Introduction
Ligophorus Euzet & Suriano, 1977 is a monogenean genus belonging to the family Dactylogyridae Yamaguti, 1963, which shows strong specificity to 17 mugilid fishes throughout the world (Soo and Lim, Reference Soo and Lim2015). The genus now contains more than 50 nominal species (Soo and Lim, Reference Soo and Lim2012; El Hafidi et al., Reference El Hafidi2013a,Reference El Hafidib; Kritsky et al., Reference Kritsky, Khamees and Ali2013; Sarabeev et al., Reference Sarabeev2013), most of which have been reported from the Mediterranean Sea, Black Sea, and Red Sea; coasts of South America; coasts of Japan and China; and the Sea of Malaysia (the last species was reported from here in 2012–2015) (Euzet and Suriano, Reference Euzet and Suriano1977; Fernandez-Bargiela, Reference Fernandez-Bargiela1987; Hu and Li, Reference Hu and Li1992; Dmitrieva and Gerasev, Reference Dmitrieva and Gerasev1996; Pan, Reference Pan1999; Zhang et al., Reference Zhang, Yang and Liu2001; Sarabeev et al., Reference Sarabeev, Balbuena and Euzet2005; Rubtsova et al., Reference Rubtsova2006, Reference Rubtsova, Balbuena and Sarabeev2007; Abdallah et al., Reference Abdallah, de Azevedo and Luque2009; Marcotegui and Martorelli, Reference Marcotegui and Martorelli2009; Siquier and Nunez, Reference Siquier and Nunez2009; Dmitrieva et al., Reference Dmitrieva2012; Soo and Lim, Reference Soo and Lim2012, Reference Soo and Lim2015).
Morphology and morphometric analyses are used for species identification by measurement of sclerotized structures of the haptor (dorsal and ventral bars, dorsal and ventral hamuli) and reproductive organs (penis, accessory piece, and vagina) (Siquier and Nunez, Reference Siquier and Nunez2009; Sarabeev et al., Reference Sarabeev2013). Between 2012 and 2015, off the islands of Malaysia in the Strait of Malacca and the Andaman Sea, 13 Ligophorus species were reported as new species collected from mugilid fishes (Soo and Lim, Reference Soo and Lim2012, Reference Soo and Lim2015; Soo et al., Reference Soo, Tan and Lim2015). This evidence shows the possibility of high diversity of Ligophorus in the seas of South-east Asia. Moreover, 13 Ligophorus species have been determined as the systemic value of anchor morphometry, and a significant correlation has been found between anchor shape and size and the species’ phylogenetic relationships (Khang et al., Reference Khang2016).
In this study, we explored the Ligophorus species collected from mugilid fishes off the coast of the Andaman Sea, Satun Province, southern Thailand, which is close to the Sea of Malaysia. We differentiated two Ligophorus species by their morphological characteristics. The molecular phylogenetic relationships of Ligophorus spp. were inferred using large-subunit nuclear ribosomal DNA (28S rDNA) and nuclear ribosomal internal transcribed spacer 1 (ITS1) sequences. The haptoral anchor structures of the new species were discussed in terms of evolution with the 13 Ligophorus species reported from Sea of Malaysia.
Materials and methods
Host and parasite collection
Mugilid fishes were caught from the coastal La-Ngu district, Satun Province (6°45′N, 99°50′E) from December 2015 to March 2016. The fishes were kept on ice and transferred to the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, within 12 hours. They were identified according to Turan et al. (Reference Turan2011) and Crosetti and Blaber (Reference Crosetti and Blaber2015). Monogeneans were removed from the gills of the fish under a stereomicroscope and then washed with 0.9% normal saline: 20 specimens were preserved in 75% ethanol and kept at –20°C for molecular study; 14 were fixed in 70% ethanol for morphological study; four were stained with carmine, dehydrated through a graded ethanol series, cleared in xylene, and mounted in Canada balsam for observation of general anatomy; and the remaining 10 were flattened between a slide glass and a cover slip, and a small amount of ammonium picrate–glycerin (APG) was added for observation of the sclerotized parts. Several weeks later, the APG specimens were dehydrated, cleared, and mounted in Canada balsam. Half of the APG specimens were lightly digested with gastric juice (0.5 ml of HCl and 1.0 g of pepsin in 100 ml of distilled water) before adding APG, as the sclerotized parts of the worms were not fully flattened after alcohol fixation. The digested specimens became fully flattened after being fixed in alcohol (fig. 1).

Fig. 1. Photographs of sclerotized parts of Ligophorus satunensis n. sp., mounted in APG medium after being fixed in 70% ethanol and lightly digested in gastric juice. (a) Haptor; scale bar 20 μm. (b) Male copulatory organ; scale bar 10 μm.
Morphological analysis
The 14 worms fixed in 70% ethanol were observed microscopically with the help of differential interference contrast. They were identified mainly on the basis of the sclerotized hard parts of the haptor (marginal hooks, ventral and dorsal hamuli, ventral and dorsal bars), reproductive organs (penis, accessory piece, and vagina) (Zhang et al., Reference Zhang, Yang and Liu2001; Soo and Lim, Reference Soo and Lim2012, Reference Soo and Lim2015; Sarabeev et al., Reference Sarabeev2013; Soo et al., Reference Soo, Tan and Lim2015). The specimens were measured using digital photo equipment (DS-Fi1 and DS-L2, Nikon, Japan). All figures were drawn using a drawing tube. All measurements of the new species are presented as a range, with mean in parentheses, in micrometres (μm) and the number of specimens to describe the differential morphology of the new species. The terminology and method of measurements followed those reported by Sarabeev et al. (Reference Sarabeev2013).
