Introduction
Tomato (Solanum lycopersicum) is the second most common vegetable after potato in global food production (Gondal et al., Reference Gondal, Ijaz, Riaz and Khan2012; Vignesh et al., Reference Vignesh, Rajamohan, Balabaskar and Anandan2021), and the People's Republic of China (PCR) is the largest area of tomato cultivation in the world (Diao et al., Reference Diao, Larsen, Kamvar, Zhang and Liu2019). Sichuan Province, located in southwestern PCR, is a major production area for tomato cultivation. This important crop for the human diet is affected by many pathogens during its growth, which are responsible for high economic losses. Among them, plant-parasitic nematodes are one of the most important pathogens on tomato; for example, Meloidogyne, Pratylenchus, Tylenchorhynchus and Helicotylenchus can all infect tomato, causing severe damage and yield loss (Hou, Reference Hou2001; Talwana et al., Reference Talwana, Sibanda, Wanjohi, Luambano-Nyoni, Massawe, Manzanilla-López, Davies, Hunt and Sikora2016). Root-lesion nematodes of the genus Pratylenchus are the most economically damaging plant-parasitic nematodes on grains, fruits and vegetables (Jones et al., Reference Jones, Haegeman and Danchin2013). To date, according to taxonomic studies, there are more than 100 valid species of root-lesion nematodes (Janssen et al., Reference Janssen, Karssen, Couvreur, Waeyenberge and Bert2017). Pratylenchus species are migratory endoparasites that can cause yield losses of up to 85% (Nicol et al., Reference Nicol, Turner, Coyne, Den, Hockland, Maafi, Jones, Gheysen and Fenoll2001), and yield losses are even higher when nematodes have synergistic interactions with certain soilborne plant pathogens (Jones & Fosu-Nyarko, Reference Jones and Fosu-Nyarko2014). Pratylenchus scribneri is one of the most important root-lesion nematodes and is a known economic pathogen that infects a variety of crops, including corn (Zea mays L.), soybean (Glycine max L.), barley (Hordeum vulgare L.), potato (Solanum tuberosum L.), sugarcane (Saccharum officinarum L.), tobacco (Nicotiana tabacum L.), tomato, strawberry (Fragaria x ananassa Duch.) and onion (Allium cepa L.) (Castillo & Vovlas, Reference Castillo and Vovlas2007; Li et al., Reference Li, Wang, Liu, Lu, Wang and Li2019).
Among the Pratylenchus species found to be associated with vegetables are P. zeae, P. scribneri, P. neglectus, P. loosi and P. brachyurus (Talwana et al., Reference Talwana, Sibanda, Wanjohi, Luambano-Nyoni, Massawe, Manzanilla-López, Davies, Hunt and Sikora2016). Pratylenchus brachyurus and P. delattrei have also been reported on tomato in Cape Verde (Flis et al., Reference Flis, Dobosz, Rybarczyk, Wasilewska-Nascimento, Kubicz and Winiszewska2018).; Siddiqui et al. (Reference Siddiqui, Sher and French1973) listed 22 host plants of P. scribneri in California, including tomato. It has also been reported that several species of Pratylenchus can infect tomato roots in the PCR, having obvious brown spots and serious rot in roots, causing great losses to tomato production. Based on the morphological characteristics, four species of Pratylenchus were identified in the rhizosphere soil of tomato in Henan Province: P. coffeae; P. scribneri; P. fallax; and P. helophilus (Li et al., Reference Li, Feng and Xu1985). It has also been reported that P. scribneri was isolated from the rhizosphere of tomato in Shandong Province, PCR (Liu & Liu, Reference Liu and Liu2007). However, the identification of most of the Pratylenchus species was only based on morphological characters and lacked molecular data, and further analysis of their pathogenicity to tomato has not been reported. In this study, a purified root-lesion nematode isolate from Sichuan Province was identified on the basis of morphological and molecular markers or characters, and the pathogenicity of this isolate on tomato was assessed in pot experiments. Both morphometric characters and molecular markers revealed that the species of root-lesion nematode from Sichuan Province was P. scribneri, and it has strong pathogenicity to tomato. To the best of our knowledge, this is the first report of P. scribneri on tomato in Sichuan Province, PCR, using morphological and molecular characters. These are also the first molecular data obtained from P. scribneri on tomato in the PCR, and the pathogenicity of P. scribneri to tomato was studied for the first time. The purpose of this study was to understand the pathogen species of root-lesion nematodes on tomato in the PCR, which provides a scientific basis for the detection and control of tomato root-lesion nematode diseases.
