Introduction
Clonorchis sinensis infection causes clonorchiasis and human cholangitis, and is highly prevalent in China, Korea, Taiwan, Vietnam and Japan (Rim, Reference Rim2005; Sripa et al., Reference Sripa, Kaewkes, Intapan, Maleewong and Brindley2010). In humans, C. sinensis infection causes eosinophilic pulmonary infiltrations, producing 35% eosinophils in a leucocyte count, with a positive skin test and C. sinensis-specific IgG antibody response (Lee et al., Reference Lee, Jin, Lee, Choi and Yum2003). Humans are infected by consuming undercooked fish containing C. sinensis metacercariae. Upon reaching the small intestine, the metacercariae exit and migrate towards the bile duct, causing obstruction of the bile duct and other diseases, including bacterial infections, inflammation, periductal fibrosis, hyperplasia and cholangiocarcinoma (Quan et al., Reference Quan, Matsumoto, Lee, Timothy, Lee, Kim, Joo and Lee2004; Hong & Fang, Reference Hong and Fang2012). Capillaria hepatica is another zoonotic parasite that is found worldwide and causes hepatic capillariasis, a serious liver disorder with remarkable elevation of eosinophil counts (Ferreira & Andrade, Reference Ferreira and Andrade1993; Juncker-Voss et al., Reference Juncker-Voss, Prosl, Lussy, Enzenberg, Auer and Nowotny2000; Klion, Reference Klion2015). More than 180 mammalian species (including humans) can be hosts of this pathogen (Fuehrer, Reference Fuehrer2014; Sharma et al., Reference Sharma, Dey, Mittal, Kumar and Hira2015). Humans are infected by ingesting embryonated eggs of C. hepatica in food, water or soil contaminated with faeces (Center for Disease Control, 2011). First-stage larvae (L1) hatch from embryonated eggs, and the L1 larvae bore through the intestinal wall and are carried to the liver by the hepatic portal vein. Larvae develop to sexually mature adults, laying eggs in the liver parenchyma, causing hepatic capillariasis (Ferreira & Andrade, Reference Ferreira and Andrade1993; Center for Disease Control, 2011).
A number of reports have shown that eosinophils are required for the clearance of primary Strongyloides stercoralis infections and secondary Nippostrongylus brasiliensis and Trichinella spiralis infections (Vallance et al., Reference Vallance, Matthaei, Sanovic, Young and Collins2000; Knott et al., Reference Knott, Matthaei, Giacomin, Wang, Foster and Dent2007; Chu et al., Reference Chu, Kim, Lee, Lee, Kim and Quan2016). The deposition of the eosinophil granule proteins major basic protein 1 (MBP-1), eosinophil peroxidase (EPO), eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP) on helminth surfaces can kill Brugia sp. in vitro (Hamann et al., Reference Hamann, Gleich, Checkel, Loegering, McCall and Barker1990).
Since both C. sinensis and C. hepatica have a wide host range and high infection rates, there is a high possibility that they can co-infect a host. Also, until now, there is no report regarding the basic immunology induced by co-infections by two parasites in the same liver. An understanding of co-infection-induced resistance, antibody response, eosinophil response, lymphocyte proliferation and cytokine production would have a significant impact in providing novel information on the basic immunology of parasitic co-infections.
In this study, we assessed antibody responses, eosinophil counts, lymphocyte proliferation, cytokine production and worm burden reduction of C. sinensis that developed in the case of super-infection with C. hepatica in rats previously infected with C. sinensis. We found that a remarkable eosinophil elevation was induced by co-infection with C. hepatica in C. sinensis-infected rats. Significantly higher levels of lymphocyte proliferation and cytokines were detected in co-infected rats compared to single C. sinensis-infected rats. Interestingly, no cross-reactive antibodies were observed between C. sinensis and C. hepatica, indicating that eosinophil elevation, lymphocyte proliferation and cytokine production might contribute to the worm reduction during C. sinensis/C. hepatica co-infection.
