Introduction
Aquatic ecosystems have been subject to an increasing number of anthropogenic pressures in recent decades. Aquatic organisms are exposed to a number of stressors, natural and man-made, such as variations of physical and chemical parameters (rainfall, temperature and salinity), dietary changes, changes in habitat availability and increased exposure to contaminants in the supply of nutrients (eutrophication) (Adams & Greeley, Reference Adams and Greeley2000). Thus, tools that increase our understanding of these impacts are of the utmost importance.
In order to evaluate and quantify the effect of environmental stressors on the health of aquatic systems, researchers have been using bioindicators, defined as organisms or communities whose vital functions correlate so closely with certain environmental factors that they can be used as indicators in assessing a given area (Markert et al., Reference Markert, Breure and Zechmeister2003). Field approaches are required to provide an integrated evaluation of these ecosystems, allowing the detection of cumulative and/or synergistic effects of environmental impacts on the community (Adams et al., Reference Adams, Bevelhimer, Greeley, Levine and The1999).
The parasites of aquatic organisms are ubiquitous and hidden components of ecological communities, which are closely related to several characteristics of the biotic and abiotic environment in which they exist. Thus, parasites have attracted increasing interest from researchers as potential indicators of environmental quality, due to the variety of forms that respond to pollution of anthropogenic origin, such as eutrophication, oil spills, heavy metals, acid precipitation, domestic sewage, and agricultural and industrial pollution (Landsberg et al., Reference Landsberg, Blakesley, Reese, Mcrae and Forstchen1998; Sures, Reference Sures2004). The effects of stressors on communities of parasites are varied and can be positive or negative: pollution can both increase parasitism and be fatal to certain species, leading to a decrease in the number of parasites. Abiotic factors such as temperature, dissolved oxygen, salinity and pH can influence the temporal and spatial occurrence of parasites, particularly helminth fish parasites (Chubb, Reference Chubb1979). Stressors can favour parasitism, for example, if the defence mechanisms of the host are adversely affected, increasing in this way the susceptibility of the host, or simply by increasing the density of final and intermediate hosts, such as the eutrophication phenomenon, which favours the occurrence of invertebrates commonly used as intermediate hosts in the life cycle of digenean helminths (Sures, Reference Sures2004). For example, eutrophication can increase parasitism, while heavy metals may reduce it. Ciliates and nematodes are sensitive indicators of eutrophication and thermal effluents, while digeneans and acanthocephalans are good indicators of heavy metals (Lafferty, Reference Lafferty1997). Poulin (Reference Poulin1992) showed that parasite communities are influenced indirectly by pollutants that are toxic to fish and intermediate hosts, and directly by environmental factors that are toxic to parasites and their free-living stages. Thus, the response of each species of parasite to every stressor should be investigated in various systems.
Considering the increasing urbanization on the Sangradouro River to the south of Florianópolis, Santa Catarina Island, the area provides an excellent opportunity for the study of parasites as bioindicators. The host species we chose for the study is a fish known as ‘cará’ Geophagus brasiliensis (Kner, 1865) (Perciformes: Cichlidae), one of the most abundant species in the area and one of the most widely distributed along the river.
According to criteria developed by Overstreet (Reference Overstreet1997) and corroborated by Sures (Reference Sures2004), host species of fish parasite biomarkers should be common, abundant, easy to sample and of relatively small size. The cará has high commercial value among hobbyists around the world, due to its lovely colour. The species is widely distributed along the coastal basins of eastern and southern Brazil and Uruguay, occurring in different types of aquatic systems, such as canals, lakes, lagoons and estuaries (Garcia et al., Reference Garcia, Raseira, Vieira, Winemiller and Grim2003). Animal and plant materials are part of the diet of this omnivorous fish, its mainstays being molluscs, vascular plants, crustaceans and detritus (Bastos et al., Reference Bastos, Condini, Varela Junior and Garcia2011). There are several studies on the parasitic fauna of G. brasiliensis; according to Eiras et al. (Reference Eiras, Takemoto and Pavanelli2010), more than 30 species of parasite have been described for this host. Regarding studies on parasite ecology, we highlight the work of Azevedo et al. (Reference Azevedo, Martins, Bozzo and Moraes2006), Madi & Ueta (Reference Madi and Ueta2009), Bellay et al. (Reference Bellay, Ueda, Takemoto, Lizama and Pavanelli2012) and Rassier et al. (Reference Rassier, Pesenti, Pereira Junior, Silva, Wendt, Monteiro and Aires Berne2015). Madi & Ueta (Reference Madi and Ueta2009) point to the monogenean subfamily Ancyrocephalinae as indicators of environmental quality in reservoirs of the State of São Paulo.