DNA extraction, PCR, and DNA sequencing
Genomic DNA was extracted from two worms of the newly described species and preserved using a Tissue Genomic DNA mini kit (Geneaid, New Taipei City, Taiwan) according to the manufacturer's instructions. The purified genomic DNA was then used as a template in polymerase chain reaction (PCR). Primer combinations followed those reported by Blasco-Costa et al. (Reference Blasco-Costa2012). A portion (D1–D2) of 28S rDNA was amplified using U178F (5′-GCACCCGCTGAAYTTAAG-3′) (Lockyer et al., Reference Lockyer, Olson and Littlewood2003) and LSU1200R (5′-GCATAGTTCACCATCTTTCGG-3′) (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000). PCR was conducted in a total volume of 50 μl using a TopTaq™ Master Mix Kit (QIAGEN, Hilden, Germany), which contains TopTaq DNA polymerase, a PCR buffer, 1.5 mm MgCl2, and 200 μm of deoxyribonucleotide triphosphate (dNTP). PCR amplicons were obtained under the following thermocycling conditions: 95°C for 4 minutes of initial denaturation; 35 cycles at 95°C for 1 minute, 50°C for 1 minute, and 72°C for 1 minute; and a final extension at 72°C for 10 minutes. For PCR amplification of the ITS1 region, the primers used were designed from the complete sequence of rDNA of Ligophorus spp. in GenBank (table 1). The primers were Ligo_ITS1_F (5′-CTGAGAAGATGACCGAACTTG-3′) and Ligo_ITS1_R (5′-GTTGTACAGTGTGGATAGGC-3′). PCR amplicons were again obtained under the following thermocycling conditions: 95°C for 3 minutes of initial denaturation; 35 cycles at 94°C for 40 s, 56°C for 30 s, and 72°C for 45 s; and a final step at 72°C for 4 minutes. The PCR amplicons were electrophoresed in 1.0% agarose gel, stained with ethidium bromide, and visualized with a UV transilluminator. Each PCR amplicon was sequenced using the amplification primers in an ABI prism (Macrogen, Seoul, Korea). The sequences obtained were checked individually by the BLAST program (McGinnis and Madden, Reference McGinnis and Madden2004) to confirm the correct target. The electropherogram of each sequence was examined for sequence accuracy by BioEdit version 7.0 (Hall, Reference Hall1999).
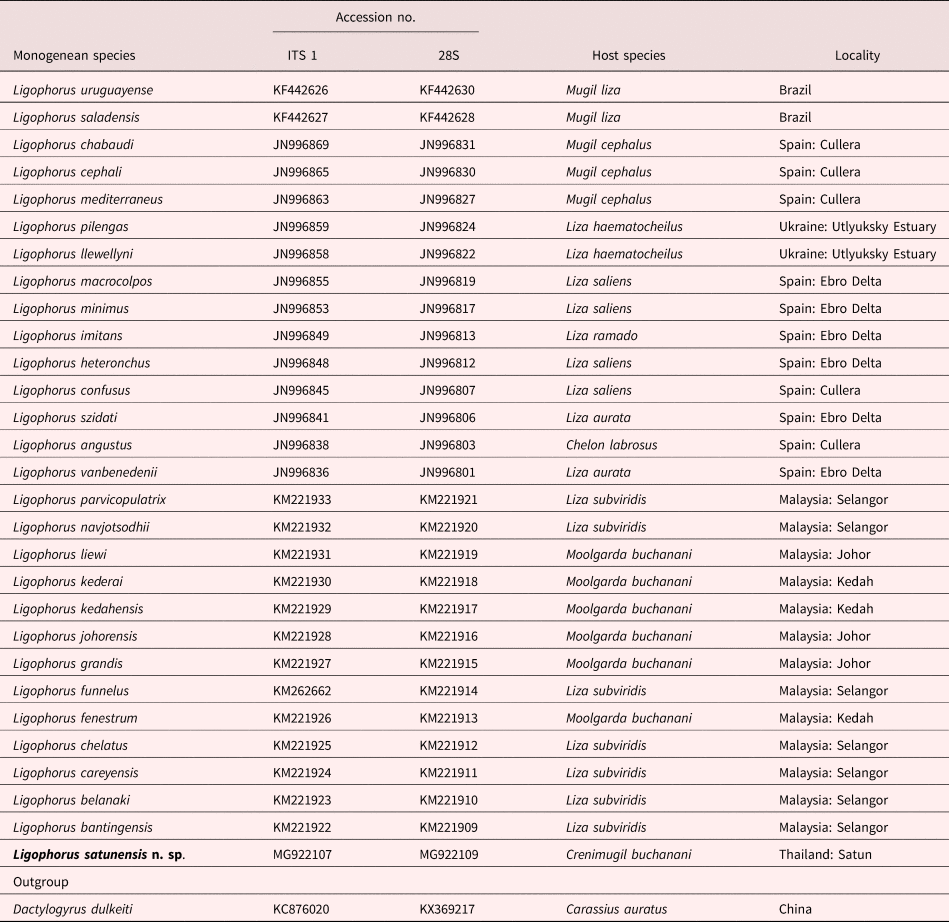
Table 1. List of Ligophorus spp. and the sequences used in the study. Hosts and countries of the collection sites are given.