Materials and methods
Animals and parasites
Sprague–Dawley (SD) rats (females, 8 weeks old) and New Zealand white rabbits (males, 2–4 months old) were purchased from Samyook Animal Center, Osan City, Kyonggi-do, Korea. White rabbits were infected with C. sinensis metacercariae to generate C. sinensis adult worms. Clonorchis sinensis metacercariae were collected from the freshwater fish Pseudorasbova parva by digesting muscles with pepsin–HCl, followed by filtration through layers of gauze. To obtain C. hepatica eggs, the liver tissues of house rats (Rattus norvegicus) infected with C. hepatica were digested with pepsin–HCl at 37°C, as described by Kim et al. (Reference Kim, Joo and Chung2007). The eggs were isolated by repeated filtration and cultured in 0.5% formalin solution at 30°C until embryonation. The embryonated eggs were kept at −20°C for egg antigen preparation and infection of rats, as described previously (Lee, Reference Lee1964; Kim et al., Reference Kim, Joo and Chung2007).
Antigen preparation
Adults of C. sinensis were collected from rabbit liver, and the excretory–secretory antigen (ES Ag) of C. sinensis was obtained as described by Chu et al. (Reference Chu, Kim, Lee, Lee, Joo, Lee, Lee, Zheng and Quan2014). Embryonated eggs of C. hepatica were homogenized for the preparation of egg antigen (egg Ag). The homogenized eggs were centrifuged at 10,000 rpm for 60 min, and the supernatant was collected. The protein concentration was determined and samples were stored at −70°C until use.
C. sinensis infection and subsequent infection with C. hepatica
Sprague–Dawley (SD) rats (females, 8 weeks old) were used. Groups of rats were primarily infected with 50 C. sinensis metacercariae (CS group, six rats). After 1 month, six rats with primary infections were subsequently challenge infected with 1000 embryonated eggs of C. hepatica (CS + CH group, six rats), at the same time as previously uninfected rats were given a single infection with C. hepatica (CH group, six rats). Uninfected rats were used as controls (naïve group, six rats). Thus, at 1 month after C. hepatica infection, and at 2 months after C. sinensis infection, rats (naïve, CS, CS + CH and CH groups) were sacrificed. Embryonated eggs of C. hepatica were isolated as described above (Animals and parasites section) and counted using a haemocytometer. The oral infection route was used for both C. sinensis and C. hepatica.
Serum antibody responses
Serum samples were collected from the ophthalmic venous plexus of rats 1 month after C. sinensis or C. hepatica infection (fig. 1). Clonorchis sinensis (CS)- or C. hepatica (CH)-specific total IgG, IgG1 and IgG2a antibody responses were determined in the naïve, CS and CS + CH rats, using enzyme-linked immunosorbent assay (ELISA). Serially diluted sera were used for ELISA and the data from 1:100 serum dilutions are shown in the fig. 2. Plates (Nunc MaxiSorp flat-bottom 96-well plate; Thermo Fisher Scientific, Waltham, Massachusetts, USA) were coated with 100 μl of C. sinensis ES Ag or C. hepatica egg Ag (4 μg/ml), and horseradish peroxidase (HRP)-conjugated anti-rat IgG, IgG1 and IgG2a (Bio-Rad, Hercules, California, USA) were used as secondary antibodies, as described previously (Chu et al., Reference Chu, Kim, Lee, Lee, Joo, Lee, Lee, Zheng and Quan2014).
Eosinophil counts
Whole blood was collected from rats at 1 month after primary infection and subsequent co-infection (fig. 1). Whole blood was stained with Discombe's solution. A 20-μl aliquot of individual blood was immediately added into 180 μl of Discombe's solution (5:5:90 acetone : 1% aqueous eosin : distilled water) and mixed well. Eosinophils in whole blood were counted using a haemocytometer.
Fig. 1. Experimental schedule. Rats (n = 12) were primarily infected with 50 metacercariae of C. sinensis (CS) and, after 4 weeks, were challenge infected with 1000 eggs of C. hepatica (CH). Blood was collected for antibody and eosinophil responses. Rats were sacrificed and lymphocyte proliferation, cytokine production and worm burden were determined at week 4 after C. hepatica challenge. The animal experiment was repeated twice independently.
Lymphocyte proliferation assay
Spleens were collected from individual rats at week 4 after C. hepatica infection, and single-cell suspensions were prepared using 70% and 50% Percoll gradients. Cells were incubated in 96-well flat culture plates (5 × 106 cells/well) for 3 days at 37°C in the presence of 5% CO2. Cells in 100 μl of RPMI-1640 were stimulated with 100 μl of 5 μg/ml C. sinensis ES Ag or phytohaemagglutinin (PHA). At day 3 after incubation, the cells were pulsed for 16 h with 0.5 μCi/well of [3H]thymidine (Amersham, Piscataway, New Jersey, USA) and then harvested on glass-fibre filters with a semiautomatic cell harvester (Skatron, Norway). Incorporated radioactivity was determined by liquid scintillation counting (LKB 1214 Rackbeta; American Instrument Exchange, Haverhill, Massachusetts, USA). The lymphocyte proliferation was expressed as background-subtracted geometric means (cpm, counts per minute).
