Introduction
The Echinococcus genus includes zoonotic endoparasites of the Taeniidae family that affects humans as well as domestic and wild mammals. Echinococcosis or hydatidosis is associated with low socio-economic levels and lack of health education (Martínez et al., Reference Martínez, Cáceres and Canals2016). This affliction, considered a neglected disease by World Health Organization (WHO), is little known, poorly studied, and it remains asymptomatic and undetected in many cases (WHO, 2018).
This zoonosis is distributed worldwide, and more than one million people are affected by the illness. South America has been recognized as an endemic area with hyperendemic regions, especially in countries like Argentina, southern Brazil, Uruguay, Chile and mountainous regions of Peru and Bolivia (Álvarez-Rojas, Reference Álvarez-Rojas2016; Cucher et al., Reference Cucher, Macchiaroli and Baldi2016). In Ecuador, this parasitism is distributed with a low prevalence compared to neighbouring countries. The country reported 144 cases between the years 2007 and 2017 (Berger, Reference Berger2019), of which 15 were registered in the hospitals of Guayas and Pichincha provinces (INEC, 2017).
Species of Echinococcus recognized worldwide are E. granulosus sensu lato (s.l.), E. multilocularis, E. vogeli, E. oligarthra and E. shiquicus (Álvarez-Rojas, Reference Álvarez-Rojas2016; Cucher et al., Reference Cucher, Macchiaroli and Baldi2016). Echinococcus granulosus s.l. is described as a cryptic complex (Nakao et al., Reference Nakao, Lavikainen, Yanagida and Ito2013), and is composed of ten genotypes: E. granulosus sensu stricto (s.s.) (G1/G2/G3), E. equinus (G4), E. ortleppi (G5), E. canadensis (G6/G7/G8/G10) and E. felidis (‘lion strain’) (Álvarez-Rojas et al., Reference Álvarez-Rojas, Romig and Lightowlers2014; Roming et al., Reference Roming, Ebi and Wassermann2015; Cucher et al., Reference Cucher, Macchiaroli and Baldi2016). Finally, the G9 genotype described by Scott et al. (Reference Scott, Stefaniak, Pawlowski and McManus1997) is now considered to belong to the G7 genotype.
The adult form of E. granulosus s.l. is established in the small intestine of infected canids (definitive hosts), which releases embryonated eggs into the faeces. The intermediate host (livestock) ingests these eggs from the environment. The oncosphere penetrates the intestine, and migrates through the circulatory system into various organs, especially the liver and lungs where it develops into the metacestodes. Internally, the mature cyst generates protoscoleces for asexual reproduction. The definitive host becomes infected by ingesting organs infected with cysts. Then, the protoscoleces adhere to the intestinal mucosa, and develop into adult stages. The human is infected by the parasite (accidental intermediate host) when ingesting food and/or contaminated water, or by direct contact with the faeces of infected dogs (CDC, 2002; Álvarez-Rojas, Reference Álvarez-Rojas2016; Pavletic et al., Reference Pavletic, Larrieu and Guarnera2017).
In this research, we analysed the hydatid sand of a metacestode found in a female pig for human feed. The parasite species was identified by morphometric analysis, molecular amplification by polymerase chain reaction (PCR) and sequencing of amplicons.
Materials and methods
Hydatid treatment
Infected porcine liver was collected from an abattoir in Quito, Ecuador. It belonged to an adult female Yorkshire pig with a normal size and low weight. The female pig came from a backyard hatchery of Rumiñahui canton. The tissue was carried immediately to the laboratory at a temperature between 4 and 8°C.
In the laboratory, cysts were removed from the surface of the liver parenchyma. The hydatid fluid was drained by aspiration, and was centrifuged at 3500 RPM for 5 min. The hydatid sand was stored at −80°C for further analysis.
Morphometric study
The morphometric identification was carried out according to the method described by Girard de Kaminsky (Reference Girard de Kaminsky2003) and D’Alessandro & Rausch (Reference D'Alessandro and Rausch2008), with some modifications. Briefly, the hydatid sand was homogenized. A drop of the material was settled in 0.2 ml of saline solution placed on a microscope slide. Then, the sample was covered, and was disintegrated with light pressure in a circular way. The total length of rostellar hooks (n = 30) arranged in a flat position was measured with 100× magnification in a microscope (Motic, Hong Kong, China) coupled to the software Images Plus 2.0 (Motic, Hong Kong, China).