Phylogenetic analysis
The 28S and ITS1 rDNA sequences were aligned with the other sequences of Ligoporus spp. deposited in GenBank (table 1) using CLUSTAL X (Thompson et al., Reference Thompson1997); all gap sites in the sequences were excluded by BioEdit version 7.0 (Hall, Reference Hall1999). The pairwise genetic distances between the Ligophorus spp. sequences were estimated using the P distance method. The partition homogeneity test was then conducted using PAUP*4.0b version 10 (Swofford, Reference Swofford2001) in order to confirm congruency of the 28S and ITS1 rDNA marker before generating the combined data set for phylogenetic tree construction. Significant incongruence after multiple comparisons of DNA partitions was determined at P > 0.02.
The best fit of the nucleotide substitution model was determined using jModelTest v. 2.0 (Posada, Reference Posada2008). Tamura 3-parameter + gamma distribution of rate parameters (G) was the suitable model used to construct the maximum likelihood (ML) tree, which was assessed with 1000 bootstrap replicates using MEGA 6.0 (Tamura et al., Reference Tamura2014). Bayesian posterior probabilities (BPP) were calculated for combined 28S and ITS1 rDNA sequences using implements of the GTR+I+G as the best-fit model. Default parameter values, such as topology branch lengths, nucleotide frequencies, nucleotide substitution rate, proportion of invariable sites, and γ-distribution, were used for priors. Four simultaneous Markov chains were run for 1,000,000 metropolis-coupled generations, starting with random initial trees and sampling every 100 generations. The convergence between runs was estimated visually by plotting the number of generations versus log-likelihood values and using the potential scale reduction factor provided in the ‘sump’ output of MrBayes (Ronquist et al., Reference Ronquist2012). After discarding results from the first 25% generations as ‘burn in’, majority-rule consensus trees were generated from the remaining trees. Node support was assessed using BPPs.
Results
Ligophorus satunensis n. sp. (figs. 2, 3Aa–e, Ba–e, Ca–d, 4Aa–d, Ba–d & 5a–e)
Taxonomic summary
Type host. The bluetail mullet, Crenimugil buchanani (Bleeker, 1853).
Site of infection. Gills.
Type locality. The Andaman Sea, Satun Province, southern Thailand (6°45′N, 99°50′E).
Dates of collection. 21 December 2015, 2 February 2016 and 23 March 2016.
Prevalence and intensity range. Prevalence 85%; intensity range 85–115 worms per fish.
Specimens deposited. Holotype and paratypes, MPM Coll. No. 21041, and 21042 and 21043, respectively, at the Meguro Parasitological Museum, Tokyo, Japan; other paratypes, TMTRE.260, at the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Etymology. The specific name refers to the type locality.
Material studied. 14 specimens.

Fig. 2. Whole worm and anatomy of L. satunensis n. sp., all carmine-stained paratypes. Photograph of a whole worm, ventral view; scale bar 100 μm. Line drawings: left, dorsal view; right, enclosed part of the photograph; scale bar 20 μm. (Accessory piece of the male copulatory organ is not drawn.) Abbreviations: GP, genital pore; Int, intestine; MG, Mehlis’ gland; Ov, ovary; PGC, prostate gland cell; PR, prostatic reservoir; SR, seminal receptacle; SV, seminal vesicle; T, testis; U, uterus.
Description
General anatomy based on stained specimens
Body elongated, 912–1328 (1094) (n = 3) X 145–279 (196) (n = 4), dorsoventrally flattened, narrowed in peduncle connecting body proper and haptor. Peduncle long and uniform in width, length 112–177 (138) (n = 3), width 46–65 (55) (n = 3). Haptor, length 45–65 (56) (n = 3), width 73–82 (77) (n = 3). Three pairs of cephalic lobes on head organ, forming one terminal and two bilateral cephalic lobes. Three pairs of head organs, each opening into each pair of cephalic lobes. Head organ cells originating on both sides of intestinal bifurcation. Two pairs of eye spots in front of mouth. Mouth subterminal. Pharynx ovoid, length 52–62 (54) (n = 4), width 45–52 (49) (n = 4). Oesophagus short. Intestine bifurcated posterior to pharynx, descending on both sides of body, united posterior to testis. Testis elongated and large, occupying central body proper, length 175–332 (227) (n = 4), width 56–158 (89) (n = 4). Vas deferens emerging at anterior end of testis, ascending between intestinal branches, forming sausage-shaped seminal vesicle immediately before entering spherical base of penis. Prostatic reservoir single, elongated, extending posterior to base of penis. Penis turning full circle before reaching genital pore. Accessory piece of penis not clearly observed in the carmine-stained specimens. Prostate gland cells (PGCs), deeply stained with carmine, posterior to prostatic reservoir. Ovary elongated, length 74–80 (77) (n = 4), width 28–40 (33) (n = 4), anterior to but slightly overlapping with testis, with its posterior third bent ventrally. Oviduct arising at apical end of ovary, receiving duct from seminal receptacle, ascending to form oötype. Mehlis’ gland at base of uterus. Uterus opening at common genital pore behind intestinal bifurcation. Vagina not observed. Vitellarium extending from level of intestinal bifurcation to posterior body proper (fig. 2).
