Introduction
Synanthropic or commensal species inhabit settlements (Khlyap & Warshavsky, Reference Khlyap and Warshavsky2010) and live in close association with humans. Urbanization, characterized by high human density, is increasing globally (Gratz, Reference Gratz, Robinson, Rettich and Rambo1999) and therefore allows these species to thrive and, when compared to sylvatic wildlife, it has been suggested that synanthropic wildlife is mostly responsible for emerging zoonotic diseases (McFarlane et al., Reference McFarlane, Sleigh and McMichael2012). In addition, urbanization may bring about clumped resource partitioning, which has been demonstrated to increase parasite richness through increased contact among wildlife, while encroachment into and modification of natural landscapes promotes contact between humans and wildlife (Wright & Gompper, Reference Wright and Gompper2005).
The multimammate mouse, Mastomys coucha (Smith, 1836), is an indigenous and common murid rodent species, widely distributed in southern Africa (Skinner & Chimimba, Reference Skinner and Chimimba2005) and a known agricultural pest (Smit et al., Reference Smit, van der Bank, Falk and de Castro2001) that has adopted a synanthropic lifestyle. In urban areas, the species readily enters residential dwellings but does not nest there (Kirsten & von Maltitz, Reference Kirsten and von Maltitz2005; Kneidinger, Reference Kneidinger2008).The three matrilineal house rat species of the genus Rattus occurring in South Africa (R. norvegicus, R. rattus, R. tanezumi) have long been recognized as true commensal species (Aplin et al., Reference Aplin, Suzuki and Chinen2011) as they are mostly associated with human habitation. Coincidentally, of the approximately 66 recognized species of Rattus worldwide (Musser & Carleton, Reference Musser, Carleton, Wilson and Reeder2005) these three species are considered to be the most invasive rat species, with a cosmopolitan distribution for urban ecosystems (Kosoy et al., Reference Kosoy, Khlyap, Cosson and Morand2015).
In Africa, species of Rattus and Mastomys are frequently implicated in disease epidemiology (Gratz, Reference Gratz, Buckle and Smith1994; Taylor et al., Reference Taylor, Arntzen, Hayter, Iles, Frean and Belmain2008), and in South Africa these rodent species have been identified as hosts and reservoirs of a variety of infectious agents, including viruses (Witkowski et al., Reference Witkowski, Klempa, Ithete, Auste, Mfune, Hoveka, Matthee, Preiser and Kruger2014), bacteria (Julius et al., Reference Julius, Bastos, Chimimba and Brettschneider2012; Le Grange, Reference Le Grange2014) and parasites (Collins, Reference Collins1972; Archer et al., Reference Archer, Appleton, Mukaratirwa and Hope2011). There is, however, a dearth of information regarding their parasitic fauna, and particularly helminths. Past helminthological studies that included commensal rodents were mostly opportunistic and focused on helminth species descriptions and certain taxa (Collins, Reference Collins1972; Archer et al., Reference Archer, Appleton, Mukaratirwa, Lamb and Schoeman2017), and did not take into consideration the unknown presence of cryptic R. tanezumi which is morphologically similar to R. rattus. Only recently was an endoparasitic survey conducted on commensal R. norvegicus, R. rattus and M. natalensis in the port city of Durban, Kwa-Zulu Natal Province in South Africa (Archer et al., Reference Archer, Appleton, Mukaratirwa, Lamb and Schoeman2017).
In South Africa, where commensal species of Mastomys and Rattus are sympatric (Kirsten & von Maltitz, Reference Kirsten and von Maltitz2005; Archer et al., Reference Archer, Appleton, Mukaratirwa, Lamb and Schoeman2017), the present study provides information on their helminth species composition and prevalence where the rodent species identities were confirmed genetically. Results are discussed in context with the implications to human health and the environment.
Materials and methods
Study sites and sampling regime
Rodents were sampled between September 2010 and September 2011 and in May 2012 with baited (peanut butter, fish and oats) Sherman traps and snap traps. Trapping localities consisted of formal and informal residential homes, school grounds, office buildings, industrial buildings and smallholdings in Hammanskraal (25°24′S, 28°15′E), Pretoria (25°45′S, 28°15′E), Tembisa (26°0′S, 28°12′E), Diepsloot (25°56′E, 28°0′S) and Alexandra (26°6′S, 28°6′E), Gauteng Province, South Africa (fig. 1).