Molecular assay
Genomic DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Wisconsin, USA) following the protocols described by the manufacturer. The parasitic samples contained 50 mg of hydatid sand. The DNA was quantified in a Nanodrop 2000 (Thermo Fisher Scientific, Massachusetts, USA) with absorbance of 260–280 nm. A pure sample of protoscoleces of E. granulosus s.l. G6/G7 strain confirmed by Sanger sequencing was used as a positive control.
The PCR primers (FW 5′-TGGTTTGGCAGTGAGCGAT-3′ and RV 5′-ACTCCAATAAGCAGCACATAGACT-3′) were developed to amplify 168 bp of a DNA fragment of the ribosome of E. granulosus. Each PCR reaction was performed in a total volume of 25 µl containing 10 µl of the sample, 1.25 U GoTaq® Flexi DNA Polymerase (Promega, Wisconsin, USA), 1.25 U/μl of buffer, 2.5 mM of magnesium chloride, 320 µM of deoxyribonucleotides triphosphate (dNTPs) (Promega, Wisconsin, USA) and 0.5 µM of each primer. The PCR included an initial denaturation at 94°C for 7 min, followed by 35 cycles (94°C for 35 s, 55°C for 35 s, 72°C for 40 s) and a final elongation step at 72°C for 5 min.
The resulting samples were electrophoresed in 2% agarose gel, stained with SYBR Safe DNA 10% (Promega, Wisconsin, USA) and visualized in Gel Doc XR+ System (BioRad, California, USA). The amplification products were sent to IDgen (Quito, Ecuador) to be sequenced using the Sanger method.
Results and discussions
Parasitic samples
Porcine tissue presented unilocular cysts rooted in the liver parenchyma in all of its lobes. Macroscopically, the protuberances were of different sizes. The external surface had a yellowish white colour and soft consistency with mucous material. The excised unilocular cysts had diameters of 43, 39 and 36 mm. Morphologically, the layers identified were the pericyst, a viscous colourless laminar cover and a thick whitish germinative membrane without internal cysts. The hydatid fluid had a transparent yellowish tone.
Eckert et al. (Reference Eckert, Gemmell, Meslin and Pawlowski2001) and Agudelo-Higuita et al. (Reference Agudelo-Higuita, Brunetti and McCloskey2016) reported that metacestodes grow from the oncosphere, and are a cystic structure typically filled with a clear fluid (hydatid fluid). The post-oncospheral development takes 10–14 days. By this time, the bladder (measuring 60 µm–70 µm in diameter) consists of a nucleated germinal layer and a thin laminated layer which lacks nuclei. Most of the cysts grow slowly in size and become surrounded by host tissue (pericyst) encompassing the endocyst of metacestode origin. The endocyst consists of the outer laminated layer and the inner cellular germinal layer, which may form brood capsules and protoscoleces.
In addition, all abdominal and thoracic organs can be invaded by cysts of hepatic origin or by metastases in distant organs, unless the latter are cysts of primary location (D'Alessandro & Rausch, Reference D'Alessandro and Rausch2008; Vizcaychipi et al., Reference Vizcaychipi, Sosa, Camicia, Santillán, Casalins and Nigro2012).
Identification of the species
Invaginated protoscoleces (n = 63) have an oval shape and a total of 30 hooks arranged in a double crown on the rostellum (fig. 1).