Sclerotized parts based on APG specimens
Haptoral hard parts comprising two pairs of hamuli, two bars and seven pairs of marginal hooks. Ventral hamuli stout, with inner length 34–36 (35) (n = 9), outer length 39–42 (40) (n = 9), base 26–28 (27) (n = 9), point 7–8 (7) (n = 9), inner root 10–15 (12) (n = 9), outer root 10–14 (12) (n = 9). Dorsal hamuli longer than ventral hamuli, completely (fig. 3Bb), incompletely (fig. 3Ba,d,e), or not fenestrated (fig. 3Bc), with inner length 45–50 (47) (n = 9), outer length 45–49 (47) (n = 9), point 7–10 (9) (n = 9), inner root 10–17 (15) (n = 9), outer root 12–16 (14) (n = 9). Ventral bar straight, length 42–46 (44) (n = 8), width 6–9 (8) (n = 8). Anteromedian protuberance (AMP) consisting of a long median piece, length 22–25 (23) (n = 8), bifurcated anteriorly, distance between bifurcation 2–7 (4) (n = 8). Dorsal bar V shaped, with both ends directing horizontally or posteriorly, length 56–64 (62) (n = 8), width 6–9 (7) (n = 8). Marginal hooks of larval type, length 12–14 (13) (n = 8). Male copulatory organ consisting of penis and accessory piece. Penis strongly curved at funnel-shaped base, turning a full circle before reaching genital pore, length 84–108 (94) along curved line (n = 9), width 2–3 (2) (n = 8). Bulb, diameter 5–10 (8) (n = 8), attached to base of penis. Heel not clearly observed. Accessory piece straight, slightly tapering distally, length 23–31 (27) (n = 8), width 6–10 (9) (n = 8), consisting of non-grooved, short base and grooved distal part.
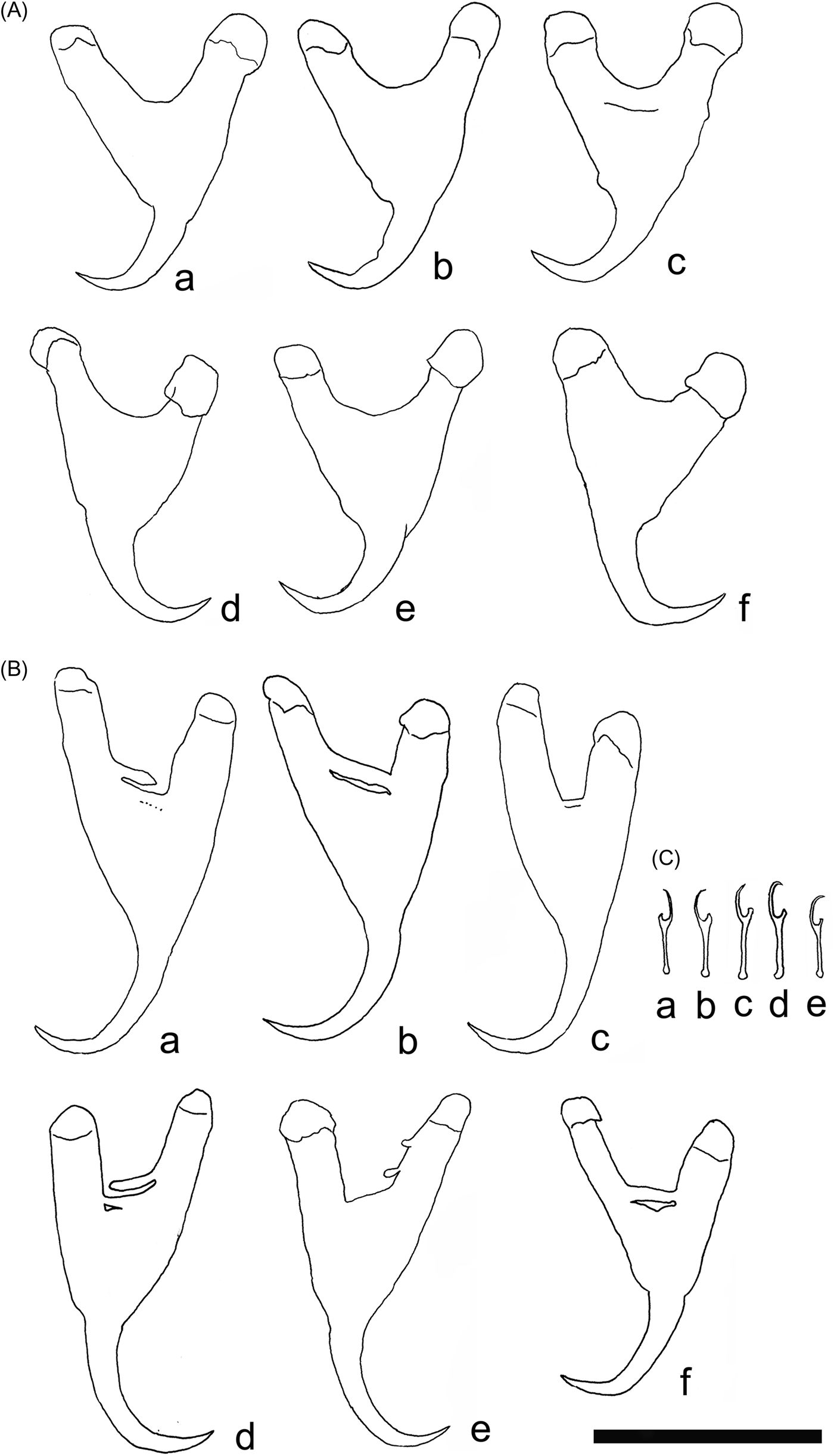
Fig. 3. Sclerotized parts of L. satunensis n. sp. and Ligophorus sp. I. (A) Ventral hamuli (a, holotype of L. satunensis n. sp.; b–e, paratypes of L. satunensis n. sp.; f, Ligophorus sp.). (B) Dorsal hamuli (a, holotype of L. satunensis n. sp.; b–e, paratypes of L. satunensis n. sp.; f, Ligophorus sp.). (C) Marginal hooks (a, holotype of L. satunensis n. sp.; b–d, paratypes of L. satunensis n. sp.; e, Ligophorus sp.). Scale bar 30 μm.