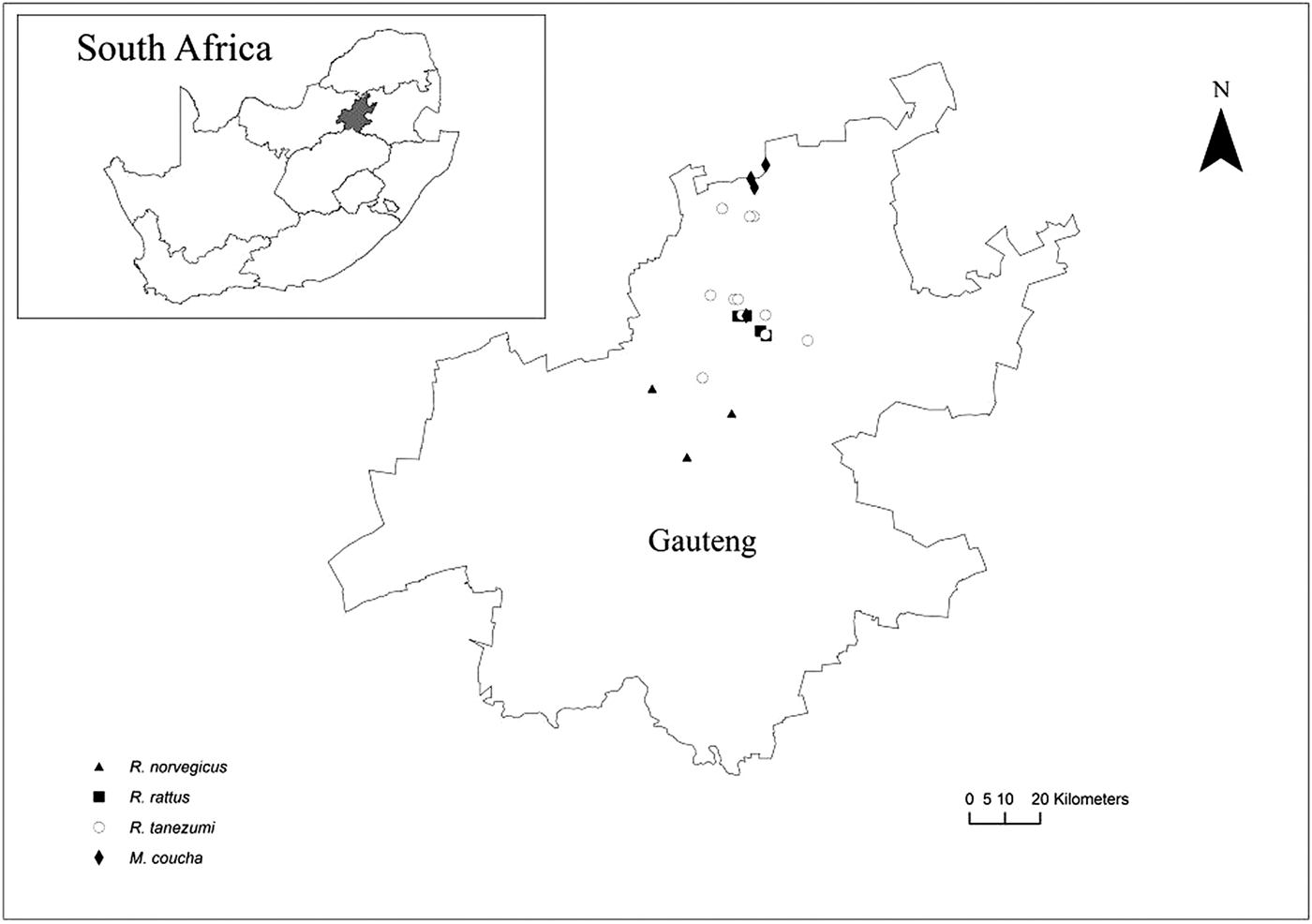
Fig. 1. Map of Gauteng Province, South Africa, showing sampling localities of indigenous Mastomys coucha and invasive Rattus species.
Rodent capture and helminth recovery
Following capture, rodents were euthanized, dissected and stored at −20°C. As part of a parallel study, sample collection was initially prioritized for tissue biopsies and not for helminth recovery; consequently, preservation of helminth material was compromised due to repeated freezing and thawing of rodent carcasses. Rodents were identified based on external morphology in accordance with published information, and morphologically cryptic species were identified by molecular typing of the mitochondrial cytochrome b (cyt b) gene region (Julius, Reference Julius2013; Le Grange, Reference Le Grange2014).
The liver, urinary bladder and gastrointestinal tract (GIT) (stomach, small intestine (SI), caecum and colon) were removed, dissected and subsequently examined under a stereomicroscope for the presence of helminths. Nematodes, acanthocephalans and metacestodes were counted, but not adult cestodes as, in many instances, only strobilar fragments without scoleces were recovered. Helminth specimens were rinsed with phosphate-buffered saline (PBS) and subsequently fixed in 70% glycerol alcohol.