Aiming to investigate the potential of the parasitic fauna of fishes as bioindicator species, the general objective of this study was to survey the parasitic fauna of ‘cará’ fish (G. brasiliensis) in the Sangradouro River, and to correlate the parasite community with the health status of the hosts and with different levels of anthropogenic impact along the river, contributing to increased knowledge and control of the impacts on the environment from human activities and the preservation of local water resources.
Materials and methods
Study area
The Sangradouro River Basin is part of the hydrographic basin of ‘Pântano do Sul’, located on the southern Santa Catarina Island, covering an area of 13.65 km2. The Sangradouro River links the Peri Lagoon and the sea and is approximately 3.5 km long, with a single stream flowing to the sea, joining the Quincas River for a bit before emptying between the beaches of ‘Armação’ and ‘Matadeiro’ in the south of the island. This river suffers from rapid urbanization along its banks, responsible for a constant supply of sewage in its course. Information on the structure and functioning of this ecosystem is scarce, even for the fish fauna, and nothing is known about the species of parasites of aquatic organisms that live there.
We chose four points along the river, from its inception in Peri Lagoon to its mouth at the beach ‘Armação’. The points closest to the Peri Lagoon have abundant vegetation on the riverbanks and little human intervention (P1 and P2). In the next section, the margins of the river are less vegetated and are influenced by a few houses (P3). Point 4 (P4) is in a region influenced by high population density and a consequent increase in the input of organic matter.
Water variables
Water temperature, pH, dissolved oxygen, conductivity and salinity were measured at each of the four sampling points using a portable multiparameter meter (model Hanna HI 9828, Hanna Instruments, São Paulo, Brazil). Water samples were taken with 250-ml plastic bottles, immersed on ice inside thermal boxes, and then transported to the laboratory. At each point, two bottles of water were collected for nutrient analysis, which was performed by the Laboratory of Biology and Cultivation of Freshwater Fish (LAPAD, CCA, UFSC). Concentrations of nutrients were determined according to Koroleff (Reference Koroleff and Grasshoff1976) for total ammonia nitrogen, Golterman et al. (Reference Golterman, Clymo and Ohnstad1978) for nitrites, Strickland & Parsons (Reference Strickland and Parsons1960) for total phosphates, and Valderrama (Reference Valderrama1981) for total phosphorus and total nitrogen. Two additional bottles of water at each point were used for analysis of total coliforms and faecal coliforms, they were sterile and supplied by the Analytical Laboratory of Food of the Department of Science and Technology (LABCAL, CCA, UFSC), which was responsible for the analyses. The method for detection and enumeration of coliforms was the most probable number technique (MPN) (Kornacki & Johnson, Reference Kornacki, Johnson, Downes and Ito2001; International Organization for Standardization, 2006). In this study, the level of pollution of the collection points was determined by the concentrations of total ammonia nitrogen, nitrites, total phosphates, total phosphorus, total nitrogen, and total and faecal coliforms in the water.
Fish sampling
The sampling, biometrics and identification of fish were conducted by the field staff of the Laboratory of Biology and Cultivation of Freshwater Fish (LAPAD, CCA, UFSC). Catches were bimonthly from April 2012 to February 2013, in the first quarter of the month, through electric fishing at the four points along the river. During sample collection, we identified the fish and then recorded the weight and total length. The fish were anaesthetized with lethal doses of eugenol (1 mg/10 l). Each individual received an identification number in the database containing data collection, sampling point, length and weight. After biometric data collection, we calculated the relative condition factor from the following equation: Kn = W/Lb, where W = total weight, L = length and b = slope of the length/weight relationship, according to Le Cren (Reference Le Cren1951) and Lima-Junior et al. (Reference Lima-Junior, Cardone and Goitein2002).
