Introduction
Elasmobranch fishes, such as rays and sharks, are apex predators in marine ecosystems and are the definitive hosts for many parasite taxa, including tapeworms. Adult tapeworms generally inhabit the spiral intestine and are the most common parasites of sharks and rays (Caira & Healy, Reference Caira, Healy, Musick, Carrier and Heithaus2004). These tapeworms are transmitted trophically, i.e. via the food chain, but their life cycle is poorly known (Williams & Jones, Reference Williams and Jones1994). Indeed, only a handful of life cycles, out of the 1000+ species of tapeworms infecting elasmobranch fishes, are known (Caira & Reyda, Reference Caira, Reyda and Rohde2005). Unfortunately, with the exception of trypanorhynch tapeworms, few host–parasite checklists include elasmobranch tapeworm larvae in teleosts beyond the convenient label of Scolex pleuronectis or S. polymorphus, which lumps together the plerocercoids (sensu Chervy, Reference Chervy2002) of many different species of marine tapeworms, such as Tetraphyllidea, Rhinebothriidea and Phyllobothriidea. Furthermore, it is almost impossible to make a link between a larval and adult cestode based on morphology alone (e.g. Aznar et al., Reference Aznar, Agusti, Littlewood, Raga and Olson2007; Jensen & Bullard, Reference Jensen and Bullard2010). Thus, the use of molecular tools to identify these tapeworm larvae seems a requirement, even in the light of the paucity of molecular data in the literature, especially regarding larval records.
Recently, the advent of molecular tools has enabled the characterization of adult cestodes in different endangered elasmobranch fishes (Poulin & Keeney, Reference Poulin and Keeney2008; Randhawa, Reference Randhawa2011; Randhawa & Brickle, Reference Randhawa and Brickle2011) and an increasing number of studies has been undertaken on their larvae since the method has proven efficient (Aznar et al., Reference Aznar, Agusti, Littlewood, Raga and Olson2007; Randhawa et al., Reference Randhawa, Saunders and Burt2007; Jensen & Bullard, Reference Jensen and Bullard2010). The decline of apex predators in our oceans could have a great impact on marine ecosystems, and especially on the abundance of populations of prey species (e.g. Myers et al., Reference Myers, Baum, Shepherd, Powers and Peterson2007). These prey species can be important vehicles for parasite transmission, thus it is important to understand the trophic links between elasmobranch fish and the teleosts they prey upon. Host–parasite checklists are important sources of ecological information and can provide insights into life cycles of parasites identified as larvae in these checklists, yet many of these are far from comprehensive (Poulin et al., Reference Poulin, Besson, Morin and Randhawa2016a). There is a regularly updated checklist of New Zealand fishes and their parasites (Hine et al., Reference Hine, Jones and Diggles2000). However, this one is also incomplete (Poulin et al., Reference Poulin, Besson, Morin and Randhawa2016a) and gaps remain to be filled before fully understanding the associations between hosts and parasites, and the identification of trophic links between the different species leading to successful parasite transmission.
Many fish species, comprising a wide array of orders, including flatfish (Pleuronectiformes), are possible prey for elasmobranch fishes (Cortés, Reference Cortés1999). Pleuronectiformes are demersal fishes, meaning that they are mainly bottom-dwelling and feed on small invertebrates, such as polychaetes and crustaceans. However, the checklist of parasites of New Zealand fishes (Hine et al., Reference Hine, Jones and Diggles2000) reports that Pleuronectiformes are very poor in parasites and that none of them host tapeworms. This might be a result of poor sampling effort (Walther et al., Reference Walther, Cotgreave, Price, Gregory and Clayton1995), either in the original records used to compile the checklist or underrepresentation of regional parasitological studies for this order of fish. As a result, a parasitological survey of a commercial species of pleuronectiform was undertaken to determine whether the depauperate parasite community reported in this checklist (Hine et al., Reference Hine, Jones and Diggles2000) is underestimated, and whether, indeed, tapeworms are absent from this parasite community. We focused on the New Zealand sole (Peltorhamphus novaezeelandiae) as it is a possible prey of apex predators, such as sharks (Cortés, Reference Cortés1999). Furthermore, since it is a commercially exploited species in New Zealand, it is assumed that its biology and ecology (including parasites) have been relatively well studied relative to those of non-commercial species.
According to the checklist of Hine et al. (Reference Hine, Jones and Diggles2000), only five species of macroparasites have been reported from this fish, including the monogenean (fluke) Neobivagina pelotretis Dillon & Hargis, 1965, the nematodes (round worms) Cucullanellus sp. and Hysterothylacium sp. (larvae and adults), and the acanthocephalan (thorny-headed worm) Aspersentis peltorhamphi (Baylis, 1944). In this study, different life stages of the same species of helminths were treated as different taxa. For instance, adult and larval Hysterothylacium aduncum (Rudolphi, 1802) nematodes were recovered from hosts examined in this study and treated as different taxa, as per most host–parasite checklists (e.g. Hine et al., Reference Hine, Jones and Diggles2000). In other New Zealand flatfish, monogeneans, trematodes, nematodes and acanthocephalans are recorded as the main macroparasites, but no tapeworm is associated with pleuronectiform fishes, with the exception of an unpublished record of a eutetrarhynchid larva from the yellowbelly flounder Rhombosolea leporina (Hine et al., Reference Hine, Jones and Diggles2000). Based on unpublished parasite surveys of other teleost species of commercial importance in New Zealand (Randhawa, pers. obs.), we expected to find at least two or three species in addition to those reported in Hine et al. (Reference Hine, Jones and Diggles2000) and, more specifically, previously unreported tapeworm larvae, many of which would use elasmobranchs as their definitive hosts. Here, we focus on a parasitological survey of the New Zealand sole in order to update the checklist of Hine et al. (Reference Hine, Jones and Diggles2000) for this fish species and to gain insights into the trophic links between this fish species and apex predators, such as elasmobranchs, that could lead to the successful transmission of these parasites.