Cytokine analysis
For the cytokine assay, supernatants of spleen-cell cultures were collected from each well by separation and stored at −20°C until use. OptEIA sets (BD Bioscience, San Jose, California, USA) were used to determine the concentration of interferon-gamma (IFN-γ), interleukin (IL)-2, IL-6 and IL-10 in culture supernatants, following the manufacturer's procedures.
Parasite burden determination of C. sinensis
Clonorchis sinensis-infected rats were sacrificed at month 1 after secondary infection with C. hepatica. Adult worms of C. sinensis were collected from the bile ducts and C. hepatica eggs were collected from livers; they were counted individually.
Statistical analysis
The antibody levels in sera, eosinophil responses in blood and lymphocyte cellular responses in spleen were recorded for each individual. Every assay was performed using at least three replicate samples, from which the arithmetic mean and standard error (SE) of the mean were calculated. A one-way analysis of variance (ANOVA) was performed. The burdens of C. sinensis adult worms were counted individually and non-parametric statistics (Wilcoxon rank sum test) were used for a valid comparison between CS and CS + CH. A value of P < 0.05 was considered significant.
Results
Sequential infection with C. hepatica in C. sinensis-infected rats induced CS-specific or CH-specific IgG, IgG1 and IgG2a antibodies
Rats were primarily infected with C. sinensis; 4 weeks later, rats were infected with C. hepatica (fig. 1). As shown in fig. 2A, significantly higher levels of C. sinensis-specific IgG antibodies were found in C. sinensis-infected and C. sinensis/C. hepatica co-infected rats compared to C. hepatica-infected rats or naïve control rats (*P < 0.01). Capillaria hepatica-infected rats showed no IgG antibody response against C. sinensis antigen. Significantly higher levels of C. hepatica-specific IgG antibodies were determined in C. hepatica-infected and C. sinensis/C. hepatica co-infected rats compared to C. sinensis-infected rats and naïve control rats (fig. 2B, *P < 0.01). Clonorchis sinensis-infected rats showed no IgG antibody response against C. hepatica antigen. Clonorchis sinensis/C. hepatica co-infected rats showed similar levels of C. sinensis-specific IgG antibody and C. hepatica-specific IgG antibody, compared to single C. sinensis or C. hepatica infection. These results indicate that no cross-reacting antibodies were elicited between C. sinensis- and C. hepatica-infected rats. Clonorchis sinensis- or C. hepatica-specific IgG isotypes IgG1 and IgG2a were also determined. As shown in fig. 2C, D, significantly higher levels of C. sinensis- or C. hepatica-specific IgG1 and IgG2a antibodies were found in single C. sinensis- or C. hepatica-infected and C. sinensis/C. hepatica co-infected rats than those in naïve rats (fig. 2C, D, **P < 0.01, *P < 0.05). Compared to IgG2a antibody responses, higher levels of C. sinensis- or C. hepatica-specific IgG1 antibodies were observed (fig. 2C, D, **P < 0.01, *P < 0.05). No significant increases of IgG1 and IgG2a antibody responses in C. sinensis/C. hepatica co-infected rats were observed compared to single C. sinensis or C. hepatica infection. No cross-reactivities of IgG1 and IgG2a antibodies were found between C. sinensis- and C. hepatica-infected rats.