Materials and methods
Nematode isolate sampling and culturing
Five soil samples were collected from the rhizosphere of tomatoes (cv. Maohong 801), which had weak growth in a field near Shizishu village in Jingtang County of Chengdu city, Sichuan Province, PCR. Root-lesion nematodes were extracted using the modified Baermann funnel method (Hooper et al., Reference Hooper, Hallman, Subbotin, Luc, Sikora R and Bridge2005). To obtain purified isolates, individual females were isolated, sterilized with 0.3% streptomycin sulphate and transferred to carrot discs, prepared according to the methods described by Reise et al. (Reference Reise, Huettel and Sayre1987) and Kaplan & Davis (Reference Kaplan and Davis1990) and maintained at 25 °C in the dark (Li et al., Reference Li, Wang, Liu, Lu, Wang and Li2019; Wang et al., Reference Wang, Liu, Xia, Hao, Wang, Li and Li2021). The selected purified root-lesion nematode SC isolate was used for subsequent morphological and molecular identification.
Morphometric identification
Nematodes were heat-killed and fixed in FG solution (formalin:glycerin:water = 10:1:89) (Xie, Reference Xie2005). The fixed specimens were transferred to anhydrous glycerine according to the methods described by Seinhorst (Reference Seinhorst1959) and mounted on permanent slides. Measurements and light photomicrographs of nematodes were performed using a Nikon Eclipse Ti-S inverted microscope (Japan). Images of key morphological features were processed by Photoshop CS5 software. The De Man formula was used to calculate the measurements. All measurements are in micrometres unless otherwise stated.
Molecular characterization and phylogenetic relationships
The DNA of one individual nematode was extracted using liquid nitrogen, followed by proteinase K (Wang et al., Reference Wang, Zhang and Gu2011). The internal transcribed spacer region (ITS) rDNA gene was amplified using primers TW81 (5′-GTTTCCGTAGGTGAACCTGC-3′) and AB28 (5′-ATATGCTTAAGTTCAGCGGGT-3′) (Subbotin et al., Reference Subbotin, Sturhan, Chizhov, Vovlas and Baldwin2006). The D2-D3 region of the 28S rDNA gene was amplified using primers D2A (5′-ACAAGTACCGTGAGGGAAAGTTG-3′) and D3B (5′-TCGGAAGGAACCAGCTACTA-3′) (De Ley et al., Reference De Ley, Felix, Frisse and Steven1999). The mitochondrial cytochrome oxidase I (mtDNA-COI) gene was amplified using primers JB3 (5’-TTTTTTGGGCATCCTGAGGTTTAT-3’) and JB5 (5’-AGCACCTAAACTTAAAACATAATGAAAATG-3’) (Liu et al., Reference Liu, Wang, Li, Li, Xue and Ma2018). Polymerase chain reaction (PCR) amplifications were carried out in 25 μL of reaction mixture with the following components: 1 × KOD FX PCR buffer’ 0.4 mm of each dNTP; 0.3 μM of each primer; 3 μL of DNA template; and 0.5 units of KOD FX (Toyobo, Japan). The reaction protocol was as follows: predenaturation at 94 °C for 2 min, followed by 35 cycles (denaturation at 98 °C for 10 s, annealing at 58.2 °C (ITS rDNA) or 51.7 °C (28S rDNA) or 52 °C (mtDNA-COI) for 30 s, extension at 68 °C for 90 s) and final extension at 72 °C for 10 min (Wang et al., Reference Wang, Xie, Li, Wu and Xu2016). The PCR products were purified using the Biospin Gel Extraction Kit (BioFlux, PCR), cloned into pJET 1.2/blunt cloning vectors (Thermo Scientific, USA) and then sequenced by Sangon Biotech Co., Ltd. (Shanghai, PCR). The newly obtained sequences were deposited in the GenBank database (NCBI).
The P. scribneri sequences were aligned with other Pratylenchus species published in GenBank using the nucleotide BLAST program in NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Nacobbus aberrans, Tylenchorhynchus dubius and Meloidogyne incognita were chosen as the outgroup taxa according to the results published by Nguyen et al. (Reference Nguyen, Le, Nguyen, Nguyen, Liebanas and Trinh2017) and Handoo et al. (Reference Handoo, Skantar, Kantor, Hafez and Hult2020). Multiple alignments of the sequences were performed using Clustal W in MEGA 7 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). Phylogenetic relationships were established with Bayesian inference (BI) using MrBayes 3.1.1 (Huelsenbeck & Ronquist, Reference Huelsenbeck and Ronquist2001). The best-fit model (GTR + I + G) was selected by the Akaike information criterion using MrModeltest 2.3 (Nylander, Reference Nylander2004). BI analysis was initiated with a random starting tree and was run with four Markov chains for 1,000,000 generations. The Markov chains were sampled at intervals of 100 generations. After discarding burn-in samples, the remaining samples were retained to generate a 50% majority rule consensus tree. Posterior probabilities were given on appropriate clades.