Materials and methods
Sample collections
Fish were collected by commercial fishermen of the Echo F/V landing in Port Chalmers, Otago, South Island, New Zealand. Fish were brought to the Botany Department at the University of Otago, where they were kept refrigerated until dissected. We dissected 28 soles fished off Kaka point (near Nuggets; approximately 46.5°S, 170.3°E), in the Catlins, South Islands, New Zealand, to a maximum of approximately 80 m depth. All dissections were performed within 4 days after fishing, in order to keep soles fresh and to collect living parasites. The outer surface, mouth and gills of each fish were examined. After opening the body cavity, we collected internal organs and kept them in fish saline (2 parts seawater:9 parts distilled water) to keep parasites alive. Each fish was filleted and the flesh was examined by candling for any encapsulated parasites. Parasites were collected and fixed either in ethanol (100%) for molecular analysis or in hot formalin (4%) for morphological analysis.
Molecular analyses
Genomic DNA was extracted from three individuals for each parasite species from each of the different organs in different fish using standard protocols (Devlin et al., Reference Devlin, Diamond and Saunders2004). Different gene regions for respective parasite groups were amplified via the polymerase chain reaction (PCR) using known primers. For tapeworms and one of the trematodes, the region of the large subunit of ribosomal DNA (LSU) known as the X-region (Harper & Saunders, Reference Harper and Saunders2001) was amplified using primers T01N and T13N (Harper & Saunders, Reference Harper and Saunders2001), using protocols described in Randhawa et al. (Reference Randhawa, Saunders, Scott and Burt2008). For nematodes, a portion of the internal transcribed spacer (ITS) region of the ribosomal cistron and cytochrome oxidase subunit II (COX2) gene were amplified using primers 93 and 94 (Nadler et al., Reference Nadler, D'Amelio, Dailey, Paggi, Siu and Sakanari2005), and 210 and 211 (Nadler & Hudspeth, Reference Nadler and Hudspeth2000), respectively, using PCR protocols described in the respective papers. In nematodes, the ITS region alone may not be sufficient for species identification, hence the use of two genes to increase the likelihood of a specific identification. For acanthocephalans, we targeted the cytochrome oxidase subunit I (COI) marker using primers LCO1490a and HCO2198, as described in García-Varela & Pérez-Ponce de León (Reference García-Varela and Pérez-Ponce de León2008). All PCRs were performed using BioLine DNA polymerase (Total Lab Systems, Auckland, New Zealand). Amplicons were purified using ExoSap PCR pre-sequencing purification kits (GE Healthcare, Auckland, New Zealand). A representative subset of PCR products was sent to Macrogen in Korea to be cycle-sequenced bi-directionally using the PCR primers on a 96-capillary ABI 3730XL DNA analyser (Applied Biosystems, Foster City, California, USA), except for the tapeworm LSUs, which were cycle-sequenced bi-directionally using the PCR primers in addition to internal primers T16 and T30 (Harper & Saunders, Reference Harper and Saunders2001). Sequence data were edited using Sequencher 5.4™ (GeneCodes, Ann Arbor, Michigan, USA) and screened using BLASTn (McGinnis & Madden, Reference McGinnis and Madden2004). Sequences are available from GenBank under accession numbers KY909254–KY909274. Unused portions of individual worms sent for sequencing were preserved in 95% ethanol to serve as hologenophores (sensu Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) and deposited with the Otago Museum, Dunedin, New Zealand (OMNZ; IV85644–IV85652) and the New Brunswick (NB) Museum, Saint John, NB, Canada (NBM-010547–NBM-010554). Additional vouchers were deposited with the New Brunswick Museum (NBM-010450–NBM-010546).
Statistical analyses
The species accumulation and saturation curves were estimated using the specpool function in the vegan package (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2016) implemented in the program R (R Development Core Team, 2015). The Morisita's index of overlap between species was calculated using the function niche.overlap with morisita as the designated method in the spaa package (Zhang, Reference Zhang2016) implemented in the program R. This index takes into account the relative abundance of each species, with values near ‘0’ indicating little overlap and values tending towards ‘1’ corresponding to a high degree of overlap between species, and is not sensitive to sample size (Morisita, Reference Morisita1959).
Results
Of the 28 fish we dissected, 13 were males and 15 were females, ranging in size (total length) from 26.5 to 38.0 cm. All fish were infected with at least three different parasite species. A total of 13 different parasite species, including two taxa we were unable to identify, were present in the fish (table 1). Parasites were recovered from the digestive tract, liver, gonads and mesenteries. However, no parasites were recovered from the mouth, eyes, gills, body surface and flesh. On average, each fish was infected by 48.64 parasites (±25.0; range 10–113 invidivual parasites) from 4.14 species (±1.08; range 3–6 species). The species accumulation curve shows that our sampling effort was good enough to identify most macroparasites present in the sole (fig. 1), with the species pool being estimated at 14 species.