Morphological identification of helminths
Cestodes were stained with aceto-alum carmine, cleared in clove oil and mounted in Entellan® (International Institute of Parasitology (IIP), 1996). Nematodes and acanthocephalans were cleared in lactophenol containing Horen's trichrome (IIP, 1996). Microscopic examination was conducted with an Olympus BX 50 (Olympus, Tokyo, Japan) compound microscope and images taken with an attached Imaging CC12 digital camera (Soft Imaging System, Münster, Germany). Scanning electron microscopy (SEM) was conducted with a JEOL 6000 F scanning electron microscope (JEOL, Tokyo, Japan) following dehydration of specimens in an ethanol series and subsequent drying by the critical-point technique. Helminth specimens were identified to the lowest taxonomic level possible with the aid of published taxonomic keys for nematodes (Anderson et al., Reference Anderson, Willmott and Chabaud1974), cestodes (Khalil et al., Reference Khalil, Jones and Bray1994) and acanthocephala (Petrochenko, Reference Petrochenko and Epstein1971). Voucher specimens of Moniliformis moniliformis (S/2017/4.1), Hydatigera taeniaeformis (S/2017/4.2-4.3), Hydatigera parva (S/2017/4.4), Eucoleus sp. (S/2017/4.5-4.7), heligmonellid sp. (S/2017/4.8-4.9), Heterakis spumosa (S/2017/4.10), Mastophorus muris (S/2017/4.11-4.12), Nippostrongylus brasiliensis (S/2017/4.13-4.14), Aspiculuris tetraptera (S/2017/4.15-4.16), Syphacia muris (S/2017/4.17-4.18), Protospirura sp. (S/2017/4.19-4.20), Syphacia obvelata (S/2017/4.21-4.22), Trichosomoides crassicauda (S/2017/4.23) and Trichuris sp. (S/2017/4.24-4.25) are deposited at the National Collection of Animal Helminths, Onderstepoort Veterinary Institute, Agricultural Research Council, Onderstepoort, South Africa.
Molecular identification of helminths
Genomic DNA was extracted using QIAamp DNA minikit (Qiagen, Hilden, Germany) following the manufacturer's protocol, with minor adjustments. A subset of cestodes (n = 12), one sample from three individuals each of M. coucha, R. norvegicus, R. rattus and R. tanezumi hosts, which could not be identified to the species level morphologically, was subjected to molecular identification. For cestode identification by polymerase chain reaction (PCR), general universal primer sets, characterizing the 28S–D3 (≈400 bp) and genus-specific primer sets of the cytochrome c oxidase subunit 1 (COI) (≈ 800 bp) gene regions, from the ribosomal and mitochondrial genomes, were used (Whiting et al., Reference Whiting, Carpenter, Wheeler and Ard1997; Foronda et al., Reference Foronda, López-González, Hernández, Haukisalmi and Feliu2011). Cosmopolitan nematodes of the Heligmonellidae (heligmonellids) and Oxyuroidea (oxyurids) were initially identified based on morphology, but, because these groups include numerous morphologically similar species (Durette-Desset & Digiani, Reference Durette-Desset and Digiani2012; Khalil et al., Reference Khalil, Lashein, Morsy and Abd El-Mottaleb2014), they were also subjected to molecular typing using primer sets characterizing the ribosomal 18S gene region (Holterman et al., Reference Holterman, van der Wurff, van den Elsen, van Megen, Bongers, Holovachov, Bakker and Helder2006). PCRs were carried out in a 25 μl volume consisting of 1 μl each of forward and reverse primers, 1 U EmeraldAmp® Taq polymerase (Takara, Shiga, Japan) and 3–4 μl DNA template. Amplified products were viewed with electrophoresis on a 1.5% agarose gel and purified using Roche PCR Product Purification Kit (Roche Diagnostics, Basel, Switzerland). Identities were confirmed by direct sequencing with the aforementioned primers.
Helminth molecular and phylogenetic analyses
DNA sequencing proceeded with a BigDye v. 3.1 terminator cycle-sequencing kit (Perkin–Elmer, Waltham, Massachusetts, USA), with each PCR primer. Samples were run on an ABI 3130 sequencer (Applied Biosystems, Foster City, California, USA) and sequence chromatograms were viewed and edited in Mega 5 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011) prior to performing BLAST nucleotide searches against the National Center for Biotechnology Information (NCBI) GenBank database. Helminth species identities were confirmed by phylogenetic analyses and included reference sequences with the maximum sequence similarities in the GenBank database. For each nematode and cestode alignment, the best-fit model of sequence evolution was selected using the Akaike Information Criterion (AICC) in jModelTest (Posada, Reference Posada2008). Phylogenies were inferred using maximum likelihood (ML) and neighbour-joining (NJ) trees, with nodal support assessed by 10,000 non-parametric bootstrap replications, also performed in Mega 5 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011), while Bayesian Inference (BI) tree analyses were performed with MrBayes v.2.1.3. and run over 10,000,000 generations.