Remarks
L. satunensis n. sp. is characterized by fenestrated dorsal hamuli, a ventral bar with a long, bifurcated AMP without lateral pieces, a penis with a funnel-shaped base, and its accessory piece consisting of a non-grooved short base and a grooved distal part. The seminal receptacle was connected with the oviduct. No sclerotized tube was found around the seminal receptacle, suggesting the vagina is unarmed. Considering these morphological features, L. satunensis n. sp. is most similar to L. fenestrum Soo & Lim, 2012 in the fenestrated dorsal hamuli, a ventral bar with a long AMP and no lateral pieces, and an un-armed vagina. However, the present new species is different from L. fenestrum in the dorsal hamuli (up to two layers of fenestrations vs six layers), ventral hamuli (non-fenestrated vs fenestrated), penis (turning a full circle before reaching the genital pore vs turning less than half a circle), and the penis's accessory piece (non-grooved basally and grooved distally vs simply grooved along its entire length).
A bulb is attached to the funnel-shaped base of the penis. The bulb seems non-sclerotized, as it is highly variable in size and shape (fig. 5), suggesting its inflatable nature. Soo and Lim (Reference Soo and Lim2012) described the penis in L. fenestrum as having a bilobed base. From their drawing of the male copulatory organ, however, the two lobes, consisting of one spherical lobe and another smaller, ornamented one, are probably the bulb and the heel, respectively.
We noted the intraspecific morphological variations of the dorsal and ventral hamuli, dorsal and ventral bars, and male copulatory organ of L. satunensis n. sp. (figs. 2–4). Notably, variations in the shape of the dorsal hamuli were most significant (fig. 3C), with complete, incomplete, or almost no fenestration. With these variations, we did not measure the base length of the dorsal hamuli, as it was not possible to measure the length accurately.

Fig. 4. Sclerotized parts of L. satunensis n. sp. and Ligophorus sp. II. (A) Ventral bar (a, holotype of L. satunensis n. sp.; b–d, paratypes of L. satunensis n. sp.; e, Ligophorus sp.); (a), (d) and (e) are dorsally oriented, and (b) and (c) are ventrally oriented. (B) Dorsal bar (a, holotype of L. satunensis n. sp.; b–d, paratypes of L. satunensis n. sp.; e, Ligophorus sp.). Scale bar 30 μm.
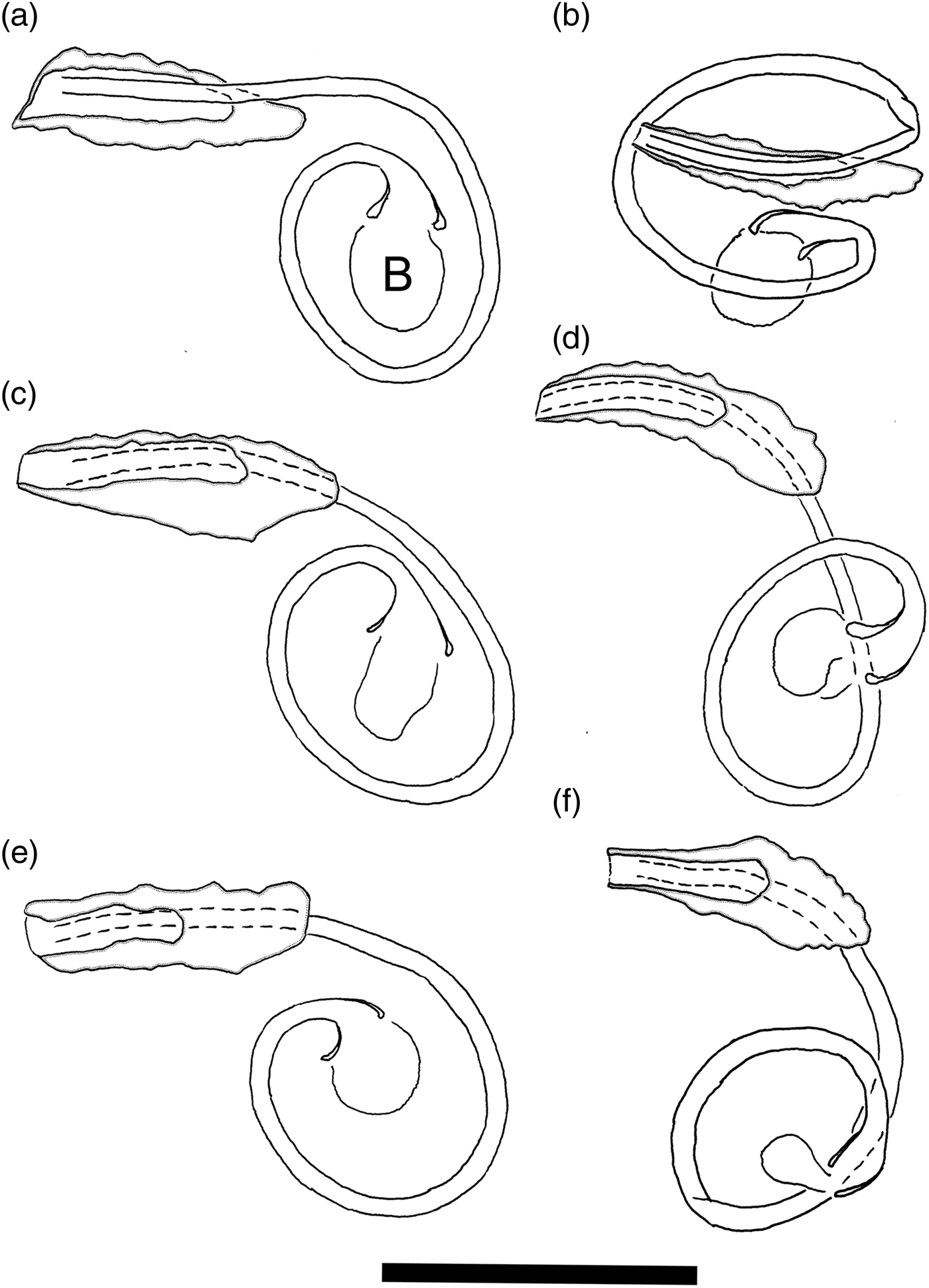
Fig. 5. Sclerotized parts of L. satunensis n. sp. and Ligophorus sp. III. Male copulatory organ. (a) Holotype of L. satunensis n. sp. (b–e) Paratypes of L. satunensis n. sp. (f) Ligophorus sp. Scale bar 30 μm.