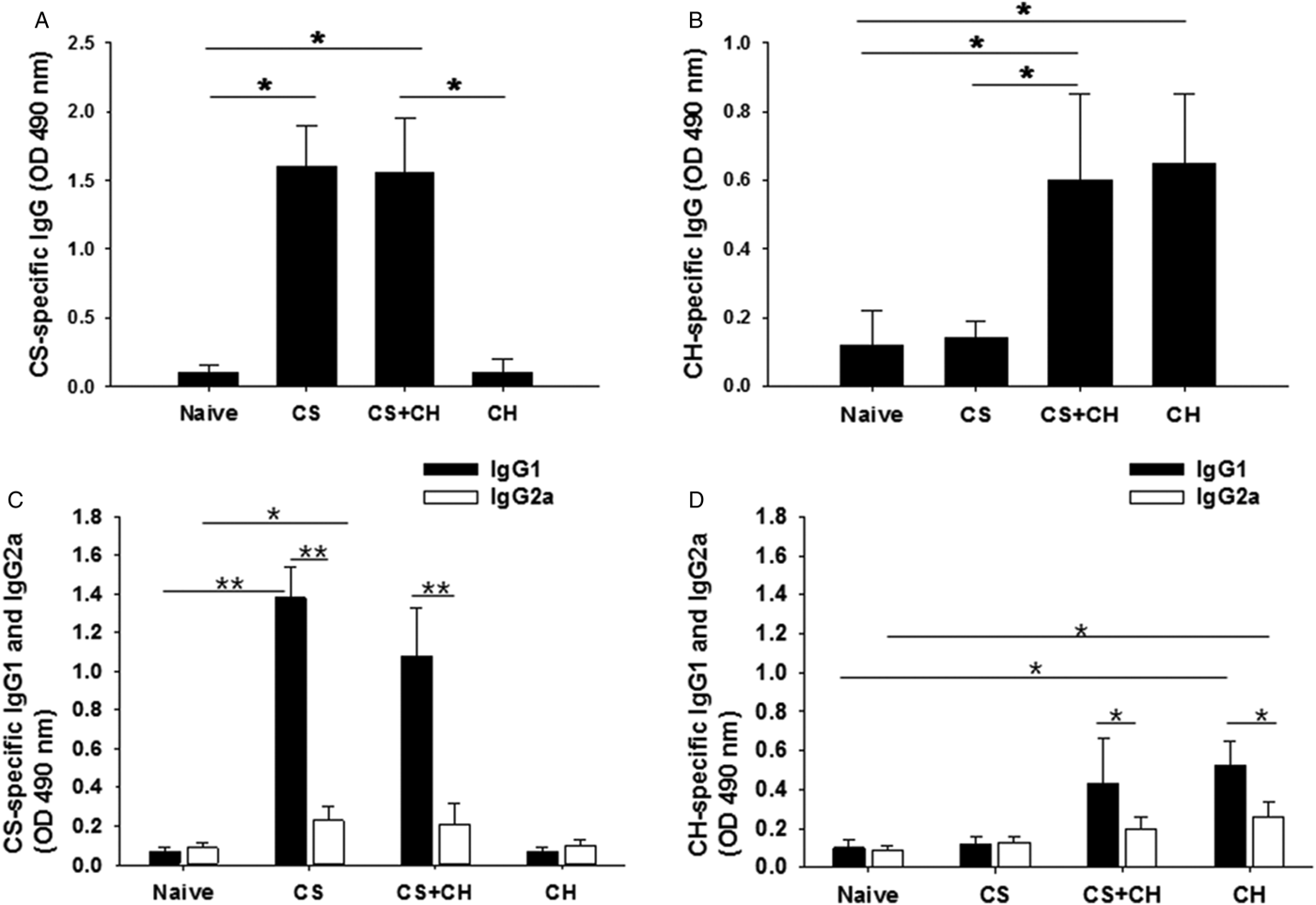
Fig. 2. Clonorchis sinensis- or C. hepatica-specific IgG, IgG1 and IgG2a antibody responses. Clonorchis sinensis-specific IgG (A) and C. hepatica-specific IgG levels (B), from rats at week 4 after primary (CS, CH rats) and post-challenge infection (CS + CH rats), were determined by ELISA. Significantly higher levels of C. sinensis- specific IgG antibodies were found in the sera of C. sinensis-infected and co-infected rats (A). Significantly higher levels of C. hepatica-specific IgG antibodies were found in the sera of C. hepatica-infected and co-infected rats (B). Importantly, no cross-reacting antibody was observed between C. sinensis and C. hepatica infections. Clonorchis sinensis-specific and C. hepatica-specific IgG1 and IgG2a antibody responses were also determined (C, D). Clonorchis sinensis-specific IgG1 and IgG2a (C) and C. hepatica-specific IgG1 and IgG2a (D), from rats at week 4 after primary (CS, CH rats) and post-challenge infection (CS + CH rats), were determined by ELISA. Significantly higher levels of C. sinensis-specific IgG1 antibodies were found in the sera of C. sinensis-infected and co-infected rats compared to IgG2a (C). Significantly higher levels of C. hepatica-specific IgG1 antibodies were found in the sera of C. hepatica-infected and co-infected rats compared to IgG2a (D). No cross-reacting antibody was observed between C. sinensis and C. hepatica infections. The error bars represent SE.