Statistical analysis
The distributions of the parasite communities among the rodent hosts were quantified using mean intensity and prevalence of respective helminths (Bush et al., Reference Bush, Lafferty, Lotz, Shostak and Journal1997) and calculated in Quantitative Parasitology (web version) (Reiczigel & Rózsa, Reference Reiczigel and Rózsa2005). Chi-square tests for differences in infection prevalence among rodent species were calculated using Statistica v.12 (Statsoft Inc., Tulsa, Oklahoma, USA). To quantify diversity and abundance of the parasite communities among the rodent hosts, infracommunity parameters (Holmes & Price, Reference Holmes, Price, Anderson and Kikkawa1986), namely species richness (d), evenness, Berger–Parker index and Brillouin's index, were assessed, and were calculated using PAST v.3.12 (Hammer et al., Reference Hammer, Harper and Ryan2001).
Results
Helminth composition
Strobilar stages of the dwarf tapeworm, Hymenolepis nana (Hughes, Reference Hughes1941) were recovered from the SI of R. norvegicus and those of Inermicapsifer madagascariensis (Baylis, Reference Baylis1949; Collins, Reference Collins1972), were recovered from the SI of M. coucha and identified based on morphological criteria. The prevalence of these among the rodent species could not be assessed as all the scoleces could not be recovered and/or the strobilae were too deteriorated for a conclusive identification. Molecular typing of cestodes detected only the presence of the rat tapeworm, Hymenolepis diminuta, in R. rattus and R. norvegicus based on a 791 bp COI mRNA phylogeny. Sequences were deposited in GenBank (accession nos: KY462775–79) (see supplementary fig. S1a). Two taeniid metacestodes were recovered, namely strobilocerci of H. taeniaeformis (syn. Taenia taeniaeformis) (Loos-Frank, Reference Loos-Frank2000) from the liver parenchyma of R. rattus, R. norvegicus and R. tanezumi, and coenurostrobilocerci of H. parva (syn. Taenia parva) (Loos-Frank, Reference Loos-Frank2000) which were attached to the serosal surfaces of the abdominal cavity in M. coucha only.
The acanthocephalan, M. moniliformis (Petrochenko, Reference Petrochenko and Epstein1971) was recovered from the SI of R. rattus only. The nematode composition among the rodent species is summarized in table 1. A spiruroid, Mastophorus muris (Wertheim, Reference Wertheim1962; Chabaud, Reference Chabaud, Anderson, Chabaud and Willmott1975) was found in the SI and stomachs of all three Rattus species. The 18S rRNA phylogeny, comprising 1155 bp (supplementary figure S1b) and submitted to GenBank (accession nos: KY462823–29), confirmed the morphological identification of N. brasiliensis (Tubangui, Reference Tubangui1931; Durette-Desset, Reference Durette-Desset, Anderson and Chabaud1983) recovered from the SI of R. norvegicus, S. muris (Petter & Quentin, Reference Petter, Quentin, Anderson, Chabaud and Willmott1976) from the caeca and colons of R. tanezumi, A. tetraptera from R. rattus and R. tanezumi and S. obvelata (Petter & Quentin, Reference Petter, Quentin, Anderson, Chabaud and Willmott1976) from M. coucha. Syphacia muris was also present in R. rattus, based on mounted specimens, but a clear sequence could not be obtained, possibly due to mixture of A. tetraptera in the same host sample.
Table 1. Total and proportional abundance (in parentheses) of helminths* in four synanthropic murid rodent species in Gauteng Province, South Africa. Oxyurids include both Aspiculuris teraptera and Syphacia muris species.
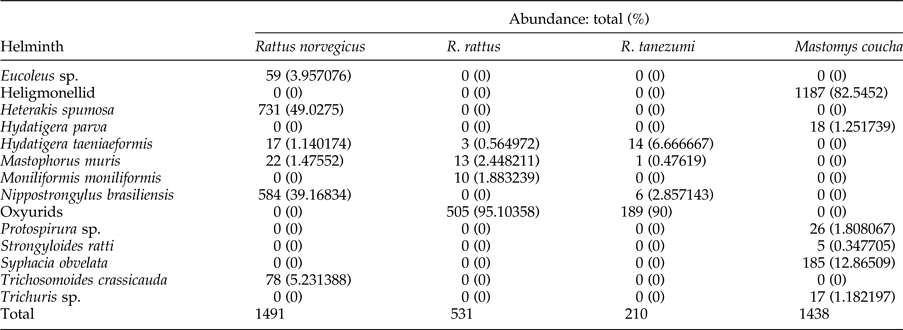
* Excludes strobilar stages of cestodes.
Additionally, molecular typing confirmed the presence of a heligmonellid in the SI of M. coucha, closely related to N. brasiliensis based on the 18S rRNA phylogeny. This heligmonellid had a conspicuous hypertrophied left ala present in both sexes. Body length ranged between 3.60 and 5.18 mm for females and between 2.47 and 2.97 mm for males. Eggs oval 54.37–64.36 (60.31) × 29.36–42.77 (34.77) μm and spicules of nearly equal lengths, with right spicule 353.69–422.74 (401.11) μm and left spicule 374.02–429.55 (416.84) μm.