Ligophorus sp. (figs. 3Af, Bf, Ce, 4Ae, Be & 5f)
Taxonomic summary
Type host. The bluetail mullet, C. buchanani (Bleeker, 1853).
Site of infection. Gills.
Locality. The Andaman Sea, Satun Province, southern Thailand (6°45′N, 99°50′E).
Date of collection. 23 March 2016.
Specimen deposited. MPM Coll. No. 21044 at the Meguro Parasitological Museum, Tokyo, Japan.
Material studied. One specimen.
Description of sclerotized parts
Ventral hamuli stout, with inner length 36, outer length 39, base 28, point 10, inner root 11, outer root 12. Dorsal hamuli longer than ventral hamuli, fenestrated (fig. 3Bf), with inner length 39, outer length 38, base 25, point 8, inner root 13, outer root 16. Ventral bar straight, length 43, width 8. AMP comprising short median piece, 16 long, bifurcated anteriorly, distance between bifurcations 2. Dorsal bar V shaped, length 56, width 5. Marginal hooks of larval type, length 12. Penis strongly curved, with funnel-shaped base, length 88 along the curved line, width 2. Bulb, 4 in diameter, attached to base of penis. Heel not clearly observed. Accessory piece straight, slightly tapering distally, length 24, width 9, comprising non-grooved short base and grooved distal part.
Remarks
This specimen was similar to L. satunensis n. sp. but was different in some sclerotized parts: a shorter AMP of the ventral bar (16 vs 22–25), shorter and more slender dorsal hamuli (39 vs 45–50 inner length; 38 vs 45–49 outer length). No significant differences were found in other sclerotized parts. As only one specimen was available for this study, we report it here as an unidentified Ligophorus.
Phylogenetic analysis
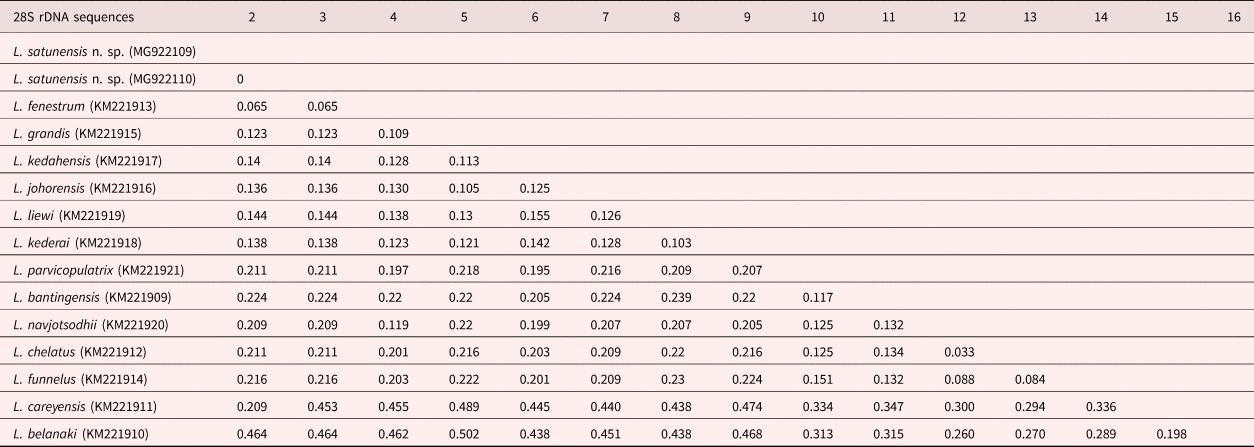
The ML and Bayesian inference (BI) methods using combinations between 28S and ITS1 rDNA sequences showed two main clades. Clade I comprised many Ligophorus spp. from South America, Europe and South-east Asia (L. belanaki Soo & Lim, 2015 and L. careyensis Soo & Lim, 2012), whereas Clade II represented Ligophorus from South-east Asia. Ligophorus satunensis n. sp. was monophyletically related to L. kederai Soo & Lim, 2015, L. kedahensis Soo & Lim, 2012, L. liewi Soo & Lim, 2015, L. johorensis Soo & Lim, 2015, L. grandis Soo, Tan & Lim, 2015, and L. fenestrum in Clade II-3 and was most closely related to L. fenestrum with high bootstrap support (fig. 6). Ligophorus satunensis n. sp. and L. fenestrum were genetically different for 28S and ITS1 rDNA sequences at 0.065 and 0.106, respectively (tables 2 and 3).