Parasitological analyses
First, the eyes and gills were removed from fish and placed in vials containing 5% formalin solution, for the later collection of parasites. Then fish were opened by a ventral midline incision, and their body cavity and internal organs were observed in Petri dishes with saline solution under a stereomicroscope. The necropsy of fish and parasitological analyses followed Eiras et al. (Reference Eiras, Takemoto and Pavanelli2006). The ecological terminology followed Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Statistical analyses
A generalized linear model (GLM) was constructed using as response variables the abundance of the two most abundant parasite species (quasi-Poisson distribution). When a species was found at more than one site of infection, as in the case of Pandosentis aff. iracundus, the total abundance was used, adding the parasites found in the stomach and intestines of the fish. As possible predictors of the models, we considered the season, total length, weight and relative condition factor (Kn) of fish, dissolved oxygen, total phosphorus, total ammonia nitrogen and faecal coliforms. We selected the best models based on the backwards selection procedure and considered predictors significant for the model at α = 0.05. We performed the analyses in the programming environment R, version 2.7.2 (R Development Core Team, 2010).
Results
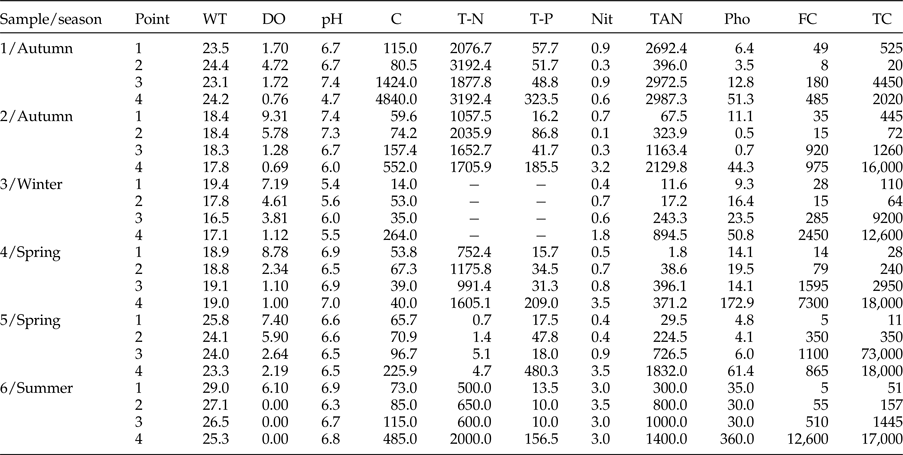
The average values for the parameters measured in the field and in the laboratory to determine the water quality are shown in table 1.
Table 1. Mean values of abiotic variables, nutrient concentration, and concentration of total and faecal coliforms in water samples collected at four points along the Sangradouro River, Florianópolis (SC), in bimonthly samples from April 2012 to February 2013. WT, Water temperature (°C); DO, dissolved oxygen (mg/l); C, conductivity (μS/cm); T-N, total-N (μg/l); T-P, total-P (μg/l); Nit, nitrite (μg/l); TAN, total ammonia nitrogen (μg/l); Pho, phosphate (μg/l); FC, faecal coliforms (MPN/100 ml); TC, total coliforms (MPN/100 ml).
A total of 137 fish specimens (G. brasiliensis) were collected from the four points of collection in the Sangradouro River: 37 at P1, 34 at P2, 41 at P3, and 25 at P4. The mean total length of the fish was 96.4 ± 38.8 mm (31.0–256.0 mm), and the mean weight was 22.9 ± 28.8 g (0.4–153.3 g); values of Kn were between 0.73 and 1.31.
Among the 137 examined fish, 114 (83.2%) were parasitized by at least one species of parasite. The study area is a small river strongly affected by the human occupation of its margins, so we captured very few fish species and individuals in our collections, probably due to the extreme conditions of this aquatic system. Two species of ectoparasites and two species of endoparasites were recorded. The parasite species, sites of infection/infestation and respective parasitological indices are shown in table 2. Additionally, we found metacercariae encysted on the fins, but it was not possible to quantify them due to the extremely high number.
Table 2. Species of parasites separated by groups, sites of infestation/infection, season (S) (Au, autumn; Wi, winter; Sp, spring; Su, summer; To, total), point of collection (P1–P4) and their parasitological indexes (P, prevalence; MI, mean intensity; MA, mean abundance; ± standard deviation) found in the host Geophagus brasiliensis in the Sangradouro River, Florianópolis, collected bimonthly from April 2012 to February 2013.