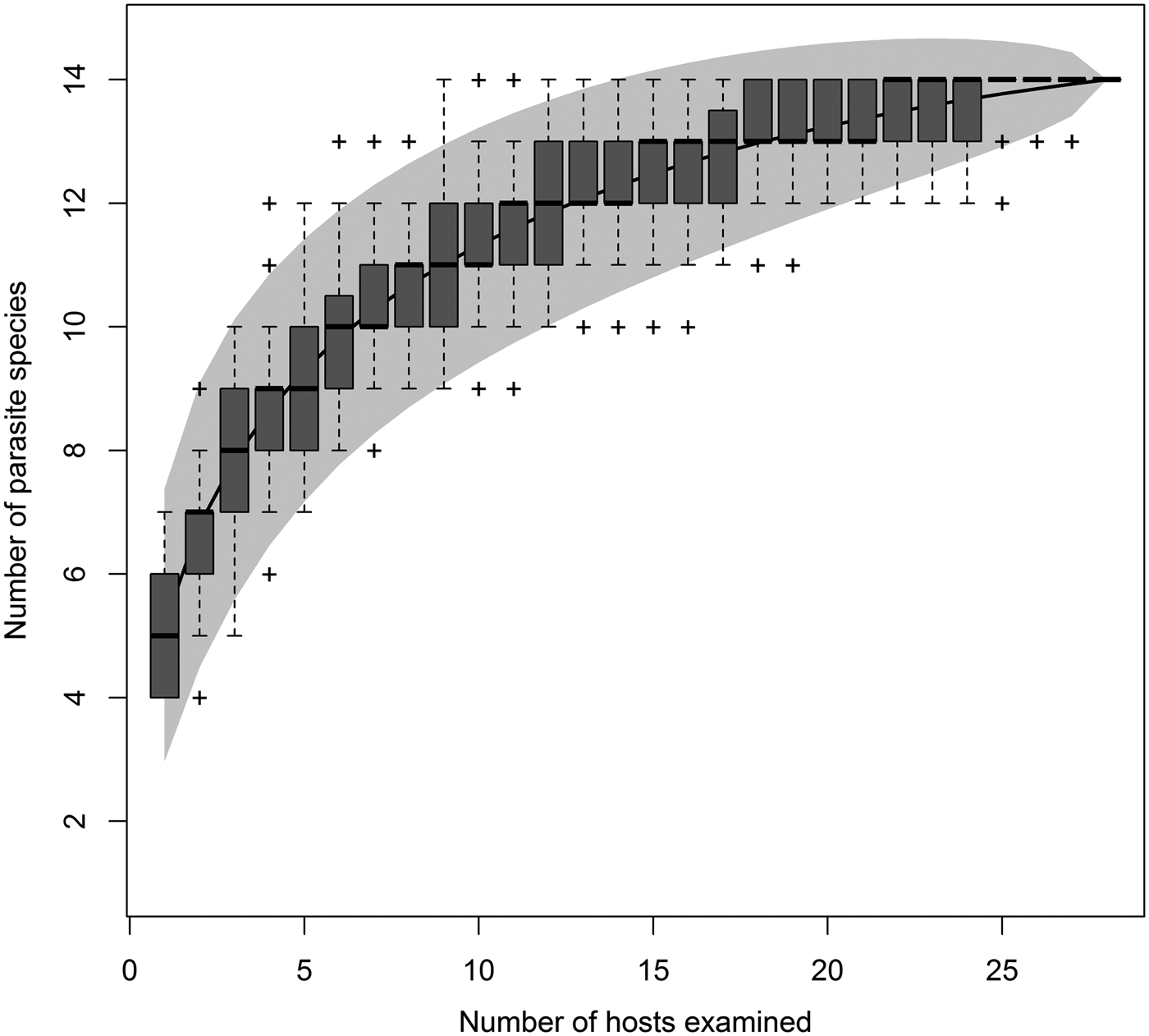
Fig. 1. Species accumulation curve describing the number of parasite species recovered as a function of number of New Zealand soles examined. The grey shaded area corresponds to the 95% confidence interval. In this survey, 13 of the 14 predicted species were recovered.
Table 1. Parasite community of the New Zealand sole (Peltorhamphus novaezeelandiae) (N = 28) from waters off Kaka point (near Nuggets), in the Catlins, New Zealand.
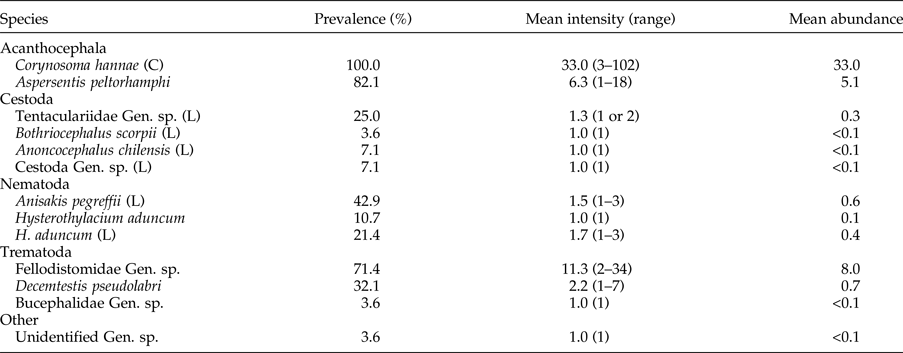
C, cystacanth; L, larvae.