Pathogenicity assays
Seeds of tomato (cv. Maohong 801) were purchased from Qiule, Zhengzhou, PRC, placed in 0.75% sodium hypochlorite solution for disinfection for 30 min, washed with sterile water and sown in pots with sterilized sand soil. Thirty days after sowing, five seedlings with the same growth status (one/pot) were selected for the pathogenicity assays. These studies were performed with the P. scribneri SC isolate extracted from carrot discs and adjusted to a suspension with 500 nematodes/ml. A 2 mL suspension containing 1000 nematodes was then pipetted into three small holes 5 cm deep. For the blank controls, 2 mL of sterile water was pipetted into holes. To permit undisturbed root invasion by P. scribneri, the seedlings were not watered during the first three days after inoculation (Hahn et al., Reference Hahn, Sarah, Boisseau, Vines, Wright and Burrows2010). Seventy-five days after inoculation, plant growth parameters, the number of nematodes, and the reproduction factor (Rf) were assessed. The symptoms of root-lesion nematode infection on tomato roots were also photographed. Tomato plants were removed from the pots and nematodes were extracted from soil and roots using the modified Baermann funnel method. The total number of nematodes (Pf) was the sum of nematodes isolated from soil and roots, and then the Rf was calculated. There were five replications for the inoculation period, and the experiment was performed twice. According to the method described by Byrd et al. (Reference Byrd, Kirkpatrick and Barker1983), the nematodes in the roots were stained and then detected under a microscope.
Results
Morphometric identification
The morphological characters analysed in females were as follows: entire body; anterior region; lip region; junction of genital gland and intestine; lateral line; anterior end of genital gland; post-vulval region and ovary, and tail region (fig. 1). The morphometric measurements of the SC isolate of P. scribneri (table 1) were consistent with P. scribneri as described previously (Roman & Hirschmann, Reference Roman and Hirschmann1969).
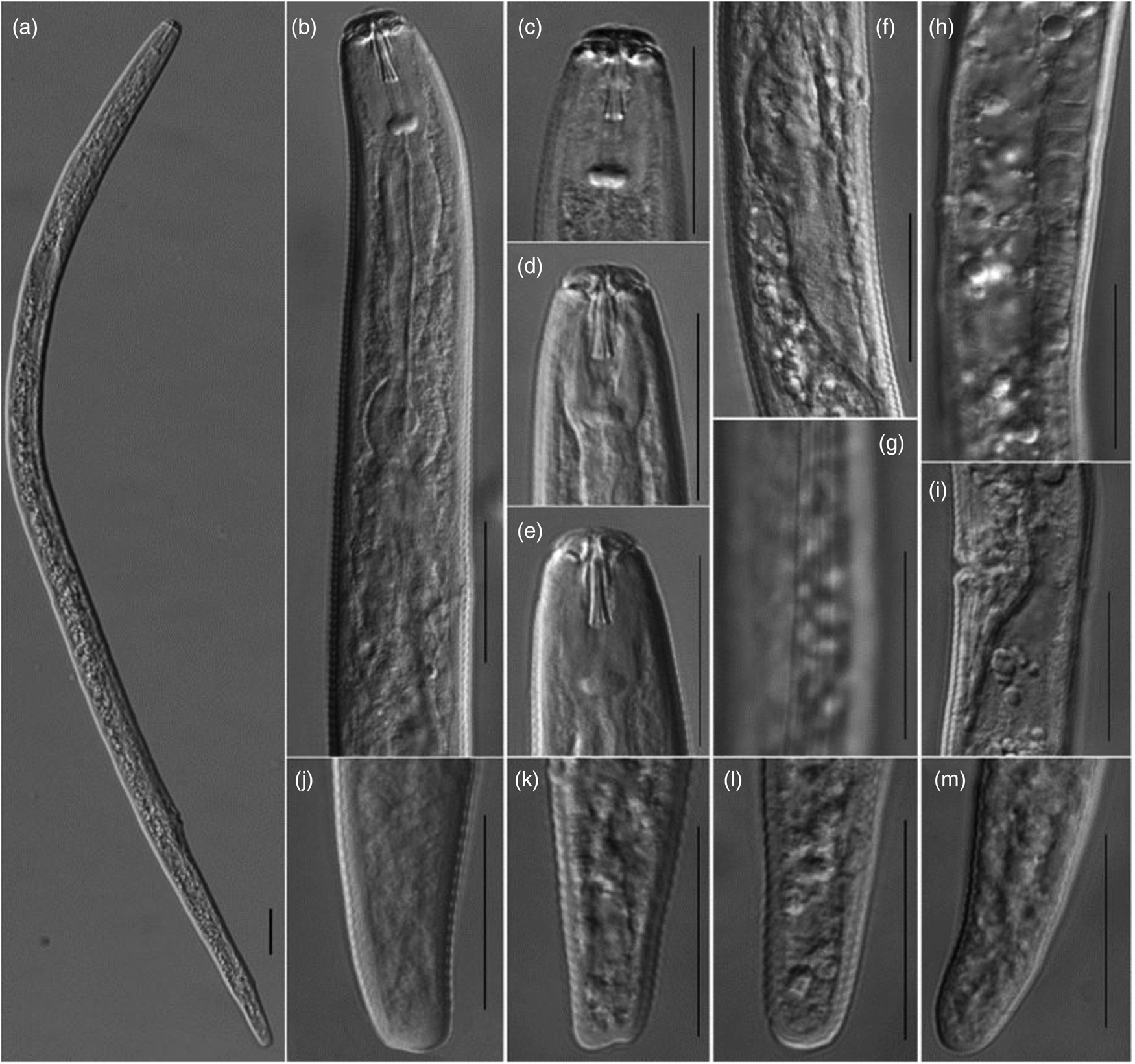
Fig. 1. Light micrographs of Pratylenchus scribneri from tomato in Sichuan Province, People's Republic of China. Females (a–m): (a) entire body; (b) anterior region; (c–e) lip region; (f) junction of genital gland and intestine; (g) lateral line; (h) anterior end of genital gland; (i) post-vulval region and ovary; and (j–m) tail region. Scale bars: 50 μm (a, n) and 20 μm (b–m).