Sequential infection with C. hepatica in C. sinensis-infected rats induced remarkable increases of eosinophil levels in peripheral blood
Whole-blood samples were collected as indicated in the Materials and methods section, and eosinophil profiles were counted. As shown in fig. 3, single infection with C. hepatica (CH) or C. sinensis (CS) in rats produced significantly higher levels of eosinophil counts compared to naïve controls, and C. hepatica (CH) infection produced much higher levels than did C. sinensis (CS) infection (*P < 0.05). Importantly, subsequent infection with C. hepatica in C. sinensis-infected rats (CS + CH) showed a remarkable increase in eosinophil counts (271 ± 81/μl) compared to single infections (154 ± 100/μl) (fig. 3, *P < 0.05, **P < 0.01). These results indicate that C. hepatica infection in helminthic co-infections is critically effective in increasing eosinophil counts in peripheral blood.

Fig. 3. Eosinophil responses. Eosinophils were counted from blood samples of rats (n = 12) at week 4 after infection with C. sinensis or C. hepatica in a single infection (CS, CH) or co-infection (CS + CH). Eosinophil levels were raised significantly in CS, CH and CS + CH rats compared to naïve controls (*P < 0.05). Significantly raised eosinophil levels were detected in CS + CH co-infected rats compared to CS or CH rats (**P < 0.01; *P < 0.05). The error bars represent SE.
Sequential infection with C. hepatica in C. sinensis-infected rats induced higher levels of lymphocyte proliferative response
Lymphocyte proliferation is the process whereby lymphocytes begin to replicate after their antigen receptors recognize an antigen. As seen in fig. 4, a higher degree of lymphocyte proliferation was found in spleens from CS and CS + CH rats when stimulated with C. sinensis ES Ag or PHA, compared to unstimulated cells or those from naïve rats (*P < 0.05). Interestingly, cells from CS + CH rats showed remarkably higher levels of lymphocyte proliferation compared to cells from CS rats (*P < 0.05). These results indicate that a significantly higher level of lymphocyte proliferation is induced by subsequent infection with C. hepatica.

Fig. 4. Lymphocyte proliferation. Rats (n = 12) were primarily infected with 50 metacercariae of C. sinensis and, after 4 weeks, rats were challenge infected with 1000 eggs of C. hepatica. Four weeks after post-challenge infection, rats were sacrificed and lymphocytes were collected from spleens. Each group was stimulated by the C. sinensis ES Ag (CS Ag) and the positive control, phytohaemagglutinin (PHA). No stimulator was added to the cell-alone group. The experiment was carried out in triplicate. Significant increases of lymphocytes (cpm) were determined in CS and CS + CH rats (*P < 0.05). The error bars represent SE
Cytokine production
Cytokine production is an indicator of cellular immune responses. To compare cytokine production levels in states of single infection (CS) and co-infection (CS + CH) and its relationship with worm burden, spleens were collected at week 4 post-challenge and cytokine production levels (IFN-γ, IL-2, IL-4 and IL-10) were determined in response to stimulation with C. sinensis ES Ag. As shown in fig. 5, significantly higher levels of the cytokines IFN-γ, IL-4 and IL-10 were produced in the CS + CH rats following C. sinensis ES Ag stimulation compared to the CS rats or naïve control rats, indicating that cytokine responses are stimulated upon subsequent infection.