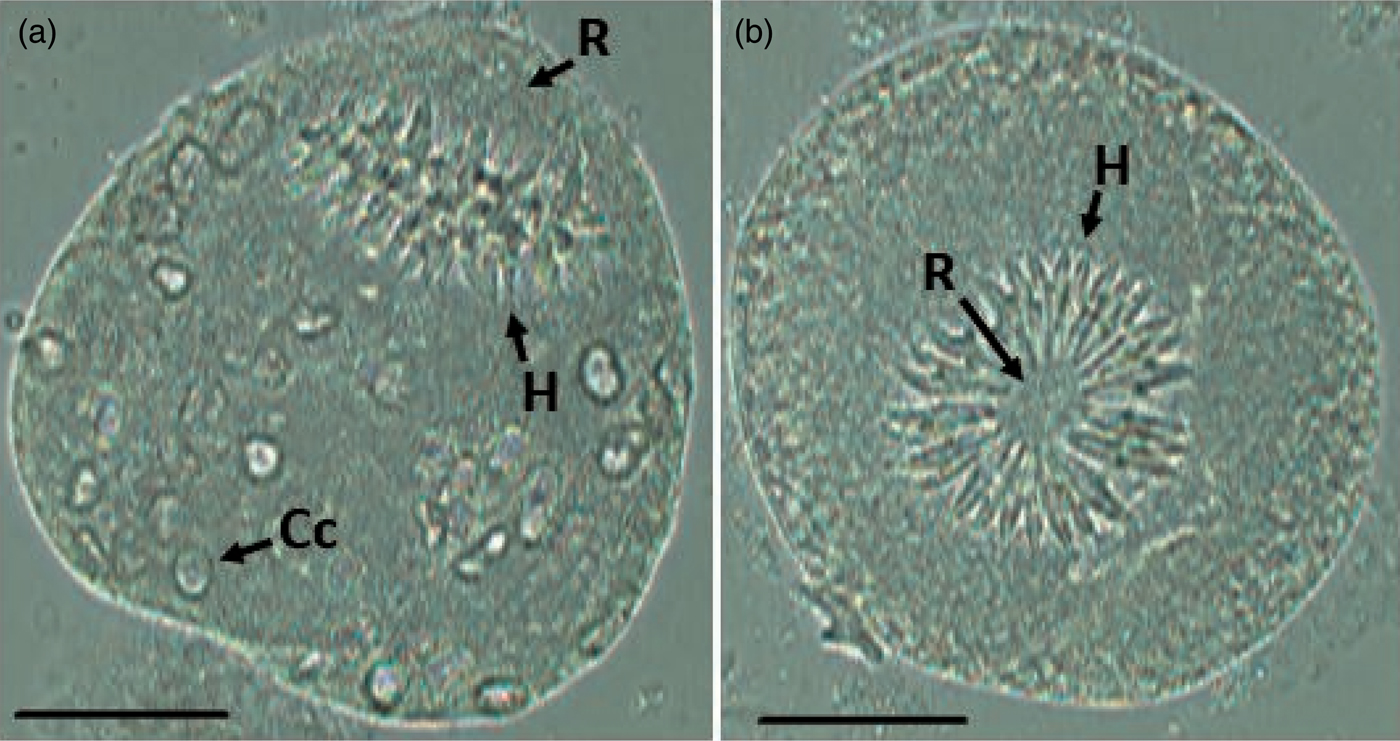
Fig. 1. Invaginated protoscolex visualized freely in the hydatid fluid of a pig. (a) Side view; (b) front view. Scale bars: 50 µm. Abbreviations: H, hooks; R, rostellum; Cc, calcareous corpuscles.
The time of development of the hydatids is variable, and it may take several months before protoscoleces are produced (fertile metacestode). There may be several thousand protoscoleces within a single cyst of E. granulosus. Each single protoscolex is capable of developing into a sexually mature adult worm. Not all metacestodes produce protoscoleces (sterile metacestode). When protoscoleces are ingested by a suitable definitive host, following the action of pepsin in the stomach, they evaginate in the upper duodenum in response to a change in pH, exposure to bile and to increased temperature. They then develop into the sexually mature adult tapeworm, approximately four to six weeks after infection, depending on the species and strain, and on the susceptibility of the host (Eckert et al., Reference Eckert, Gemmell, Meslin and Pawlowski2001; Rosales et al., Reference Rosales, Gavidia, Lopera, Barrón, Ninaquispe, Calderón and Gonzáles2008; Agudelo-Higuita et al., Reference Agudelo-Higuita, Brunetti and McCloskey2016).
After crushing non-adult parasites, the microscopic observation defined numerous large rostellar hooks arranged in a flat plane. Morphologically, they had a curved back with a characteristic slit in the handle–edge division, and the heel was projected in the middle of the hook (fig. 2). In addition, the hooks (n = 30) had an average length of 27.367 µm (standard error = 0.319 µm).