Table 1. Morphometrics of females of Pratylenchus scribneri.

Notes: all measurements are in μm and in the form of mean ± standard deviation (range). n: number of specimens measured; L: body length; a: body length/greatest body width; b: body length/length from the lips to the junction of oesophageal gland and intestine; b’: body length/length from the lips to oesophageal gland end; c: body length/tail length; c’: tail length/tail diameter at anus; V: distance of vulva from the lips × 100/body length; and DGO: distance between dorsal oesophageal gland opening and stylet knobs.
Female: body stout, straight or slightly bent ventrally after heat relaxation; labial region slightly offset from body, composed of two annuli of approximately the same height, anterior one distinctly narrower than second and inner part of lateral lips narrower than outer part; stylet robust, with rounded knobs that vary little in shape, stylet length 15.1–16.0 μm; orifice of dorsal pharyngeal gland approximately 2.5–3.3 μm posterior to stylet base; lateral fields with four longitudinal lines, occasionally a fifth line may be present at mid-body; excretory pore immediately posterior to hemizonid; pharyngeal gland overlapping intestine ventrally or ventrolaterally; anterior genital branch consisting of ovary with oocytes in single file except for a short zone near anterior end; short oviduct; empty spermatheca; post-vulval uterine sac generally 18.3–26.3 μm body diameter long; vulva–anus distance generally 2.2–3.5 times tail length; and tail tapering slightly, terminus mostly broadly rounded, varying from somewhat narrower to almost truncate, usually with 21–25 annuli.
Male: not found.
Molecular characterization and phylogenetic relationships
Primers TW81/AB28, D2A/D3B and JB3/JB5 were used to amplify the rDNA-ITS gene, the D2-D3 region of the 28S rDNA gene and the mtDNA-COI gene of root-lesion nematode SC isolates, respectively (fig. 2). The obtained ITS rDNA, D2-D3 region of 28S rDNA and mtDNA-COI sequences were submitted to GenBank for a BLAST search. The obtained ITS rDNA sequences (GenBank Accession Numbers MZ203866, OK021634, OK021635 and OK021636) showed 99%–100% identity with P. scribneri sequences available in GenBank; the D2-D3 region of the 28S rDNA sequences (MZ215788, OK021637, OK030711 and OK030712) shared more than 99% identity with several P. scribneri sequences available in GenBank; and the mtDNA-COI sequences (MZ203870, OK021638, OK021639 and OK030210) shared more than 99% identity with several P. scribneri sequences available in GenBank.