The heligmonellid and oxyurid nematodes had the highest mean intensity of infection (>8) (table 2). Trichosomoides crassicauda (Thomas, Reference Thomas1924), recovered from the bladder, H. spumosa (Hartwich, Reference Hartwich, Anderson, Chabaud and Willmott1974) recovered from SI and caecum, and Eucoleus sp. (Gibbons, Reference Gibbons2010) recovered from the stomach mucosa were only found in R. norvegicus. Trichuris sp., recovered from the caecum, Strongyloides ratti from the SI, and a spiruroid nematode species, Protospirura sp. (Yamaguti, Reference Yamaguti1961) (fig. 2), recovered from the stomach and SI, were found in M. coucha only. The 18S rRNA sequence of Protospirura sp. was deposited in GenBank under accession no. KY462830.

Fig. 2. Protospirura sp.: (a) cephalic end, (b) submedian lobe with denticles, (c) large lateral lobe with denticles and (d) ventral side of male caudal end showing spicules.
Table 2. Mean infection intensity and prevalence of each helminth* sampled from synanthropic Rattus norvegicus, R. rattus, R. tanezumi and Mastomys coucha in urban areas of Gauteng Province, South Africa, with lower and upper CI limits displayed in parentheses. Oxyurids include both Aspiculuris teraptera and Syphacia muris species.
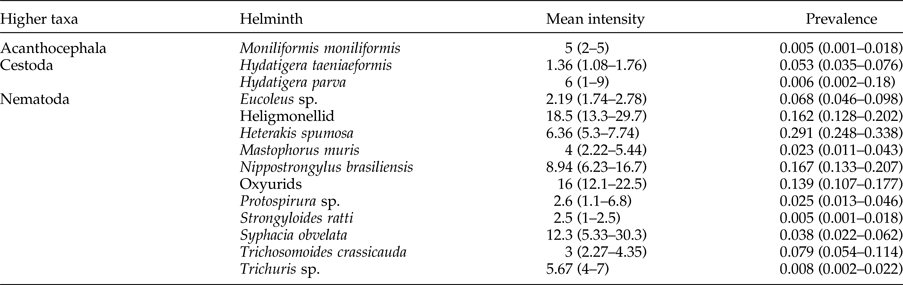
* Excludes strobilar stages of cestodes.
Helminth infracommunity structure
Apart from strobilar stages of cestodes which could not be counted, 3670 nematode, acanthocephalan and metacestode specimens were recovered from 395 rodents. Overall gastrointestinal helminth prevalence was high (≥70%) and helminth infracommunities were distinct (P < 0.05) for all rodent species, namely R. norvegicus (n = 240), R. rattus (n = 40), R. tanezumi (n = 31) and M. coucha (n = 84) (table 3). Within rodent species, comparisons between nematode