Fig. 2. Large rostellar hook of Echinococcus granulosus. Scale bar: 10 µm. Abbreviations: S, slit; E, edge; Ha, handle; He, heel.
Regarding the morphometric assessment of rostellar hooks, Eckert et al. (Reference Eckert, Gemmell, Meslin and Pawlowski2001) states that E. oligarthrus and E. granulosus have dimensions between 25.4 and 27.3 µm, and 22.6 and 27.8 µm, respectively, coinciding with the dimensions obtained in this study. To differentiate, Girard de Kaminsky (Reference Girard de Kaminsky2003) and D’Alessandro & Rausch (Reference D'Alessandro and Rausch2008) mention that the edge of the hook of E. oligarthrus has a straight back, and the handle measures half the length of the hook, whereas the edge of E. granulosus is longer than the handle.
The molecular amplification by conventional PCR defined bands from samples of hydatid sand (fig. 3). The 168 bp sequences of the amplified products were aligned with BLAST using the sequences present in the National Center for Biotechnology Information (NCBI). The results revealed an identity of 100% for E. granulosus s.l. strain G6/G7 (E. canadensis G6/G7) mitochondrial partial 12S rRNA (accession number HG975348.1).

Fig. 3. Agarose gel of molecular amplification of Echinococcus canadensis G6/G7 samples 1: Positive control; 2: no template control; 3–5: hydatid sand samples. M, molecular weight marker.
Echinococcus granulosus s.l. is composed of numerous variants initially identified by Smyth & Davies (Reference Smyth and Davies1974) who called them physiological strains. Since then, more strains were identified and several works have shown that they differ in many features (Álvarez-Rojas et al., Reference Álvarez-Rojas, Romig and Lightowlers2014; Roming et al., Reference Roming, Ebi and Wassermann2015).
Nakao et al. (Reference Nakao, Lavikainen, Yanagida and Ito2013) found that E. granulosus s.l. is cosmopolitan and is focused in endemic areas of South America. Cucher et al. (Reference Cucher, Macchiaroli and Baldi2016) and Pavletic et al. (Reference Pavletic, Larrieu and Guarnera2017) identified E. granulosus s.s. (G1), E. ortleppi (G5) and E. canadensis (G7) genotypes in samples of livestock and humans in Brazil. Genotypes E. granulosus s.s. (G1) and E. canadensis (G6/G7) were found in samples of population and livestock of Peruvians. Genotypes of E. granulosus s.s. (G1/G2/G3), E. ortleppi (G5) and E. canadensis (G6/G7) were analysed in samples of tissue from livestock and humans in Argentina. With respect to the worldwide situation of human cystic echinococcosis, E. granulosus s.s. (G1 genotype) accounts for most of the global burden, followed by E. canadensis (G6 and G7 genotypes).
Based on this, the presence of the E. granulosus s.l. strain E. canadensis G6/G7 has been demonstrated in Ecuador, since other documents only described macroscopic findings of hydatid cysts in livestock. Moreover, the few related reports available in the country concern clinical cases of polycystic hydatid disease caused by E. vogeli (D’Alessandro et al., Reference D'Alessandro, Rausch, Cuello and Aristizábal1978; Calvopiña et al., Reference Calvopiña, Cartagena, López, Guerrero, Amunarriz, Guderian and Guevara1993). These cases are distributed in focused provinces of continental Ecuador.
Acknowledgements
The authors would like to acknowledge the support and collaboration of all researchers of the National Program for the Multidisciplinary Approach of Neglected Parasitism in Ecuador (PROPAD), especially MSc Eliana Champutiz. In addition, we would like to express our gratefulness to PhD Emmanuel Quentin, PhD Patricio Ponce and Pablo Ordoñez for the revision of this article.
Financial support
This work was supported by the National Secretary of Superior Education, Science, Technology, and Innovation (SENESCYT), Ecuador (grant number 20130372, 2013).
Conflicts of interest
None.
Ethical standards
All procedures in the abattoir was realized by a Veterinary and Zootechnics Medical, and the ethical standards were in accordance with Ecuadorian regulations.