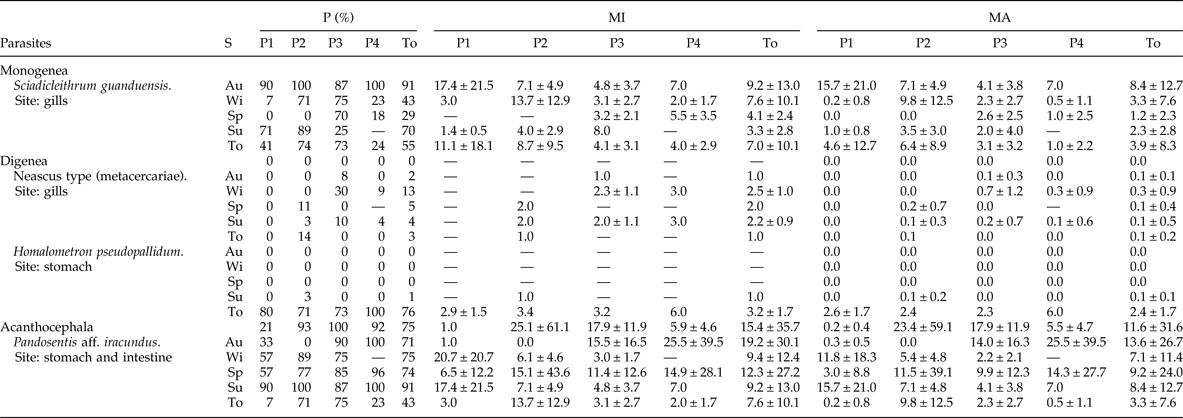
The most prevalent ectoparasite species was the monogenean Sciadicleithrum guanduensis (55.5%). Among endoparasites, Pandosentis aff. iracundus was the most prevalent species (74.5%).
Regarding the water variables, in order to eliminate collinearity between the predictor variables in the model, we limited the analyses to the main water quality parameters as indicators of organic pollution: dissolved oxygen, total ammonia nitrogen, total phosphorus and faecal coliforms.
According to the model using the monogenean S. guanduensis as the response variable (table 3), the variables with significant influence on the abundance of the parasite were season (highest values in autumn) (fig. 1a), total length and Kn of fish, dissolved oxygen and faecal coliforms (fig. 2). On the other hand, for the endoparasite P. aff. iracundus, the highest abundance was recorded in winter (fig. 1b), and the concentrations of dissolved oxygen, total ammonia nitrogen and faecal coliforms were more important in explaining the abundance of parasites (table 3, fig. 3).
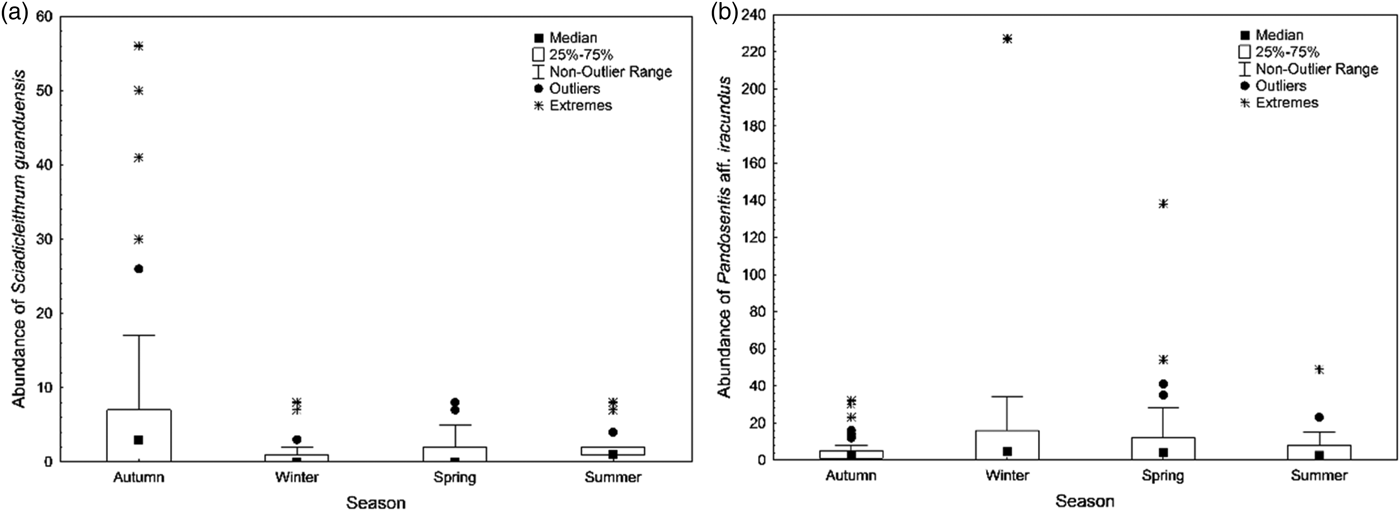
Fig. 1. Abundance of Sciadicleithrum guanduensis (a) and Pandosentis aff. iracundus (b) parasites of Geophagus brasiliensis captured in the Sangradouro River (Florianópolis, SC, Brazil), in different seasons.