Not only was the number of parasite species encountered higher than expected, but the diversity was also greater. In this study, we recovered parasites assignable to at least 10 different families: 2 families of acanthocephalans, 2 families of nematodes, 3 families of trematodes and at least 3 families of cestodes. Table 1 shows the abundance of each parasite species found in the 28 soles. As per previous studies (see Hine et al., Reference Hine, Jones and Diggles2000), we recovered the acanthocephalan A. peltorhamphi and nematodes from the genus Hysterothylacium (both larvae and adults). However, we also found previously unreported species: 1 nematode, 3 trematodes (flukes) and several cestodes at larval stages (table 1). The identities of the following previously unreported taxa were confirmed by molecular identification. The nematode was assignable to Anisakis pegreffii Campana-Rouget & Biocca, 1955, with 601 bp quality sequence length for the COX2 and 99.2 to 99.8% sequence similarity with A. pegreffii GenBank accession numbers AB517563, KC809997 and KF972438. One of the trematodes was assignable to the Bucephalidae, with 1366 bp quality sequence length for the LSU and 95.2% sequence similarity with Dollfustrema hefeiensis Liu, 1999 (KT273386), whereas the trematodes assignable to Decemtestis pseudolabri Manter, 1954 and the fellodistomid were identified morphologically. The majority of tapeworm larvae were assignable to the Tentaculariidae, i.e. Heteronybelinia yamaguti (Dollfus, 1960) (1024 bp quality sequence length; 97.1% sequence similarity with FJ572932), Kotorella pronosoma (Stossich, 1901) (1428 bp quality sequence length; 97.5% sequence similarity with DQ642788), Nybelinia indica Chandra, 1986 (1253 and 1265 bp quality sequence length; 97.1 and 97.3% sequence similarity with FJ572930) and Tentacularia coryphaenae Bosc, 1802 (1835 bp quality sequence length; 95.8% sequence similarity with AF286976). Two tapeworm larvae were assignable to Anoncocephalus chilensis (Riggenbach, 1896) (1768 bp quality sequence length; 100.0% sequence similarity with DQ925320) and one to Bothriocephalus scorpii (Müller, 1776) (1799 bp quality sequence length; 99.0% sequence similarity with AF286942). PCRs failed for a further two parasites; one was clearly a larval tapeworm, whereas the other remains unidentified at the class level. Furthermore, with the use of DNA sequence data we have been able to provide a species identification to nematodes previously reported as Hysterothylacium sp. to H. aduncum (both larvae and adults). The quality sequence length was 1009 bp for the ITS and 98.4% sequence similarity with H. aduncum GenBank accession number JQ934881. We also discovered larval acanthocephalans (cystacanths) assignable to Corynosoma hannae Zdzitowiecki, 1984 in all the soles we dissected. We provided samples to Hernandez-Orts et al. (Reference Hernández-Orts, Smales, Pinacho-Pinacho, García-Varela and Presswell2017) who generated sequence data (KX957726). Overall, acanthocephalans were the most commonly recovered parasites from our sampled fish, both numerically and in terms of overall prevalence. Except for the unidentified parasite and cestode larva, bucephalid trematode, larval B. scorpii and A. chilensis, all the other eight species were found in prevalences above 10% and some, such as both acanthocephalan species and fellodistomid trematodes, were highly prevalent in this sole (over 70%) (table 1).
The effect of fish total length on total parasite abundance was assessed using generalized linear models with quasipoisson error to accommodate the overdispersed and discrete nature of the data, and revealed no significant relationship (P = 0.707; t = −0.38, df = 27). The same was true when partitioning the data according to sex (P = 0.587; t = 0.56, df = 12 and P = 0.153; t = −1.52, df = 14 for males and females, respectively). The same analyses were repeated for individual parasite taxa and the abundance of only two were affected by host length. These were A. pegreffii (P = 0.012; t = 2.71, df = 27 and P = 0.026; t = −2.58, df = 12 for the whole population and in male fish, respectively) and B. scorpii larvae (P = 0.019; t = 2.68, df = 14 in female fish only). The effect of fish total length on species richness (defined as the total number of parasite species infecting a host individual) was assessed using generalized linear models with Poisson error to accommodate our counts, and revealed no significant relationship (P = 0.786; z = −0.27, df = 27). The same was true when partitioning the data according to sex (P = 0.887; z = −0.14, df = 12 and P = 0.619; t = −0.50, df = 14 for males and females, respectively).
The three most prevalent and abundant taxa, i.e. C. hannae, A. peltorhamphi and the fellodistomid trematode, had the highest degree of overlap, with A. peltorhamphi (0.63), C. hannae (0.63) and A. pegreffii (0.61), respectively (table 2).
Table 2. Summary of niche overlap between parasite species of the New Zealand sole (Peltorhamphus novaezeelandiae) (N = 28) from waters off Kaka point (near Nuggets), in the Catlins, New Zealand. Values correspond to Morisita's index of overlap, with values trending towards ‘0’ corresponding to little niche overlap and values tending towards ‘1’ corresponding to high degree of niche overlap between species.