and cestode prevalence revealed that R. norvegicus (χ2 = 21.49; df = 1; n = 228; P < 0.05) and M. coucha (χ2 = 47.10; df = 1; n = 79; P < 0.05) displayed higher gastrointestinal nematode than cestode prevalence, while within R. rattus and R. tanezumi these differences were not statistically significant (fig. 3).
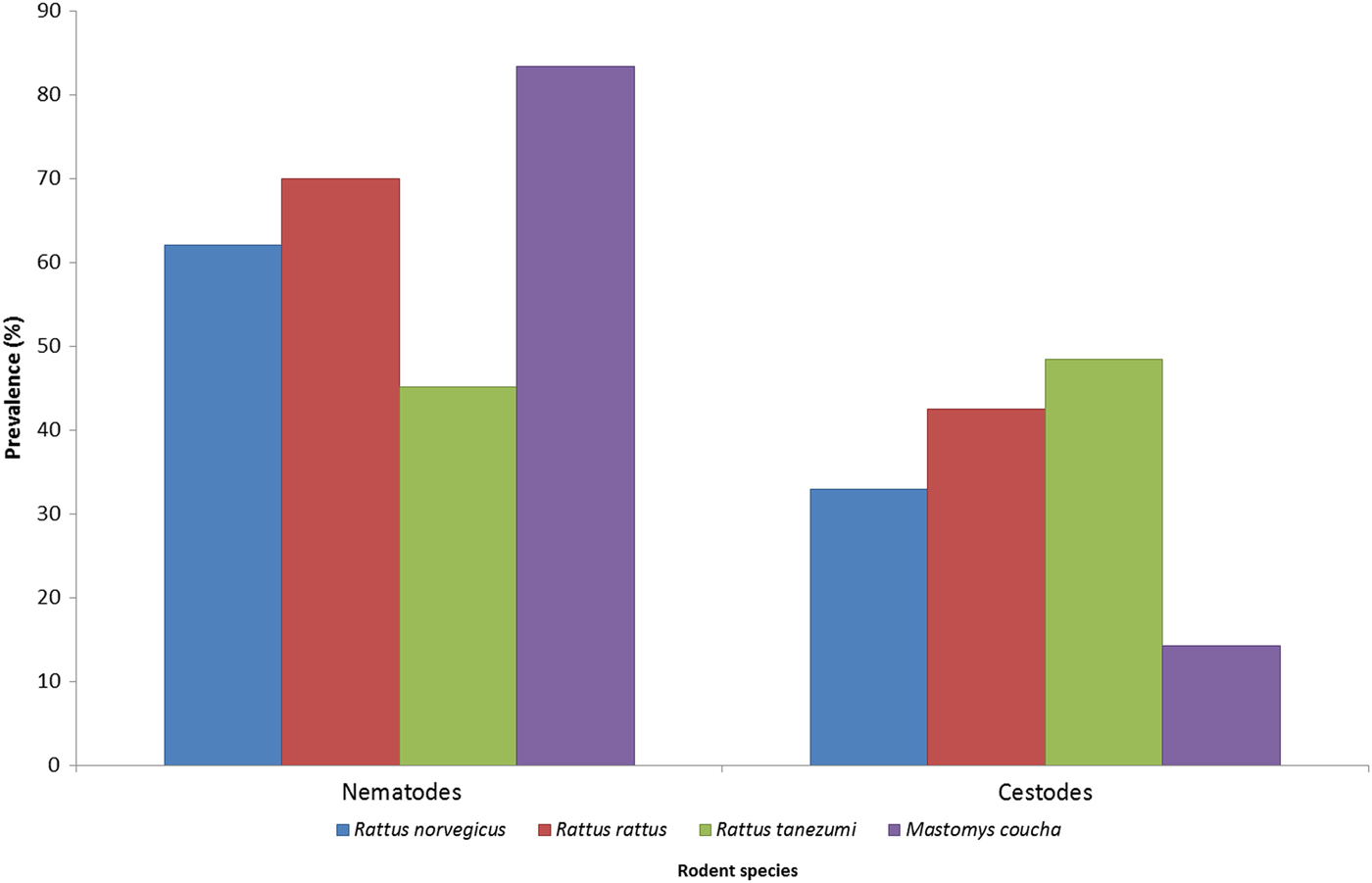
Fig. 3. Differences in nematode and cestode prevalence (%) within synanthropic Rattus norvegicus, R. rattus, R. tanezumi and Mastomys coucha in urban areas of Gauteng Province, South Africa.
Table 3. Infracommunity parameters of the helminth fauna* of synanthropic indigenous and invasive murid rodents in urban Gauteng Province with lower and upper CI limits displayed in parentheses.
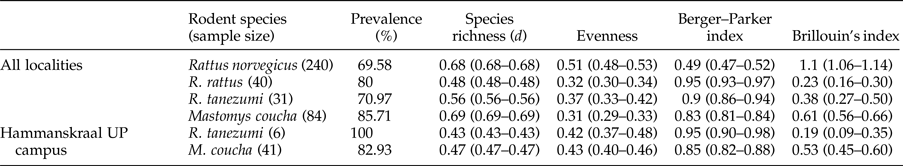
* Excludes strobilar stages of cestodes.
One locality each in Hammanskraal and Pretoria where Rattus species and M. coucha co-occurred contained sufficient sample sizes for comparative analyses between the invasive and indigenous rodents. At the Hammanskraal locality, R. tanezumi (n = 6) had similar total helminth and nematode prevalence to M. coucha (n = 41) while the cestode prevalence of R. tanezumi was significantly higher than that of M. coucha (χ2 = 12.00; df = 1; n = 47; P < 0.05). Similarly, at the Pretoria locality R. rattus (n = 32) and M. coucha (n = 18) had similar total helminth and nematode prevalence yet the cestode prevalence of R. rattus was statistically significantly higher than that of M. coucha (χ2 = 4.52; df = 1; n = 50; P < 0.05). Diversity indices at the Hammanskraal locality (table 3) showed that R. tanezumi had lower alpha diversity (Brillouin's index) than M. coucha, yet both species have similar species richness (d) and are predominantly infected by one or more helminth species (Berger–Parker index).