Fig. 2. Polymerase chain reaction amplification of the internal transcribed spacer region (ITS) rDNA gene, the D2-D3 region of the 28S rDNA gene and the mtDNA-COI gene of Pratylenchus scribneri. M: DL2000 marker; 1: ITS rDNA; 2: D2-D3 region of 28S rDNA; and 3: mtDNA-COI.
The phylogenetic tree based on the ITS rDNA gene (fig. 3), which contained 58 ingroups and one outgroup taxon, revealed that the newly obtained sequence of the SC isolate is clearly different from other Pratylenchus species and forms a 100% supported clade with P. scribneri. The phylogenetic tree based on the D2-D3 region of the 28S rDNA gene contained 51 ingroups and one outgroup taxon, showing that the newly obtained sequence of this isolate is clearly different from other Pratylenchus species and formed a highly supported clade with P. scribneri (100%) (fig. 4). The Bayesian phylogenetic tree generated from the mtDNA-COI region dataset contained 46 ingroups and one outgroup taxon, showing that the newly obtained sequence of the SC isolate is clearly different from other Pratylenchus species and formed a highly supported clade with P. scribneri (fig. 5). These results confirmed that the sequence of this root-lesion nematode isolate formed a highly supported clade with all the reported P. scribneri sequences.

Fig. 3. Bayesian tree of Pratylenchus as inferred from internal transcribed spacer region gene rDNA gene sequences under the GTR + I + G model. Posterior probabilities greater than 50% are given for appropriate clades. Newly obtained sequence is indicated in boldface type.

Fig. 4. Bayesian tree of Pratylenchus as inferred from the D2-D3 region of 28S rDNA gene sequences under the GTR + I + G model. Posterior probabilities greater than 50% are given for appropriate clades. Newly obtained sequence is indicated in boldface type.

Fig. 5. Bayesian tree of Pratylenchus as inferred from mtDNA-COI gene sequences under the GTR + I + G model. Posterior probabilities greater than 50% are given for appropriate clades. Newly obtained sequence is indicated in boldface type.
Pathogenicity assays
Seventy-five days after nematode inoculation, obvious disease symptoms were observed, including reduced plant growth and height and chlorotic leaves, compared with the uninoculated control plants. The plant height, fresh shoot weight and fresh root weight were 43.50 cm, 28.27 g and 4.44 g, respectively, which were significantly lower than those in uninoculated control plants (P < 0.05) (table 2). Due to the infection and damage of P. scribneri, the roots of tomato plants decreased significantly and showed distinct brown lesions (fig. 6a). At the initial stage of nematode infection, tomato roots showed small and light brown spots (fig. 6b) that became larger and brown or dark brown (fig. 6c, d), eventually leading to the whole roots appearing in a large area of necrosis and rot (fig. 6e). A large number of root-lesion nematodes were extracted from the rhizosphere soil and roots of the tomato plants. The average number of P. scribneri nematodes extracted from each inoculated plant was 4293. Also, the Rf of P. scribneri in tomato soil and roots reached 4.29 (table 2). According to the measuring standards of Goo & Sipes (Reference Goo and Sipes1997), tomato is a suitable host plant for the P. scribneri SC isolate. Staining results showed a large number of P. scribneri nematodes and eggs in the tomato root tissues, which indicated that P. scribneri can propagate and complete its life cycle in tomato roots (fig. 6f–i).

Fig. 6. Symptoms of tomato roots infected by Pratylenchus scribneri. (a) CK: healthy plant with a good root system; 1: tomato roots infected by P. scribneri 75 days after inoculation’ (b) early symptoms of small and light brown spots infected by P. scribneri; (c) expanding lesions on tomato roots; (d) deep brown spots on roots seriously infected by P. scribneri; (e) severely rotted root system caused by P. scribneri; (f–i) photomicrograph of P. scribneri within tomato roots; (f) healthy roots; (g, h), a large number of P. scribneri and eggs in the infected tomato roots; and (i) P. scribneri in tomato root cells. Scale bars: 50 μm (A, B, C) and 25 μm (D). n = nematode; e = egg; and s = stylet.
Table 2. Effects of Pratylenchus scribneri SC-1 isolate on tomato growth, final population density (Pf) 75 days after inoculation and reproduction factor (Rf).