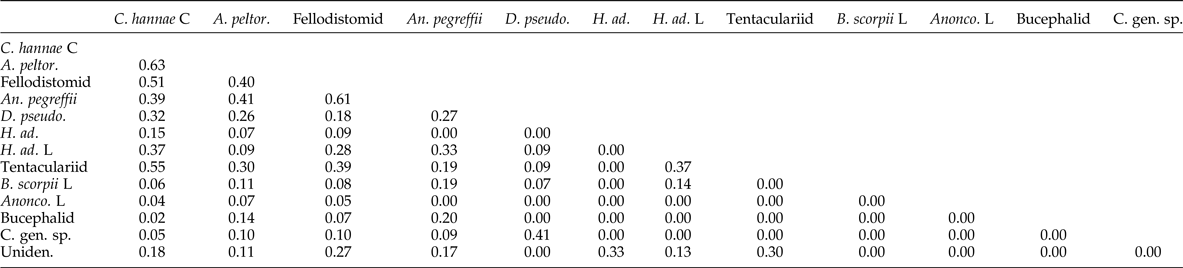
C, cystacanth; L, larva; C. hannae, Corynosoma hannae; A. peltor., Aspersentis peltorhamphi; Fellodistomid, fellodistomid trematode; An. pegreffii, Anisakis pegreffii; D. pseudo., Dicemtestis pseudolabri; H. ad., Hysterothylacium aduncum adult; H. ad. L, Hysterothylacium aduncum larvae; Tentaculariid, tentaculariid cestode; B. scorpii L, Bothriocephalus scorpii larvae; Anonco. L, Anoncocephalus chilensis larva; Bucephalid, Bucephalid trematode; C. gen. sp., unidentified cestode larva; Uniden., unidentified parasite.
Discussion
The present survey suggests that all parasites of New Zealand (NZ) sole were not discovered in previous studies. Considering that the checklist is only based on previous papers describing parasites in different fish, it suggests that some specimens might have gone undetected or been discarded if they were of no interest for research. When looking at the literature it appears that each family of parasites reported in the checklist of Hine et al. (Reference Hine, Jones and Diggles2000) has been reported in this fish from a single paper. Therefore, gaps in the checklist might be due to a lack of expertise or interest in some classes of parasites, such as trematodes and cestodes. For instance, it is somewhat surprising that a trematode species with 82% prevalence in the present survey was not detected in previous studies. The discovery of larval cestodes, adult trematodes and new records of nematodes confirms the point highlighted by Poulin et al. (Reference Poulin, Besson, Morin and Randhawa2016a) that there is still a lot to be done to update checklists. As the checklist of Hine et al. (Reference Hine, Jones and Diggles2000) is not complete, it is almost impossible to use this resource to assess parasite richness across host species and identify the different trophic links as potential routes for parasite transmission.
The NZ rough skate (Dipturus nasutus) is the only skate species present in waters surrounding the South Island of NZ and it is a host to many tapeworms (Hine et al., Reference Hine, Jones and Diggles2000): Acanthobothrium filicolle (Zschokke, 1887) and A. wedli Robinson, 1959 (Order Onchoproteocephalidea); Clydonobothrium elegantissimum (Lönnberg, 1889), C. leioformum Alexander, 1963 and Echeneibothrium sp. (Order Rhinebothriidea); and Echinobothrium coenoformum Alexander, 1963 (Order Diphyllidea) (Alexander, Reference Alexander1963; Hewitt & Hine, Reference Hewitt and Hine1972). Generally, skates do not prey on flatfish (Cox & Francis, Reference Cox and Francis1997). However, a recent diet study recovered flatfish from the gut contents of several NZ rough skates (Randhawa, pers. obs.). Therefore, it is at least plausible that the NZ sole could be an intermediate host for parasites of the NZ rough skate. Of the tapeworm larvae recovered, only the tentaculariid trypanorhynch parasitizes elasmobranchs. Trypanorhynchs have not been reported previously in this skate species, despite over 100 skates being dissected in recent surveys by Randhawa and his lab (Randhawa, pers. obs.), implying that there is another definitive host for these trypanorhynch larvae in New Zealand's marine ecosystem. In addition to the NZ rough skate, NZ waters off the south-east coast of the South Island, near where these NZ soles were collected, is home to eight relatively common species of sharks: Carcharodon carcharias (great white shark), Cephaloscyllium isabellum (New Zealand draughtboard shark), Galeorhinus galeus (tope shark), Isurus oxyrhinchus (mako shark), Lamna nasus (porbeagle shark), Notorhynchus cepedianus (seven-gill shark), Prionace glauca (blue shark) and Squalus acanthias (spiny dogfish). All these species are definitive hosts to various tapeworm species and all, except C. isabellum, host trypanorhynchs (Randhawa & Poulin, Reference Randhawa and Poulin2010). In fact, six of the eight species are known hosts to trypanorhynchs of the family Tentaculariidae (family affiliation of trypanorhynch larvae identified during this survey) (Randhawa & Poulin, Reference Randhawa and Poulin2010): C. carcharias, G. galeus, I. oxyrhinchus, N. cepedianus, P. glauca and S. acanthias (see Linton, Reference Linton1905; Joyeux & Baer, Reference Joyeux and Baer1936; Sao Clemente & Gomes, Reference Sao Clemente and Gomes1992; Beveridge & Campbell, Reference Beveridge and Campbell1996; Palm, Reference Palm1999; Palm & Beveridge, Reference Palm and Beveridge2002; Knoff et al., Reference Knoff, Sao Clemente, Pinto, Lanfredi and Gomes2004; Gomes et al., Reference Gomes, Knoff, Sao Clemente, Lanfredi and Pinto2005; Palm & Walter, Reference Palm and Walter2005). However, no adult tentaculariids have been reported previously from NZ elasmobranch fishes, despite larvae being reported from a variety of teleost fishes (Hine et al., Reference Hine, Jones and Diggles2000), including Arripis trutta (kahawai), Emmelichthys nitidus (redbait), Katsuwonus pelamis (skipjack tuna), Nematodactylus macropterus (tarakihi), Thunnus alalunga (albacore tuna), Thyrsites atun (barracouta), Trachurus novaezealandiae (jack mackerel) and Zeus faber (john dory) (see Robinson, Reference Robinson1959; Baker, Reference Baker1971; Korotaeva, Reference Korotaeva1975; Vooren & Tracey, Reference Vooren and Tracey1976; Lester et al., Reference Lester, Barnes and Habib1985; Jones, Reference Jones1991). A survey of the literature on the diet of sharks suggests that not all these sharks feed on members of the Pleuronectidae (family affiliation of the NZ sole) (Rasmussen & Randhawa, pers. comm.). In fact, only four of the six shark species known to host tentaculariid trypanorhynchs feed on pleuronectid fish, i.e. C. carcharias, G. galeus, P. glauca and S. acanthias (Fadeev, Reference Fadeev1960; Holden, Reference Holden1966; Compagno, Reference Compagno1984; McFarlane et al., Reference McFarlane, Beamish, Saunders, Smith and Butler1984; Harvey, Reference Harvey1989; Ellis et al., Reference Ellis, Pawson and Shackley1996; Bowman et al., Reference Bowman, Stillwell, Michaels and Grosslein2000). The hypothesis that the NZ sole might be a secondary intermediate host to trypanorhynch tapeworms seems plausible based on shark diet data. However, without specific identification of the trypanorhynch species infecting NZ sole in the present study, elucidating trophic links to parasite transmission cannot be made unequivocally.