Fig. 6. Phylogenetic tree of Ligophorus spp. based on concatenated sequences of the ITS1 region and the 28S gene. Dactylogyrus dulkeiti was used as an outgroup. The ML bootstrap support/BPP is shown at each internal node. The branch length is drawn to scale, with the scale bar indicating the number of nucleotide substitutions.
Table 2. P genetic distances of 14 species of Ligophorus discovered off islands of Malaysia in the Strait of Malacca and the Andaman Sea, based on ITS1 sequences.
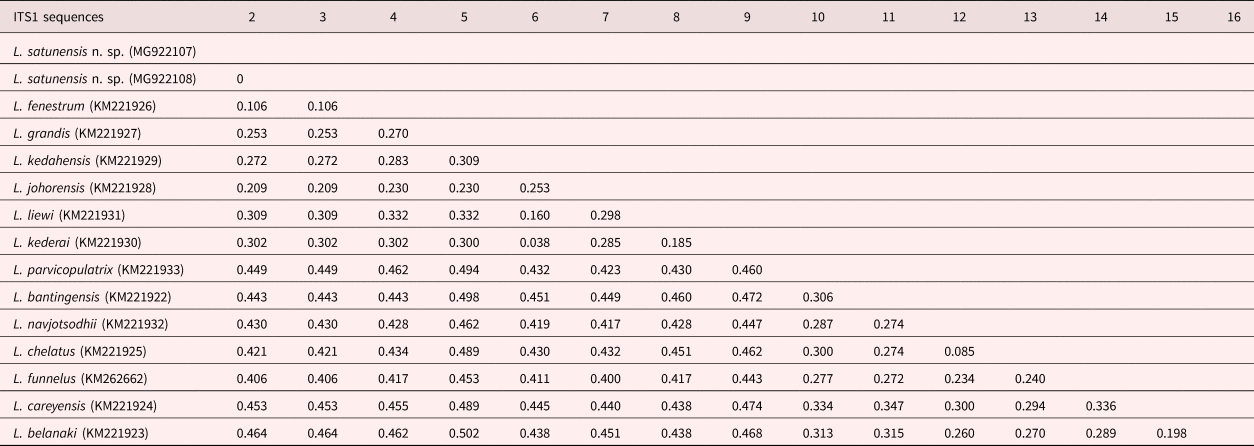
Table 3. P genetic distances of 14 species of Ligophorus discovered off islands of Malaysia in the Strait of Malacca and the Andaman Sea, based on 28S rDNA sequences.
Discussion
This study explored a new species in the genus Ligophorus, namely L. satunensis n. sp., found in the bluetail mullet, C. buchanani, caught off the coast of Satun Province. So far, 13 species of Ligophorus found in the mugilid fishes of Malaysia have been described, of which six species were recorded from C. buchanani (host name Valamugil buchanani in Soo and Lim, Reference Soo and Lim2012, Reference Soo and Lim2015, and Moolgarda buchanani in Soo et al., Reference Soo, Tan and Lim2015): L. kedahensis, L. fenestrum, L. kederai, L. liewi, L. johorensis and L. grandis. The first three species were collected from C. buchanani caught off Langkawi Island in the Andaman Sea, near our study area along the coast of Satun Province, whereas the other three were caught in the Straits of Johor. The three Ligophorus species from fishes caught off Langkawi Island were not part of this study, suggesting that host populations are different from each other despite the geographical proximity of the two sampling areas. Ligophorus satunensis n. sp. is the seventh species of Ligophorus found in C. buchanani, the fourteenth species from South-east Asia, and the first species discovered in Thailand.
So far, only sclerotized parts have been described for most new Ligophorus species. Among the valid species of Ligophorus (Soo and Lim, Reference Soo and Lim2012; El Hafidi et al., Reference El Hafidi2013a,Reference El Hafidib; Kritsky et al., Reference Kritsky, Khamees and Ali2013; Sarabeev et al., Reference Sarabeev2013), the entire bodies of only 11 species have been drawn (Euzet and Suriano, Reference Euzet and Suriano1977; Mariniello et al., Reference Mariniello2004; Sarabeev and Balbuena, Reference Sarabeev and Balbuena2004; Rubtsova et al., Reference Rubtsova2006, Reference Rubtsova, Balbuena and Sarabeev2007; Abdallah et al., Reference Abdallah, de Azevedo and Luque2009; Siquier and Nunez, Reference Siquier and Nunez2009; Soo and Lim, Reference Soo and Lim2012, Reference Soo and Lim2015; Sarabeev et al., Reference Sarabeev2013). In this study, we presented the anatomy of the reproductive organs of L. satunensis n. sp. The anatomy was consistent with the genus definition (Euzet and Suriano, Reference Euzet and Suriano1977; Sarabeev et al., Reference Sarabeev2013), except for the vagina, which was not observed, probably because fresh specimens were not available. Among less noted anatomical structures was the prostatic reservoir, which is elongated, extending posteriorly from the male copulatory organ and PGCs, which are widely distributed posterior to the reservoir. The prostate gland has been described only in L. vanbenedenii, L. domnichii and L. tainhae (Euzet and Suriano, Reference Euzet and Suriano1977; Rubtsova et al., Reference Rubtsova, Balbuena and Sarabeev2007; Abdallah et al., Reference Abdallah, de Azevedo and Luque2009).