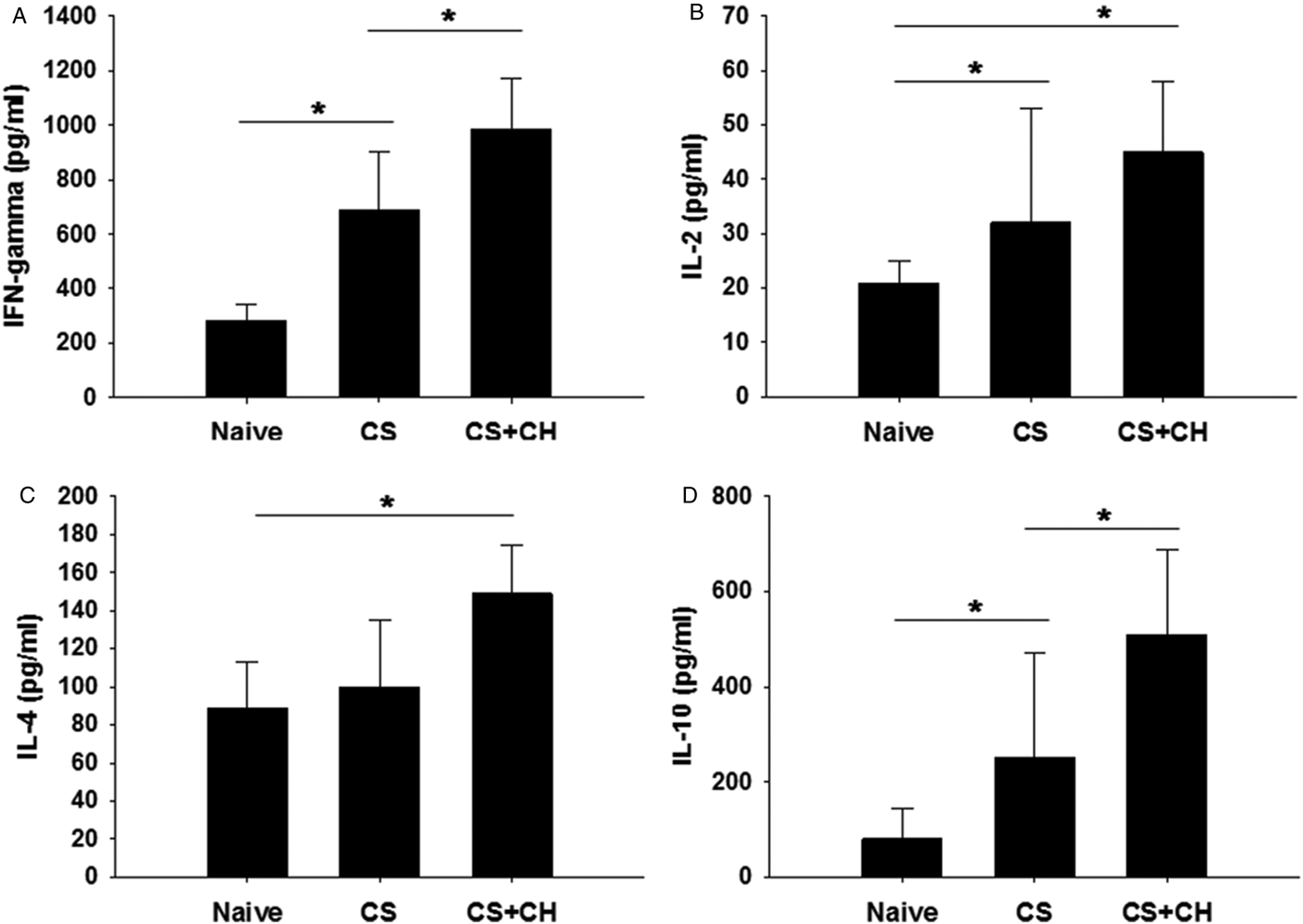
Fig. 5. Cytokine responses. Rats (n = 12) were primarily infected with 50 metacercariae of C. sinensis and, after 4 weeks, rats were challenge infected with 1000 eggs of C. hepatica. At week 4 post-challenge infection, rats were sacrificed and cytokines IFN-γ, IL-2, IL-4 and IL-10 were determined in lymphocytes of spleens from naïve, CS and CS + CH rats. Levels of IFN-γ, IL-4 and IL-10 were found to be significantly higher in CS + CH compared to CS rats (A, C, D, *P < 0.05). The error bars represent SE.
Co-infection with Capillaria hepatica induced resistance against Clonorchis sinensis infection
Adult C. sinensis worms in livers of co-infected CS + CH rats were counted, and reduced parasite loads were observed compared to those in singly infected CS rats. As shown in fig. 6A, a significant reduction of C. sinensis was found in CS + CH rats (18.2 ± 10) compared to CS rats (35.6 ± 6) (*P < 0.05). Capillaria hepatica infection was counted by determining C. hepatica eggs in the livers (fig. 6B). These results indicate that subsequent infection with C. hepatica in C. sinensis-infected rats reduced the C. sinensis worm burden.