In addition to hosting the tentaculariid larvae, other larval cestodes were recovered from our sample: B. scorpii (Bothriocephalidea) and A. chilensis (Pseudophyllidea). In NZ waters, the former is a known parasite of Pseudophycis bachus (red cod) (Robinson, Reference Robinson1959), while A. chilensis has been recovered from Genypterus blacodes (ling) (Grabda & Slósarczyk, Reference Grabda and Slósarczyk1981). Both tapeworms were recovered in the present survey as larvae in NZ sole. In NZ, red cod are known to prey on pleuronectid flatfish, but these are considered a minor component of their diet (Horn et al., Reference Horn, Forman and Dunn2012). NZ flatfish are also minor prey components of ling's diet, and the latter is also known to feed on red cod (Dunn et al., Reference Dunn, Connell, Stevens and Horn2010). This makes it unlikely that NZ sole is the main pathway for transmission of B. scorpii to red cod and A. chilensis to ling. Knowing this, NZ sole may also act as paratenic hosts, with the transmission route consisting of a predator of flatfish and a prey of sharks, such as red cod and ling. The suggestion that NZ sole may act as a paratenic host in tentaculariid life cycles is plausible, given that morid fish (red cod is affiliated to the family Moridae) are known prey to S. acanthias (Hanchet, Reference Hanchet1991) and that ophidiid fish (ling is affiliated to the family Ophidiidae) are known prey of G. galeus, N. cepedianus and P. glauca (Compagno et al., Reference Compagno, Ebert and Smale1989; Harvey, Reference Harvey1989; Ebert, Reference Ebert1991). Without specific identification of the tentaculariid larvae in this study and further parasitological surveys of both red cod and ling, the actual transmission routes for these parasites cannot be confirmed.
A single unidentified tapeworm larva was recovered during this survey, but we were unable to generate any sequence data for it. Hence, it remains unidentified, but it is considered distinct from the other tapeworm larvae we were able to identify using molecular tools, due to differences in morphology.
Like cestodes, we found cystacanths of C. hannae in our sample of NZ soles. Clearly, NZ sole is not their definitive host. As a matter of fact, adult specimens have been recovered from the large intestine of Hydrurga leptonyx (leopard seal), Arctophoca forsteri (long-nosed fur seal) and Phocarctos hookeri (NZ sea lion) (Zdzitowiecki, Reference Zdzitowiecki1984; Shiel et al., Reference Shiel, Smales, Sterrer, Duggan, Pichelin, Green and Gordon2009; Hernández-Orts et al., Reference Hernández-Orts, Smales, Pinacho-Pinacho, García-Varela and Presswell2017) from Antarctica and NZ, while immature specimens have been recovered from the fish-eating birds Leucocarbo chalconotus (Stewart Island shag), Phalacrocorax punctatus (spotted shag) and Megadyptes antipodes (yellow-eyed penguins) (Shiel et al., Reference Shiel, Smales, Sterrer, Duggan, Pichelin, Green and Gordon2009; Hernández-Orts et al., Reference Hernández-Orts, Smales, Pinacho-Pinacho, García-Varela and Presswell2017) from NZ. Pleuronectid fish are considered to be paratenic hosts for this parasite and cystacanths have been reported from our sampling of NZ sole and Colistium guntheri (NZ brill) (this study, but see Hernández-Orts et al., Reference Hernández-Orts, Smales, Pinacho-Pinacho, García-Varela and Presswell2017). It is believed that the life cycle for C. hannae might involve amphipods, with teleosts being necessary for concentrating the cystacanths for transmission up the food chain to pinnipeds, but in which no parasite development occurs (Zdzitowiecki & Presler, Reference Zdzitowiecki and Presler2001). Pleuronectid fish are relatively important prey to a number of pinnipeds worldwide (e.g. Lance et al., Reference Lance, Orr, Riemer, Weise and Leake2001; Tollit et al., Reference Tollit, Schulze, Trites, Olesiuk, Crockford, Gelatt, Ream and Miller2009; Szoboszlai et al., Reference Szoboszlai, Thayer, Wood, Sydeman and Koehn2015). With a high prevalence (100%) in both NZ sole and NZ brill, and a relatively high average intensity of infection (33 in NZ sole and 56 in NZ brill), it is likely that pleuronectid fish play an important role in C. hannae transmission to pinnipeds. However, pleuronectid fish, including the NZ sole, are minor prey components of yellow-eyed penguins and of shags (Moore & Wakelin, Reference Moore and Wakelin1997; Grémillet et al., Reference Grémillet, Argentin, Schulte and Culi1998; Rail & Chapdelaine, Reference Rail and Chapdelaine1998), the latter generally considered to be pelagic feeders. Therefore, pleuronectid fish are unlikely to contribute much to C. hannae infections in fish-eating birds. Corynosoma spp. have also been recorded in other demersal fishes, such as Macroronus novaezelandiae (hoki) and ling (Hine et al., Reference Hine, Jones and Diggles2000) and it has been suggested that these specimens may, in fact, be conspecific with C. hannae (Hernández-Orts et al., Reference Hernández-Orts, Smales, Pinacho-Pinacho, García-Varela and Presswell2017). A broader survey of teleost parasites in NZ may reveal that C. hannae is more widely distributed in terms of taxa and may allow us to establish the importance of the different teleosts in their transmission to pinnipeds, based on relative abundance of infection in different paratenic hosts.