Fenestrated hamuli are rare and known only in L. fenestrum (ventral and dorsal hamuli), L. kederai (ventral hamuli) and L. liewi (ventral hamuli) (Soo and Lim, Reference Soo and Lim2012, Reference Soo and Lim2015; Soo et al., Reference Soo, Tan and Lim2015). In this study, we found that the dorsal hamuli of L. satunensis n. sp. and Ligophorus sp. are fenestrated. However, fenestration was not as evident as in the previously known three species; in L. satunensis n. sp., the hamuli were only single layered, incomplete, or not fenestrated, and they were single layered in Ligophorus sp. As far as we know, fenestration is known only among Ligophorus species infecting C. buchanani, including the present two species. Regarding this characteristic, Khang et al. (Reference Khang2016) reported that fenestration of hamuli is invariant. The authors suggested that the larger and more robust humuli in Ligophorus species infecting C. buchanani in the open sea (Langkawi Island, close to Satun Province) may reflect the consequence of host adaptation to strong water currents.
In the phylogenetic tree, we divided Ligophorus species into two groups, Clade I and Clade II, and further divided Clade II into three subclades. Clade I comprises Ligophorus species from various mugilid species in many localities, Clade II-1 and Clade II-2 are all from Liza subviridis (Valenciennes, 1836) (now Planiliza subviridis; Valenciennes, 1836) in Malaysia, and Clade II-3 is from C. buchanani in Malaysia and Thailand. This phylogenetic relationship supported the hypothesis of host ecology correlated to the group of Ligophorus, particularly 14 species reported from Malaysia and Thailand (Khang et al., Reference Khang2016). On the basis of genetic distances and phylogenetic relationships, L. satunensis n. sp. is very close to L. fenestrum but far from L. liewi and L. kederai. This evidence supports the fact that fenestration is not a synapomorphic characteristic.
Regarding major diagnostic characteristics, the shape of sclerotized parts of Ligophorus, such as the AMP of the ventral bar and the dorsal and ventral hamuli, showed phenotypic variations within the specimens of L. satunensis n. sp (figs 3 and 4). Moreover, we also found that L. satunensis n. sp. was very similar to another species called Ligophorus sp. However, they could be differentiated by size of AMP of the ventral bar and shape and size of dorsal hamuli. Unfortunately, further description and study of the Ligoshorus sp. was not possible because we had only one specimen. This suggested that the shape of sclerotized parts of Ligophorus may be a rapid adaptation, which is affected by host size and variation in environmental conditions (Rohde, Reference Rohde1991). Rodríguez-González et al. (Reference Rodríguez-González2015) also suggested that the morphological variation reflects a host-driven plastic response, which is supported by the hypothesis of monogenean specificity through rapid speciation.
In terms of variations in sclerotized parts, many previous studies hypothesized that the difference in geographical areas and host species would affect the shape of sclerotized parts of the haptor, particularly in Ligophorus spp., because of adaptive evolution (Euzet and Suriano, Reference Euzet and Suriano1977; Euzet and Sanfilippo, Reference Euzet and Sanfilippo1983; Gusev, Reference Gusev and Bauer1985; Fernandez-Bargiela, Reference Fernandez-Bargiela1987; Dmitrieva and Gerasev, Reference Dmitrieva and Gerasev1996; Pan, Reference Pan1999; Rodríguez-González et al., Reference Rodríguez-González2016). This evidence suggests that the structure and measurement of sclerotized parts alone can pose the problem of species validity within the genus, because variation range and similarity between Ligophorus spp. may overlap each other (Dmitrieva et al., Reference Dmitrieva, Gerasev and Pron'kina2007; Sarabeev and Desdevises, Reference Sarabeev and Desdevises2014).
The discovery of a new species in the Thai and Malaysian seas indicates rich Ligophorus species diversity in South-east Asia. Further study of various mugilid species in different geographical areas is required in order to explore the diversity of the host–parasite relationship. In addition, further study of variations of sclerotized parts of the haptor, together with the phylogenetic relationship of Ligophorus species existing in Asia, is needed in order to understand the adaptation process and morphology of the host.
Acknowledgements
We would like to thank Dr Takashi Iwaki, Meguro Parasitological Museum, Japan for valuable guidance on morphological identification of helminths. We also thank Dr Makamas Sutthacheep, Marine Biodiversity Research Group, Department of Biology, Faculty of Science, Ramkhamhaeng University, Bangkok, Thailand for helpful comments. We would like to thank the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Thailand for facilitating the laboratory instruments. The manuscript was edited for English language by the Office of Research Service (ORS), Faculty of Tropical Medicine, Mahidol University, Thailand.
Financial support
This study was partially supported by a budget for research promotion from the Thai Government to Ramkhamheang University and Faculty of Tropical Medicine, Mahidol University, Thailand. The manuscript was edited for English language by the Office of Research Services (ORS), Faculty of Tropical Medicine, Mahidol University, Thailand.
Conflict of interest
None.