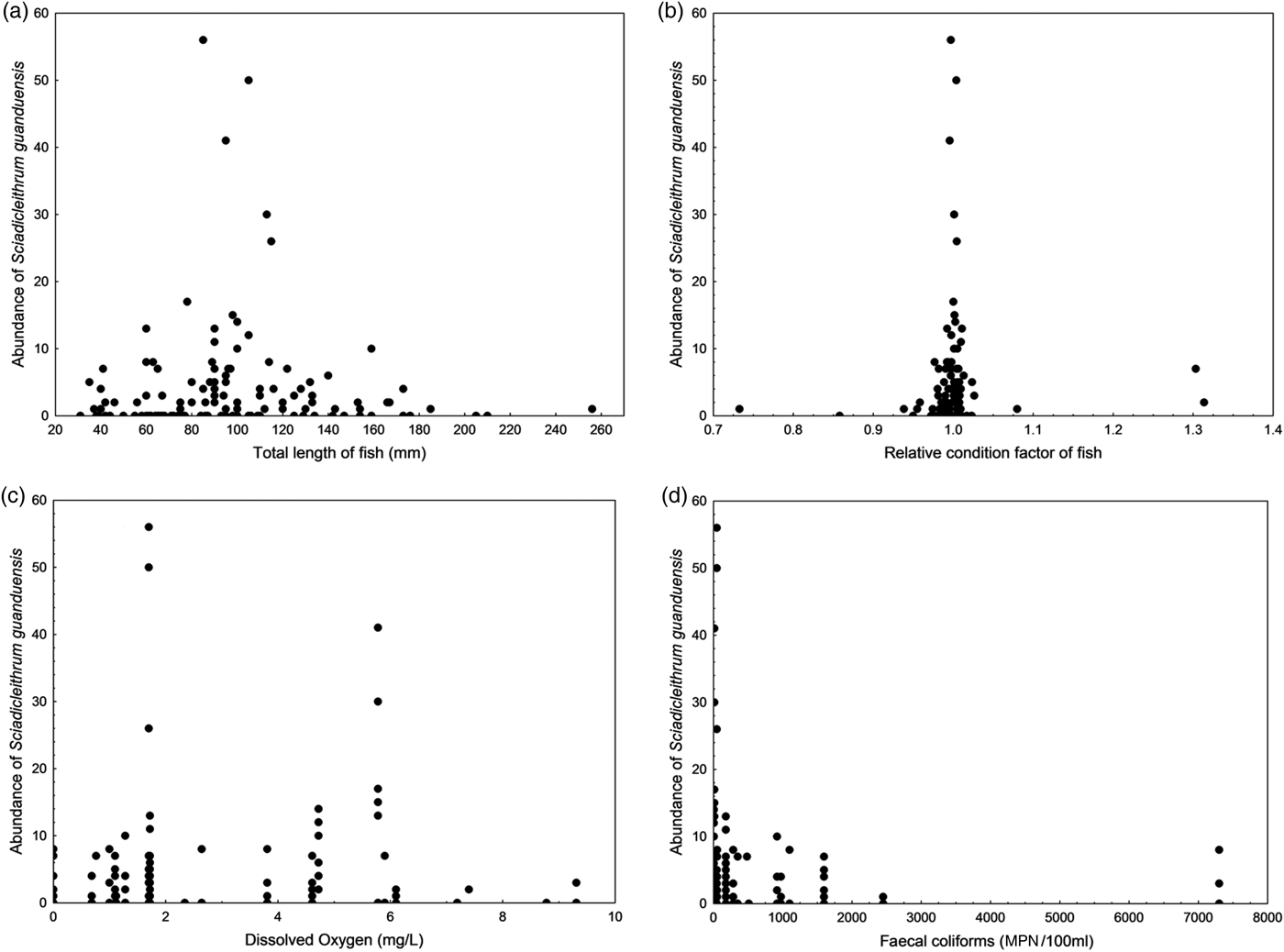
Fig. 2. Correlation between the abundance of Sciadicleithrum guanduensis and the total length of fish (a), relative condition factor of fish (b), and the concentrations of dissolved oxygen (c) and faecal coliforms (d) in the water.