The other acanthocephalan species, A. peltorhamphi, has been reported from the NZ sole previously and is included in the checklist of Hine et al. (Reference Hine, Jones and Diggles2000). The NZ sole is indeed one of the definitive hosts for this parasite as it has also been reported from the pleuronectids Rhombosolea leporina (yellowbelly flounder) and R. plebeia (NZ flounder) (Shiel et al., Reference Shiel, Smales, Sterrer, Duggan, Pichelin, Green and Gordon2009). The A. peltorhamphi cystacanths are likely acquired by the teleost fish feeding on infected amphipods (Zdzitowiecki & Presler, Reference Zdzitowiecki and Presler2001), although this has yet to be determined empirically for NZ pleuronectid fish. With an 82% prevalence and mean intensity of infection of 6.3 A. peltorhamphi per host, we can conclude that the NZ sole is a competent definitive host for this parasite. However, barring a diet study of the NZ sole, the transmission route for A. peltorhamphi will remain undetermined. Based on unquantified observations during this study, it is clear that crustaceans (including amphipods) make up the bulk of the NZ sole's diet, followed by polychaete worms. Investigating the parasite community of these prey items may shed light on intermediate host utilization by larval parasites of the NZ sole.
Although it has not been reported previously in the NZ sole (Hine et al., Reference Hine, Jones and Diggles2000), the trematode Decemtestis pseudolabri (Opecoelidae) has been reported in another NZ fish, Notolabrus celidotus (spotty) (Manter, Reference Manter1954), a wrasse-like fish of the Labridae family. We found it in 9 of 28 fish (32.1%), but recovered a mere 20 specimens, or approximately two per infected host. It is possible that this parasite is not strictly host specific and may thrive more in N. celidotus and its close relatives, although Manter (Reference Manter1954) did not report ecological data such as prevalence or intensity of infection. Both the NZ sole and spotty share a common distribution in NZ's southern coast and both feed on benthic organisms such as small crustaceans (Russell, Reference Russell1983), which suggests that both species may become infected by ingesting infected common prey. Similar feeding habits can break down barriers to host specificity (Randhawa et al., Reference Randhawa, Saunders, Scott and Burt2008), suggesting that, in some instances, ecology is more important than phylogeny in determining host specificity.
The other common trematode species recovered during this survey is an undescribed fellodistomid. Other members of the Fellodistomidae have been identified from NZ fishes: Benthotrema richardsoni Manter, 1954 (synonym of Pseudobenthotrema richardsoni) ex Pelotretis flavilatus (lemon sole), Choanomyzus tasmaniae Manter & Crowcroft, 1950 ex Paranotothenia magellanica (Maori cod), Hypertrema ambovatum (Manter, 1960) ex Simenchelys parasitica (snubnosed eel), Proctoeces subtenue (Linton, 1907) (synonym of Proctoeces maculatus (Looss, 1901)) ex Latridopsis ciliaris (blue moki), Steringotrema rotundum Manter, 1954 ex Parapercis colias (blue cod), Tergestia agnostomi Manter, 1954 ex Aldrichetta forsteri (yellow-eyed mullet) and T. magna Korotaeva, 1972 ex E. nitidus (redbait) (see Manter, Reference Manter1954, Reference Manter1960; Korotaeva, Reference Korotaeva1975). Fellodistomid trematodes have been reported in many flatfish, including the lemon sole in NZ, and other demersal fishes all around the world, and seem to be specific to demersal environments. To date, we have been unable to identify these specimens, but sequence data places them unequivocally within the Fellodistomidae (Pérez-Ponce de León, pers. comm.) and we continue working on a morphological description for our specimens. However, with 71.4% prevalence and a mean intensity of infection of approximately 11 worms per infected host, there is no doubt that the NZ sole is a competent definitive host for this trematode species. Members of the Fellodistomidae exhibit a relatively high degree of host specificity, hence it remains unclear whether an expanded survey of NZ pleuronectid fish would yield this species from other flatfish. Additionally, due to its high prevalence, it is expected that this species utilizes a common prey item of the NZ sole for its transmission. As such, we hypothesize that a survey of small crustaceans might lead to the discovery of metacercariae assignable to this species.