Discussion
The strobilar stage of I. madagascariensis has been recorded from islands of the Indian Ocean, Cuba, Venezuela, Thailand and the Philippines, and widely from Africa, and is typically a parasite of small mammals (Frean & Dini, Reference Frean and Dini2004). This constitutes the first host record from M. coucha as the cestode species has already been reported from the congener, M. natalensis (Collins, Reference Collins1972). The species has zoonotic implications, and infections in humans have been reported from Cuba, Kenya (Baylis, Reference Baylis1949), Zambia (Hira, Reference Hira1975) and South Africa (Frean & Dini, Reference Frean and Dini2004). Case reports of Frean & Dini (Reference Frean and Dini2004) were from two children in the Roodepoort and Benoni areas, respectively, forming part of the greater Johannesburg area, Gauteng Province, South Africa. The strobilar stage of H. nana, the dwarf tapeworm, is found in sylvatic and laboratory rodents, simian primates and humans (Soulsby & Mönnig, Reference Soulsby and Mönnig1982). Hymenolepis nana is the most common tapeworm infection in humans, as a result of autoinfection and humans being the primary source of infection (Soulsby & Mönnig, Reference Soulsby and Mönnig1982; Beaver et al., Reference Beaver, Rodney Clifton, Eddie Wayne and Craig1984). There are, however, at least two human prevalence reports from children in Gauteng Province (Kark & Le Riche, Reference Kark and Le Riche1944; Walker et al., Reference Walker, Dini, Walker and Frean2000). Hymenolepis diminuta, the rat tapeworm, is a common parasite of synanthropic murid rodents but is infrequently recovered from humans (Beaver et al., Reference Beaver, Rodney Clifton, Eddie Wayne and Craig1984). It has been recovered in human stool samples in South Africa (Fantham & Porter, Reference Fantham and Porter1936) but the exact localities cannot be deduced from the latter publication.
The presence of metacestodes of H. taeniaeformis and H. parva species confirms the role of synanthropic rodents as intermediate hosts. Hydatigera taeniaeformis has a cosmopolitan distribution, and is a common taeniid of the domestic cat (Abuladze, Reference Abuladze and Skrjabin1970; Loos-Frank, Reference Loos-Frank2000). It has recently been discovered to include morphologically cryptic lineages (Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016). Apart from the domestic cat, the definitive host range is broad and includes wild felids, viverrids, genets, mustelids and wild canids (Abuladze, Reference Abuladze and Skrjabin1970; Loos-Frank, Reference Loos-Frank2000). Since the metacestode was recovered from invasive Rattus species, it is likely to form part of the H. taeniaeformis sensu stricto clade, which appears to be limited to the liver parenchyma of murid rodents acting as intermediate hosts (Lavikainen et al., Reference Lavikainen, Iwaki, Haukisalmi, Konyaev, Casiraghi, Dokuchaev, Galimberti, Halajian, Henttonen, Ichikawa-Seki, Itagaki, Krivopalov, Meri, Morand, Näreaho, Olsson, Ribas, Terefe and Nakao2016). A prevalence of 5.3% (n = 311) and mean intensity of 1.36 (table 2) of H. taeniaeformis in Rattus species from the present study was comparable to that of a study in Mexico, where Rattus species are also invasive (Panti-May et al., Reference Panti-May, Hernández-Betancourt, Rodríguez-Vivas and Robles2013). While H. taeniaeformis infection is common in R. norvegicus and R. rattus (Abuladze, Reference Abuladze and Skrjabin1970), prevalence in R. tanezumi outside the native range is also confirmed from the present study. Since only two cases of infections with strobilocerci have been reported in humans, H. taeniaeformis is not regarded as a public health concern (Sterba & Barus, Reference Sterba and Barus1976). The strobilar stage of H. parva has been reported in some wild felids (Felis silvestris silvestris, F. s. lybica), genets (Genetta spp.) and the striped polecat (Ictonyx striatus) from Africa and Europe (Jones & Pybus, Reference Jones, Pybus, Samuel, Kocan, Pybus and Davis2001). The intermediate host range of the polycephalic metacestode of H. parva, also referred to as a coenurostrobilocercus, appears to be restricted to murid rodents (Mas-Coma & Feliu, Reference Mas-Coma and Feliu1977; Loos-Frank, Reference Loos-Frank2000) and has been recovered in the present study from M. coucha, which is a new intermediate host record. Other known intermediate hosts are the congener M. natalensis and other rodent species, Mus musculus, M. minutoides, Apodemus sylvaticus, Aethomys chrysophilus, Micaelamys namaquensis (Skinner & Chimimba, Reference Skinner and Chimimba2005), Thallomys paedulcus and Rhabdomys pumilio (Jones & Pybus, Reference Jones, Pybus, Samuel, Kocan, Pybus and Davis2001).
The acanthocephalan Moniliformis moniliformis, is a cosmopolitan and common parasite of R. rattus and R. norvegicus, with cockroaches acting as intermediate hosts (Schmidt, Reference Schmidt1971). A recent study in Kwa-Zulu Natal Province, South Africa reported it from R. norvegicus only (Archer et al., Reference Archer, Appleton, Mukaratirwa, Lamb and Schoeman2017), while the present study found it only in R. rattus. Human infections are rare but may present with symptoms of gastrointestinal disease and have been reported from several countries (Schmidt, Reference Schmidt1971; Andres et al., Reference Andres, English and Greiner2014).