Notes: values represent the mean of five replicates ± standard error; * indicates significant differences between two treatments based on t-test (P < 0.01). Reproduction factor (Rf) = final population density (Pf)/initial population density (Pi)
Discussion
Most vegetable crops may be attacked by one or more species of Pratylenchus, and root-lesion nematodes of the genus Pratylenchus have been reported to parasitize tomato plants at home and abroad. The accurate identification of Pratylenchus species is crucial for applying appropriate control measures. The taxonomy of the genus Pratylenchus is always difficult because of the relatively large and significant intraspecific variability in the diagnostic characters and the similarity of the diagnostic characters among different species (Ryss, Reference Ryss2002). Loof (Reference Loof1978) pointed out that a few characteristics were reliable and useful for the identification of species of Pratylenchus, such as structure of lateral fields, stylet length, lip annuli, shape of labial region, position of vulva, presence and shape of spermatheca, length of overlapping gland lobe, shape of female tail and terminus and presence or absence of males, and length of the post vulval uterine sac. Although morphology is still used for species identification of this genus, new technologies based on biochemical and molecular analyses are becoming increasingly important in nematode taxonomy and practical identification (Andrés et al., Reference Andrés, Pinochet, Hernandez and Delibes2000; De Luca et al., Reference De Luca, Fanelli, Divito, Reyes and De2004). However, these new molecular approaches should still be integrated with morphological data. In this study, a combination of morphometric characters and molecular markers was used to identify the species of the purified root-lesion nematode SC isolate. The results revealed that the species of this root-lesion nematode extracted from the rhizosphere of tomato in Chengdu City of Sichuan Province was P. scribneri. The Bayesian tree showed that the phylogenetic analysis of the ITS rDNA gene, D2-D3 region of the 28S rDNA gene and mtDNA-COI gene were consistent, which clearly separated P. scribneri from other Pratylenchus species. This is the first report of P. scribneri on tomato in Sichuan Province, PCR, using morphological and molecular data. It is also the first molecular data obtained from P. scribneri on tomato in the PCR.
Pathogenicity is usually used in plant pathology to indicate the capacity of an organism to induce disease or the amount of physiological damage caused to the host plant by the presence of a pathogen (Shaner et al., Reference Shaner, Stromberg and Lacy1992). The most obvious aboveground symptoms of root-lesion nematode disease on plants are stunting and chlorotic (yellowish) colouring, which give the field a ragged appearance. The symptoms of root-lesion nematode damage to plant roots are similar to those of other soil-borne diseases, nutrient deficiencies, insect damage and environmentally induced stress. Therefore, the damage caused by Pratylenchus species in agriculture is frequently neglected. In this study, we demonstrated that the slow decline of tomato caused by P. scribneri is a disease that fulfils Koch's postulate. The pathogenicity of P. scribneri to tomato was confirmed via pot experiments in the greenhouse, and the microscopic detection of tomato roots inoculated with P. scribneri confirmed the presence of these nematodes. Rebois (Reference Rebois and Huettel1986) reported the ectoparasitic feeding behaviour of P. scribneri on root hairs of corn, tomato and soybean. However, the endoparasitic feeding of P. scribneri on tomato roots was also demonstrated, and the entire body of the nematode and eggs were observed in the inoculated roots, which indicated that P. scribneri could propagate and complete its life cycle in root cells. The present study also showed that the P. scribneri SC isolate collected from Sichuan Province has a strong pathogenicity to tomato. These results provide an effective theoretical basis for the diagnosis and control of tomato root-lesion nematode disease. In addition, root-lesion nematodes are poikilothermic organisms, and consequently, temperature influences the rates of physiological processes, such as movement, growth and reproduction, expression of nematode damage to plants and sex determination (Freckman & Caswell, Reference Freckman and Caswell1985). Therefore, whether growing Pratylenchus-infected tomato plants under different temperature conditions will affect the pathogenicity of P. scribneri to tomato requires further investigation.
Financial support
This study was financially supported by the Science and Technology Innovation Foundation of Henan Agricultural University (Grant Number: KJCX2018A11), Key Scientific Research Projects of Colleges and Universities in Henan Province of the People's Republic of China (Grant Number: 21B210003) and the Open Project of Key Laboratory of Crop Ecophysiology and Farming System in Desert Oasis Region, Ministry of Agriculture and Rural Affairs (Grant Number: 25107020-202102).
Conflict of interest
None.
Ethical standards
All procedures contributing to this study comply with the ethical standards of the relevant national and institutional guides on the care and use of animals.