We also found nematodes, both at larval and adult stages, in the NZ sole. Third-stage larvae of A. pegreffii are common in teleosts (Mattiucci & Nascetti, Reference Mattiucci and Nascetti2008). Generally, this nematode utilizes odontocetes as their definitive hosts. Since larvae of this species are ubiquitous in marine ecosystems, it is unclear what role the NZ sole plays in their transmission, if any. However, anisakid larvae have been recovered previously from elasmobranchs in NZ: C. isabellum, G. galeus, I. oxyrhinchus, N. cepedianus, P. glauca and S. acanthias (in Hewitt & Hine, Reference Hewitt and Hine1972). Also, these have been observed in L. nasus (Randhawa, pers. comm.). A wide range of NZ teleosts are known to harbour anisakid third-stage larvae (see Hine et al., Reference Hine, Jones and Diggles2000). As mentioned above, several of these shark species are known to prey on pleuronectid fish, and so are several of the teleosts known to be infected with larval stages of this parasite. Consequently, we consider the NZ sole to be no more than a paratenic host for this parasite.
In NZ, adult and larval H. aduncum have been recovered from over 20 and 50 different fish species, respectively (Hine et al., Reference Hine, Jones and Diggles2000), including the NZ sole, making this parasite a generalist. It is not atypical to find this species at both larval and adult stages within the same fish individual (e.g. Navone et al., Reference Navone, Sardella and Timi1998), since the last two moults occur in the intestine of the definitive host (Køie, Reference Køie1993). However, only larvae or adults were recovered from individual NZ sole (niche overlap of 0; table 2). The general life cycle of this parasite generally involves an obligatory invertebrate intermediate host, with other invertebrates acting as potential paratenic hosts (Køie, Reference Køie1993) and a teleost intermediate, definitive or paratenic host (Deardorff & Overstreet, Reference Deardorff and Overstreet1980; Navone et al., Reference Navone, Sardella and Timi1998). For instance, Navone et al. (Reference Navone, Sardella and Timi1998) described the life cycle of H. aduncum from the south-west Atlantic; it involved an amphipod as obligatory intermediate host and teleosts as intermediate, paratenic or definitive hosts. Hence, given that unquantified observations during this study reveal that crustaceans (including amphipods) make up the bulk of the NZ sole's diet, it is not surprising that they host larval H. aduncum. However, based on our unquantified stomach content observations, it remains unclear as to how the NZ sole ends up hosting the adult worms. Ling, a species known to harbour adult H. aduncum, does feed on pleuronectid fish and may acquire infective stages from the NZ sole that develop into adults in this definitive host. However, the trophic role played by the NZ sole in the transmission of H. aduncum remains equivocal pending diet studies of higher-level predators in NZ waters.
At the population level, the only parasites demonstrating an increase in abundance with size were larval anisakid nematodes, suggesting that these bioaccumulate in the teleost paratenic host. In fact, anisakids are known to infect large teleosts without undergoing further moults, leading to the accumulation of large numbers of infective stages (Hammerschmidt et al., Reference Hammerschmidt, Koch, Milinski, Chubb and Parker2009) waiting to be transmitted trophically to their definitive host. However, fish were targetted by commercial fishermen for commercial sizes during this survey and this may explain why the expected positive relationship between fish size and abundance of parasites (see Poulin, Reference Poulin2007) was not observed for more species or overall in our sample. Niche overlap of anisakid larvae was greatest with the fellodistomid trematode, suggesting that the two parasites might be transmitted by a shared prey item, the identity of which remains equivocal barring a detailed parasitological study of common prey items of the NZ sole. Considering that neither anisakid larvae nor the fellodistomid trematodes are the most common parasites encountered in the NZ sole, one should not conclude that these parasites are transmitted via the primary prey items. However, both acanthocephalans demonstrated a high level of niche overlap and are very common, both in terms of prevalence and relative abundance, hence it is likely that these are transmitted via a shared primary prey item.
In conclusion, the present study reiterates the need for caution when using/interpreting host–parasite checklists (Poulin et al., Reference Poulin, Besson, Morin and Randhawa2016a). As demonstrated here, even in common fish of commercial importance, basic ecological information is lacking. Our results further suggest that tapeworm larvae may be more widespread than expected from the literature, and the lack of information regarding marine teleost species infected with larval tapeworms contributes to our lack of knowledge regarding their life cycles, particularly of those infecting elasmobranch fishes. Finally, we appeal to marine biologists to proactively undertake dietary studies of teleost fish and to seek expertise from parasitologists, who can collaborate in identifying larval parasites from these prey items (see Poulin et al., Reference Poulin, Blasco-Costa and Randhawa2016b). This is necessary to better understand the trophic interactions leading to parasite transmission and whether parasites have exploited energy flows in food webs to enhance their transmission.
Acknowledgements
We acknowledge Gerardo Pérez-Ponce de León for his help in dissection and his expertise in the identification of trematodes and acanthocephalans, and Bronwen Presswell for her expertise in the identification acanthocephalans and for her primers. We are thankful to Zac Tobias for his assistance with preparing samples for DNA sequencing. We are grateful to Vickey Tomlinson for technical support, Tina Summerfield for the use of her lab for molecular work and Gavin Heineman (Echo F/V) for specimen collection. Lab space was generously provided by the Department of Botany at the University of Otago.
Financial support
Financial support for this project was generously provided by the Department of Botany at the University of Otago. T.A.’s visit to New Zealand was supported financially by the École Normale Supérieure de Lyon and a grant from the Auvergne-Rhônes-Alpes region.
Conflict of interest
None.