Fig. 3. Correlation between the abundance of Pandosentis aff. iracundus and the concentrations of dissolved oxygen (a), total ammonia nitrogen (b), total phosphorus (c) and faecal coliforms (d) in the water.
Table 3. Predictor variables retained in the best models (GLMs), using the backwards selection criteria, with the abundance of parasites Sciadicleithrum guanduensis and Pandosentis aff. iracundus as response variables.
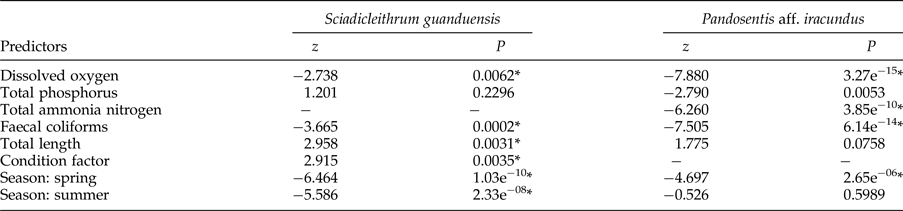
*Significant at α = 0.05.
Discussion
Parasite species
Among the four species recorded parasitizing G. brasiliensis in the present study in the Sangradouro River, two species were already recorded by Carvalho et al. (Reference Carvalho, Tavares and Luque2010) as parasitizing the same species in the Guandu River, near Rio de Janeiro State. The authors observed the presence of S. guanduensis on the gills of G. brasiliensis, with a prevalence of 10%, an intensity range of 1–31, an average intensity of 11.9, and a mean abundance of 1.2. The prevalence of S. guanduensis in this study was 55%, with an intensity of 1–56 individuals per host. The high prevalence found in the Sangradouro River compared with the Guandu River may be explained by greater contact between hosts in the Sangradouro River. The Sangradouro is a shallow and small river, only about 3.5 km in length, as opposed to the Rio Guandu, which, despite its small size under natural conditions, became raging waters after the transposition of the Paraiba do Sul River. High densities and consequent increased contact between fish are factors that contribute to host colonization by monogeneans, whereas the monoxenic cycle (without an intermediate host) of these parasites is favoured under these conditions (Pavanelli et al., Reference Pavanelli, Eiras and Takemoto1998).
Another parasite recorded in G. brasiliensis, both in the Sangradouro River and in the Guandu River, was the Neascus-type metacercariae. Carvalho et al. (Reference Carvalho, Tavares and Luque2010) recorded the parasite in the eyes and integument of fish, whereas in Rio Sangradouro we found the parasites on the gills. As the specimens were identified only to the generic level, we cannot say that they are the same species. The presence of larval stages parasitizing G. brasiliensis is an indication that the fish occupies an intermediate position in the trophic web, and should be consumed by a definitive host (another fish, birds, or piscivorous mammals), which will be parasitized by the digenean adults. Only one specimen of the digenean Homalometron pseudopallidum was observed in our study. This parasite species was recorded for the same host from Patos Lagoon, Rio Grande do Sul, Brazil, by Rassier et al. (Reference Rassier, Pesenti, Pereira Junior, Silva, Wendt, Monteiro and Aires Berne2015), also presenting low values of prevalence (2.7%) and mean abundance (0.12).
The most prevalent and abundant parasite species in our study, the acanthocephalan P. aff. iracundus, represents the first record of the genus in Brazilian waters, as well as the first record of the parasite in the host G. brasiliensis.