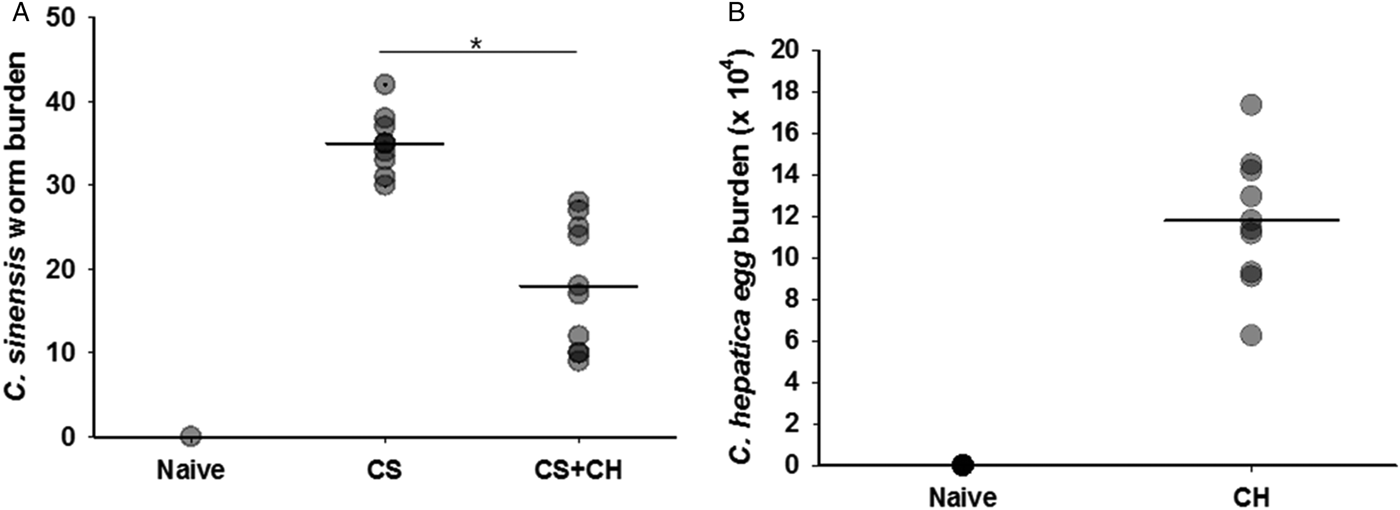
Fig. 6. Clonorchis sinensis worm burden. Rats (n = 12) were primarily infected with 50 metacercariae of C. sinensis and, after 4 weeks, rats were challenge infected with 1000 eggs of C. hepatica. At week 4 post-challenge infection, rats were sacrificed and C. sinensis adults were collected in livers and counted from CS and CS + CH rats. Significantly reduced levels of C. sinensis adults were found in CS + CH rats compared to CS rats (A, *P < 0.05). (B) Capillaria hepatica infection was determined in the rat livers. The error bars represent SE.
Discussion
In the present study, we investigated IgG antibody responses, eosinophil levels, lymphocyte proliferation, cytokine production and worm burden induced by co-infection with C. sinensis and C. hepatica, which have not been investigated previously. We found that cross-reactive C. sinensis-specific IgG antibody was not detected in C. hepatica-infected rats, while high levels of C. sinensis-specific IgG antibody were detected in CS and CS + CH co-infected rats, indicating no IgG cross-reactivity between C. sinensis and C. hepatica. However, eosinophils, lymphocytes and cytokine responses were increased significantly in CS + CH co-infected rats, indicating that these responses might contribute to C. sinensis worm reduction in CS + CH co-infected rats.
It has been reported that single infection with C. hepatica or C. sinensis induces eosinophilic liver infiltration or peripheral blood eosinophilia (Ewing & Tilden, Reference Ewing and Tilden1956; Lee, Reference Lee1964). Eosinophil elevation in parasitic helminth infection is beneficial to the host via the antibody-dependent cellular cytotoxicity system (Kazura, Reference Kazura1981; Klion & Nutman, Reference Klion and Nutman2004; Ganley-Leal et al., Reference Ganley-Leal, Mwinzi, Cetre-Sossah, Andove, Hightower, Karanja, Colley and Secor2006; Bruschi et al., Reference Bruschi, Korenaga and Watanabe2008; Cadman & Lawrence, Reference Cadman and Lawrence2010; Cadman et al., Reference Cadman, Thysse, Bearder, Cheung, Johnston, Lee and Lawrence2014). In a study of co-infections with C. sinensis and T. spiralis, single infection with T. spiralis was found to significantly increase eosinophil levels compared to co-infection, in which resistance against subsequent T. spiralis infection was seen. Intestinal pathological changes and immune responses elicited by prior infection with C. sinensis contributed to protection (Chen et al., Reference Chen, Huang, Huang, Yu, Li, Song, Li and Lu2013). In the current study, we observed that eosinophil levels were significantly increased upon subsequent infection with C. hepatica in C. sinensis-infected rats, and the pre-existing C. sinensis worm burden was reduced upon subsequent infection, indicating that the increase in eosinophil levels may contribute to the resistance against pre-existing C. sinensis. The mechanism of resistance involved in co-infection is likely to be very complicated, and more studies are needed for this mechanism to be elucidated.
