, probably acquired by the local eating habits.
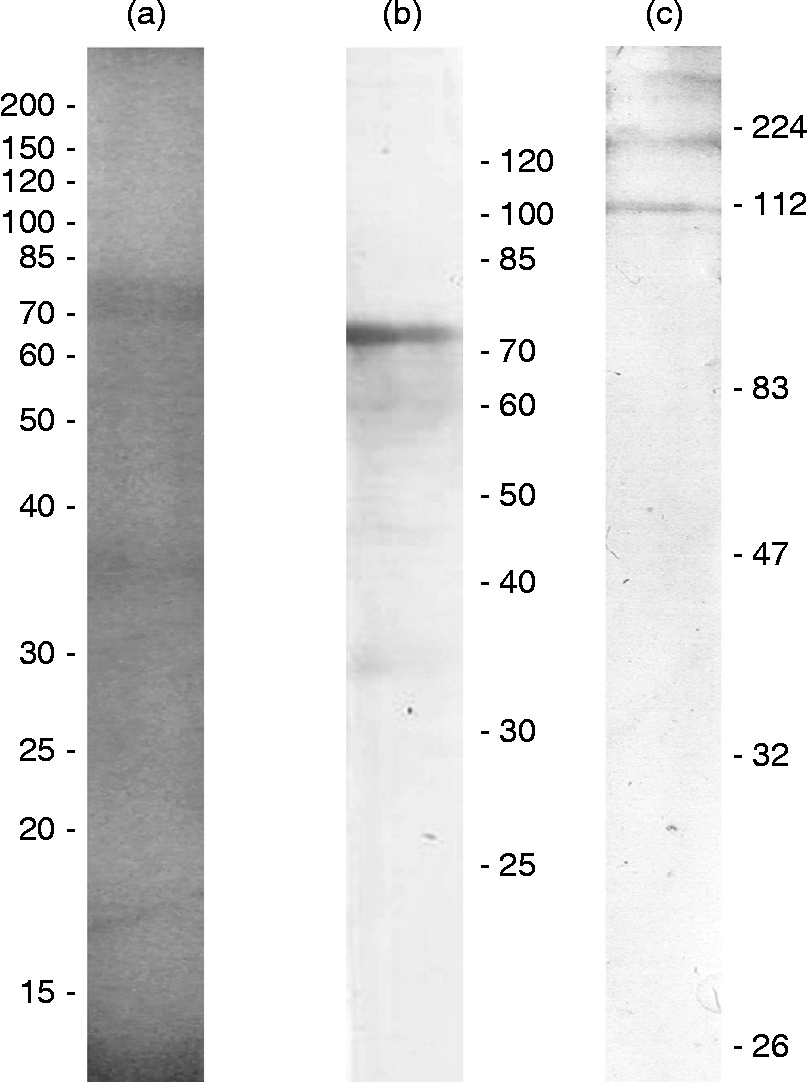
SDS-PAGE revealed a protein profile with the most evident band in the region of 75 kDa (fig. 2a). The immunoblot revealed that specific IgG recognizes proteins of two molecular weight ranges: 30–40 and 60–75 kDa (fig. 2b) and that specific IgE only binds to proteins that present at least 112 kDa (fig. 2c). No reactivity was detected when the pre-immune or control group sera were incubated with the PH-CPE membrane.

Fig. 2 Protein profiles of P. heteracanthum crude parasite extract (PH-CPE) in 12% SDS-PAGE visualized with Coomassie Blue (a) with molecular weight marker reference in kDa; and immunoblots showing IgG (b) and IgE (c) recognizing immunogenic proteins of PH-CPE in pooled sera from all sensitized mice 7 days after the second immunization.
The present results show that the immunogenicity of different proteins, present in the crude extract derived from P. heteracanthum, elicits immune responses with different T-lymphocyte helper (Th) profiles (Th1 – IgG, and Th2 – IgE) and are in agreement with previous studies. For example, Gòmez-Morales et al. (Reference Gòmez-Morales, Ludovisi, Giuffra, Manfredi, Piccolo and Pozio2008) demonstrated that both specific IgG and IgE react with proteins of the 26 kDa region of M. horridus extract, whereas the 30 kDa proteins are only recognized by IgE, and proteins between 75 and 100 kDa by IgG.
An insight into these different immune response profiles has been given by Vázquez-López et al. (Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2001), who demonstrated that crude extracts of G. gigas present stress factors to the GALT, once heat-shock proteins (hsp60 and hsp70) significantly increase 2 h after oral administration, resulting in transient, yet significant, inflammatory responses. As shown previously by Paschoal et al. (2009), timing may be more important than the antigenicity. Based on their results that hsp60 and/or hsp70 levels increase in the spleen 15–20 days after antigen inoculation, Vázquez-López et al. (Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2001) suggest that the same stress factors that act on the GALT can act systemically and may modulate the systemic immune response, inducing the production of specific IgE and IgG. In a later paper, these same authors (Vázquez-López et al., Reference Vázquez-López, De Armas-Serra, Bernardina and Rodríguez-Caabeiro2002) showed that a 24 kDa collagenase purified from the crude extract of G. gigas is the target of both GALT and systemic IgE, and participates in the potentially serious/adverse intestinal responses in murine models. Taking these results to the clinical setting, such reactions are very likely to occur in humans.
Further studies regarding cross-reactivity between different trypanorhynchs and complementary clinical trials are required to elucidate whether the immunogenic activity of PH-CPE represents a risk to human health, since the present results indicate that P. heteracanthum antigens have the potential to induce specific IgG and IgE response in experimental animals.
Acknowledgements
The authors thank Dr Gerlinde Agate Platais Brasil Teixeira for her constructive comments; and Eduardo Martins Barbosa and Marcos Fortes Telles for processing the figures.
Financial support
This research was partially supported by PROPPI–Universidade Federal Fluminense (FOPESQ-2011).
Conflict of interest
None.
Ethical standards
The study was developed according to the ethics committee on animal research standards of the Federal Fluminense University, under the registration number 038/2009. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.