Parasites × water variables
The concentrations of dissolved oxygen and faecal coliforms in the water were negatively correlated with the abundance of the monogenean S. guanduensis and the acanthocephalan P. aff. iracundus, the latter also being negatively correlated with the concentration of total ammonia nitrogen in the water. Blanar et al. (Reference Blanar, Munkittrick, Houlahan, MacLatchy and Marcogliese2009) performed a meta-analysis with the studies on pollution and parasitism in aquatic animals, showing that monogeneans usually present a negative response to contaminants in the water, whereas for acanthocephalans the difference was not significant. Mean parasite species richness and diversity indices decreased from the unpolluted site to the polluted site in the study of Madanire-Moyo et al. (Reference Madanire-Moyo, Luus-Powell and Olivier2012) on metazoan parasites of Oreochromis mossambicus in South African lakes. In their study, lakes affected by sewage, agricultural and industrial activity, and mining effluents were classified from unpolluted to polluted; the distribution of monogeneans was limited to unpolluted and moderately polluted lakes, and mean abundance of monogeneans was higher in the unpolluted sites, showing a clear influence of the water quality on this group of parasites that are in direct contact with the aquatic environment.
A negative correlation was observed between the abundance of P. aff. iracundus and concentrations of faecal coliforms and total ammonia nitrogen. Lafferty (Reference Lafferty1997) reviewed the role of fish parasites as indicators of human impacts, and concluded that acanthocephalans are negatively affected by organic pollution (sewage). On the other hand, Blanar et al. (Reference Blanar, Munkittrick, Houlahan, MacLatchy and Marcogliese2009) performed an update of Lafferty's list and found no significant differences in the number of studies that detected an effect of pollution on the population of acanthocephalans and those that did not observe any effect at all. According to Lewis & Hoole (Reference Lewis and Hoole2003), acanthocephalans can respond quickly to environmental changes and are more reliable indicators of water quality than their host fish. This is especially true for heavy-metal pollution, since acanthocephalans can reduce the levels of some metals in the gastrointestinal tissue of their host, related to the uptake of bile-bound metals by parasites (Sures, Reference Sures2003; Nachev et al., Reference Nachev, Schertzinger and Sures2013; Nachev & Sures, Reference Nachev and Sures2016).
High levels of inorganic nitrogen pollution are harmful to aquatic organisms, including fish and invertebrates (Arthur et al., Reference Arthur, West, Allen and Hedtke1987; Camargo & Alonso, Reference Camargo and Alonso2005, Reference Camargo and Alonso2006; Piedras et al., Reference Piedras, Oliveira, Moraes and Bager2006). The negative correlation found between acanthocephalans and total ammonia nitrogen concentration may be a result of the direct negative effect of this substance on the definitive host of the parasite. Our results show a decrease in the abundance of acanthocephalans with increasing concentration of oxygen in the water. The dissolved oxygen concentration is related to the water temperature, with the highest values found at lower temperatures. Likewise, the reproduction of Acanthocephala is positively correlated with temperature (Kennedy, Reference Kennedy2006). Thus, the negative correlation between the abundance of acanthocephalans and oxygen concentration could be linked to the temperature of the water and its effect on the reproduction of the parasites.
Parasites × length and condition factor of hosts
A positive correlation between the abundance of monogeneans and the total length of the hosts was observed in our study, and had been reported for other parasites and hosts (see Poulin, Reference Poulin1999), including the study of Bellay et al. (Reference Bellay, Ueda, Takemoto, Lizama and Pavanelli2012) with Sciadicleithrum frequens in G. brasiliensis. Regarding ectoparasites, changes in parasitism levels with host length are expected, because of the increase in the infection site (gill surface) area (Cezar & Luque, Reference Cezar and Luque1999). The abundance of monogeneans was also positively correlated with the condition factor of the hosts. Lizama et al. (Reference Lizama, Takemoto and Pavanelli2006) recorded 33 metazoan species parasitizing the freshwater fish Prochilodus lineatus, and found that, in general, parasitized fish presented a higher condition factor than unparasitized fish, including parasitic infection by the monogenean Rhinonastes pseudocapsaloideum; the authors proposed that the largest individuals with the highest Kn tolerate higher levels of parasitism. However, the parasitism × condition factor correlation should be considered with caution, since Lagrue & Poulin (Reference Lagrue and Poulin2015) showed that the fish body condition tended to be overestimated when parasite mass was not accounted for, and the parasite mass should be considered when estimating the condition.