Cross-reactive IgG and IgA antibody responses induced by co-infection with C. sinensis and T. spiralis were previously shown to protect against C. sinensis in co-infection (Chu et al., Reference Chu, Kim, Lee, Lee, Joo, Lee, Lee, Zheng and Quan2014). Shared antigens between C. sinensis and T. spiralis were reported to elicit cross-reactivity, thereby producing resistance against pre-existing C. sinensis infection (Chu et al., Reference Chu, Kim, Lee, Lee, Joo, Lee, Lee, Zheng and Quan2014). However, in our present co-infection study, C. sinensis-specific antibody responses were not detected in C. hepatica-infected rats. Also, C. hepatica-specific antibody responses were not found in C. sinensis-infected rats, indicating there are no cross-reactive antibody responses between C. sinensis and C. hepatica. Consistently, it has also been observed that Capillaria philippinensis antigen is not cross-reactive with sera from patients with schistosomiasis mansoni and fascioliasis (El Dib et al., Reference El Dib, Sabry, Ahmed, El-Basiouni and El-Badry2004). In the present study, similar levels of C. sinensis-specific IgG antibody responses were observed in CS and CS + CH rats, indicating that a subsequent infection with C. hepatica does not enhance C. sinensis-specific antibody responses. This indicates that cross-reactive antibodies in co-infection might not be involved in C. sinensis worm reduction upon subsequent infection with C. hepatica. In the present study, higher levels of C. sinensis- or C. hepatica-specific IgG1 and IgG2a antibodies were found in single C. sinensis- or C. hepatica-infected and C. sinensis/C. hepatica co-infected rats compared to naïve rats. It is well known that IgG2a and IgG1 are, respectively, induced by T-helper cells Th1 and Th2. Our results suggest that C. sinensis, C. hepatica and co-infection of these two helminths induced a combined Th1/Th2 immune response, which is consistent with the cytokine production seen in the present study.
Our data indicate that a high degree of lymphocyte proliferation was detectable in CS and CS + CH rats. We also found that higher levels of cytokines IFN-γ, IL-4 and IL-10 were detected in co-infection compared to single infection with C. sinensis, indicating that Th1 and Th2 cytokines may be involved in the immune response changes induced by C. hepatica infection. In murine schistosomiasis, the presence of any eggs in the liver induced a marked Th2 response that developed into granulomatous lesions (Cheever et al., Reference Cheever, Hoffmann and Wynn2000). A mixed Th1- and Th2-type immune response is induced in humans concurrently infected with Necator americanus and Oesophagostomum bifurcum (Pit et al., Reference Pit, Polderman, Baeta, Schulz-Key and Soboslay2001). The Th1 response is induced to prevent alternative macrophage activation and to limit the fibrosis-enhancing effects of the protective Th2 response (Hoffmann et al., Reference Hoffmann, Caspar, Cheever and Wynn1998; Hesse et al., Reference Hesse, Cheever, Jankovic and Wynn2000). Future studies will focus on detailed cytokine-related immune responses in C. sinensis/C. hepatica co-infected rats.
The present findings demonstrated that the worm burden of C. sinensis was significantly reduced in C. sinensis-infected rats upon subsequent infection with C. hepatica. Cross-reactive antibody responses against C. sinensis were not involved in worm reduction.
Financial support
This work was supported by a grant from the National Research Foundation of Korea (NRF) (NRF-2014R1A2A2A01004899) and a grant from the Ministry of Health and Welfare, Republic of Korea (HI15C2928).
Conflict of interest
None.
Ethical standards
All animal experiments and husbandry involved in the studies presented in this manuscript were conducted under the guidelines of the Kyung Hee University IACUC (permit number: KHUASP (SE) - 17 - 009).