All nematodes recovered in the study were rodent-specific and non-zoonotic. Nippostrongylus brasiliensis was first described from R. norvegicus in Brazil and is believed to have spread throughout the world with invasive rodents (Mawson, Reference Mawson1961; Smales, Reference Smales1997). Syphacia muris was first described from a laboratory rat while S. obvelata, common in laboratory mice, was long used as a model for pinworm infection for chemotherapeutic studies against human enterobiasis (Hussey, Reference Hussey1957). As a result S. obvelata may have spread throughout the world with laboratory rodents, eventually spreading to indigenous rodents such as M. coucha. The unidentified heligmonellid recovered from only M. coucha might belong to the genus Heligmonina, based on the presence of a hypertrophied left ala (Durette-Desset et al., Reference Durette-Desset, Brouat, Diouf and Duplantier2008), but other more distinguishing characteristics need to be examined for a conclusive identification. Mastophorus muris was present in all three Rattus species. The nematode has a cosmopolitan distribution, with cockroaches acting as intermediate hosts (Verster & Brooker, Reference Verster and Brooker1970), and its presence is therefore not unexpected in urban areas. Recorded from indigenous wild hosts in only Argentina, Australia and Madagascar (Rojas & Digiani, Reference Rojas and Digiani2003), it appears to infect mostly invasive, synanthropic rodents (Smales, Reference Smales1997). Protospirura sp. was recovered from indigenous M. coucha only and may represent a new species, but further work is needed to elucidate its taxonomy. So far, Protospirura chabaudi is the only species in the genus to be described from Africa – in R. rattus from the Congo (now the Democratic Republic of Congo (DRC)) (Vuylsteke, Reference Vuylsteke1964). Protospirura chabaudi lacks denticles on the large lateral lobe of the pseudolabia (Vuylsteke, Reference Vuylsteke1964), while the Protospirura sp. in this study clearly contains two denticles (fig. 2a–c) on each lateral lobe of the pseudolabia. With the exception of P. muricola, which has various definitive hosts and an almost global distribution (Smales et al., Reference Smales, Harris and Behnke2009), Protospirura species appear to be geographically restricted. An example is P. siamensis, which has been recorded from South-East Asia only (Ribas et al., Reference Ribas, Veciana, Chaisiri and Morand2012), although recovered from synanthropic, invasive R. tanezumi, which has an almost global distribution (Kosoy et al., Reference Kosoy, Khlyap, Cosson and Morand2015). Protospirura muricola has spicules of similar length (Smales et al., Reference Smales, Harris and Behnke2009), while the Protospirura sp. recovered in this study has spicules of markedly unequal lengths (fig. 2d). Surprisingly, P. muricola was not recorded in the present study, although it has been reported from several small mammal taxa, including M. coucha, in the DRC and appears to be widespread in Africa (Smales et al., Reference Smales, Harris and Behnke2009).
In conclusion, synanthropic, invasive Rattus species, when compared to synanthropic, indigenous M. coucha, pose a higher risk to public health in urban areas of Gauteng Province, South Africa, as they harbour helminths with zoonotic implications at a higher prevalence, which may become more relevant under poor hygienic conditions. Where R. tanezumi and M. coucha co-occurred, R. tanezumi showed lower helminth diversity and equal species richness to M. coucha. Nevertheless, both synanthropic invasive and indigenous rodents act as both definitive and intermediate hosts to helminths that can infect humans and domestic animals. The presence of a cosmopolitan nematode in an indigenous rodent that originated from laboratory or wild, globally invasive rodent hosts demonstrates the co-invasive potential of helminth parasites. Furthermore, molecular techniques were useful in supplementing and confirming morphological identities of the identified helminths and revealing the phylogenetic relationships of unidentified helminths, but would require additional work, i.e. more gene regions and larger, more comprehensive databases for genetic sequence comparison, to make further inferences. It is anticipated that the genetic sequences resulting from this study would supplement databases and be helpful for future work.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X17000761
Acknowledgements
The authors would like to express their gratitude to members of the public and field volunteers for assistance in sample collection; the staff of the Helminthology Laboratory, Department of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria for their guidance in the identification of helminth specimens and use of the facility; and the Laboratory for Microscopy and Microanalysis at the University of Pretoria for images taken with the scanning electron microscope.
Financial support
The study was supported by the National Research Foundation (NRF) DNA sequencing facility of the University of Pretoria (NRF RISP grant 2001/2012; UID: 78566) and the DST-NRF Centre of Excellence for Invasion Biology (CIB).
Conflict of interest
None.
Ethical standards
Rodents were sampled with permission from the Gauteng Directorate of Nature Conservation (Permit number: CPF6 0032) and processed with approval granted by the Animal Ethics Committee, University of Pretoria (Ethics clearance number: EC025-10).