Parasites × season
According to the model using the ectoparasite S. guanduensis as the response variable, the highest abundance was observed in autumn, and the variables total length and relative condition factor of the hosts, and the concentrations of dissolved oxygen and faecal coliforms in the water, were related to the abundance of parasites. According to Carvalho et al. (Reference Carvalho, Tavares and Luque2010), S. guanduensis showed higher prevalence and intensity in spring, confirming the expected pattern of infestation by monogeneans in the warmer months (Eiras, Reference Eiras1994). However, the peak abundance of monogeneans in autumn has been observed for some species: Diplectanum piscinarius, a parasite of Plagioscion squamosissimus in the Volta Grande Reservoir, Minas Gerais, Brazil (Martins et al., Reference Martins, Fujimoto and Moraes2000); Ligictaluridus floridanus, a parasite of Ictalurus punctatus in Mexico (Rábago-Castro et al., Reference Rábago-Castro, Sánchez-Martínez, Loredo-Osti, Gomez-Flores, Tamez-Guerra and Ramírez-Pfeiffer2011); Gyrodactylus salaris, a parasite of the Atlantic salmon (Salmo salar) (Appleby & Mo, Reference Appleby and Mo1997) and Arctic charr (Salvelinus alpinus) (Winger et al., Reference Winger, Kanck, Kristoffersen and Knudsen2008), both in Norway. According to Jansen & Bakke (Reference Jansen and Bakke1991), seasonal fluctuations in the population of monogeneans can be attributed to changes in the immune response of the hosts and in the water temperature. In a study conducted in the state of São Paulo, G. brasiliensis showed mature gonads during spring and summer, whereas autumn was the post-reproductive period (Gomiero & Braga, Reference Gomiero and Braga2007). Moreover, parental care of offspring is shown by G. brasiliensis, which could favour the increase of parasite abundance during this period due to increased sedentary behaviour and territoriality. Regarding the water temperature, autumn presented intermediate values of temperature, and this could be related to the highest abundance of monogeneans in this season. There are no studies on the production, development or viability of the eggs of Sciadicleithrum under laboratory conditions; however, for the freshwater gill monogenean Discocotyle sagittata, a parasite of Oncorhynchus mykiss, Gannicott & Tinsley (Reference Gannicott and Tinsley1998) observed the highest values of egg viability at intermediate temperatures; at the optimal temperature for transmission, the output of eggs is high and the majority of eggs develop quickly and hatch successfully.
Parasites are essential for the biodiversity and production of ecosystems; therefore, a healthy system is one that is rich in parasite species (Hudson et al., Reference Hudson, Dobson and Lafferty2006). Changes in water quality should affect the parasite community on different levels: the monoxenic species that are in direct contact with the water and their pollutants, and the heteroxenous species that use intermediate hosts to complete their life cycle, which may also be affected by the pollutants. In our study, the abundance of monogeneans and acanthocephalans were affected negatively by organic pollution, and monogenean abundance was also correlated with the length and condition factors of the hosts. We conclude that S. guanduensis and P. aff. iracundus may be sensitive to changes in water quality; the disturbed environment affected both monoxenic and heteroxenous parasites, as their abundance was negatively correlated with water variables related to organic pollution. Thus, parasite abundance can be investigated as an indicator of anthropogenic impacts on other aquatic environments.
Acknowledgements
The authors thank the field staff of the Laboratory of Biology and Cultivation of Freshwater Fish (LAPAD, CCA, UFSC) for logistical support during field activities, especially Claudia Machado MSc and Rodrigo Stallbohm.
Financial support
This work was supported by the Coordination for the Improvement of Higher Education Personnel – CAPES (A.C.F.L., postdoctoral research fellow scholarship PNPD/CAPES 23038.036282/2008-17); and the National Council of Technological and Scientific Development – CNPq (M.L.M. and A.P.O.N., productivity grants).
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
Animal handling was according to the Ethical Committee on Animal Use (CONCEA – National Council of Animal Control and Experimentation). The fish were anaesthetized according to the Ethics Committee CEUA/UFSC PP00801.








