Introduction
Sympatric pairs of whitefish belonging to the Coregonus lavaretus complex inhabit many oligotrophic lakes in the Palearctic region of the northern hemisphere. These lacustrine sympatric pairs serve as an intriguing model for investigating different aspects of fish evolutionary biology (e.g. Østbye et al., Reference Østbye, Amundsen, Bernatchez, Klemetsen, Knudsen, Kristoffersen, Næsje and Hindar2006; Solovyev et al., Reference Solovyev, Kashinskaya, Bochkarev, Andree and Simonov2019). Within such pairs, a very important feature is the demonstration of different feeding habits for each within the same lake. The relatively low level of productivity of oligotrophic lakes enforces whitefish to use food items from different taxonomical groups in order to assimilate the available food supply of such lakes efficiently. As a result, it leads to trophic diversification of whitefish into planktivorous ‘dwarf’ and benthivorous ‘normal’ forms that, consequently, decrease the competition for the food resources of the lake (e.g. Bernatchez, Reference Bernatchez, Hendry and Stearns2004). Lake Teletskoye (West Siberia, Russia) is inhabited by one such sympatric whitefish pair (Bochkarev & Zuikova, Reference Bochkarev and Zuikova2006). Coregonus lavaretus pravdinellus (Dulkeit, 1949) is a small form that may reach only around 17 cm Smith's fork length and 40 g of total body weight at its maximum age (7 years old), while C. l. pidschian (Gmelin, 1789) is a larger form (20–25 cm Smith's fork length, 150 g total body weight, at the age of 7 years) with age exceeding 14 years (Bochkarev & Zuikova, Reference Bochkarev and Zuikova2006; Bochkarev, Reference Bochkarev2009). The first mention of whitefish from Lake Teletskoye can be found in the year 1865, in the article entitled ‘About herrings from Lake Teletskoye ’ in the newspaper ‘The news of Tomsk Province/Tomskie gubernskie novosti’ written by Gulyaev (Reference Gulyaev1865). At the present time, their origin, phylogenetic relationships and taxonomic position are still disputed (Politov et al., Reference Politov, Gordon, Baldina, Litvinenko and Reshetnikov2010). According to the analysis of mtDNA they are genetically very closely related to each other and share the same haplotypes (Bochkarev et al., Reference Bochkarev, Zuykova and Katokhin2011). Although the purpose of this work is not to rectify taxonomical issues, due to the intricate taxonomical position of these fish, we will consider these fish as ‘forms’ and obviate any discussion of subspecies, etc. These two forms will be hereafter referred to as ‘benthivorous’ for C. l. pidschian and ‘planktivorous’ for C. l. pravdinellus.
Generally, the occurrence of a parasite species in a host population depends on the host's genetic factors and feeding preferences (Pulkkinen et al., Reference Pulkkinen, Valtonen, Niemi and Poikola1999; Francová & Ondračková, Reference Francová and Ondračková2011). It has been shown that parasites provide information on various aspects of their host's biology and can serve as a specific indicator of the host's habitat selection and feeding behaviour (Bertrand et al., Reference Bertrand, Marcogliese and Magnan2008). Members of the genus Triaenophorus are widely distributed in fish in the northern hemisphere (Valtonen et al., Reference Valtonen, Rintamäki and Lappalainen1989) and have a complex life cycle, with two intermediate hosts and one definitive host. It is well known that plerocercoids of this parasite invade the muscle tissue of coregonid and salmonid fishes and negatively affects the growth of this intermediate host (Kuperman, Reference Kuperman1981; Schähle et al., Reference Schähle, Medgyesy and Psenner2016). Copepods, as the first intermediate host, are infected by procercoids of T. crassus and then transmitted to the second intermediate host (coregonid and salmonid fishes). At the end, pike Esox lucius feeds on infected fish and the life cycle of T. crassus is completed (Kuperman, Reference Kuperman1981; Rosen & Dick, Reference Rosen and Dick1984; Pulkkinen et al., Reference Pulkkinen, Valtonen, Niemi and Poikola1999). In Lake Teletskoye T. crassus was found in muscles of both forms of whitefish, as the second intermediate host, and in E. lucius as a definitive host (Titova, Reference Titova1954). From this point of view, the sympatric pair of whitefish in Lake Teletskoye is an applicable model for the estimation of differences in feeding habits via assessment of their parasite community.
We hypothesized that trophic diversification of whitefish into ‘planktivorous’ and ‘benthivorous’ forms is likely to affect the transmission of T. crassus via food webs (higher in ‘planktivorous’ form than in ‘benthivorous’ one).
The main aim of the present study was to estimate the relation between different feeding habits of the ‘benthivorous’ and ‘planktivorous’ forms and the infection level of T. crassus plerocercoids in their muscles. Additionally, as cestodes are a species-rich taxa and can seriously impact fisheries, aqua- and agriculture, and wildlife conservation, this study includes work to augment existing DNA databases on these species to advance DNA barcode studies. For this, the mitochondrial cox1 region was amplified for analysis to partially sequence the cox1 gene of T. crassus in order to provide improved resolution to future phylogenetic analyses for this species of cestode.
Materials and methods
Study area and sampling
Fish were collected from the end of August to the middle of September in 2017 and 2019–2020 in the north part of Lake Teletskoye (51°79'N; 87°30'E). Lake Teletskoye is a large (223 km2) and deep (maximum 325 m) oligotrophic lake (basin of Ob River) in the Altai Mountains (Altai Republic, Russia). The water temperature of the upper 1 m of the water column ranged from 17.1 to 18.3°C. All fish were captured using gill-nets (mesh sizes 18 and 22 mm) at depths from 2 to 15 m and transported to the laboratory in the Lake Teletskoye field station of the Institute of Systematic and Ecology of Animals SB RAS. The fish were anesthetized on ice prior to sacrifice. Afterwards, the fish were identified, measured (standard length, SL) and weighed (total body weight, BW) with accuracy to 0.1 mm and 0.1 g respectively. In order to analyse the prevalence of infection of T. crassus in fish muscle, we collected 67 (SL = 214.5 ± 4.3, BW = 134.8 ± 7.6), 25 (SL = 211.4 ± 3.9, BW = 146.6 ± 8.6) and 54 (SL = 186.4 ± 4.7, BW = 89.0 ± 9.2) individuals of the ‘benthivorous’ form and 38 (SL = 136.4 ± 0.8, BW = 28.2 ± 0.5), 30 (SL = 136.1 ± 1.9, BW = 31.3 ± 1.0) and 33 (SL = 133.1 ± 1.5, BW = 26.8 ± 0.9) individuals of the ‘planktivorous’ form in the years 2017, 2019 and 2020. The fish age was estimated using scales sampled from the left side of the body between the dorsal fin and lateral line (only for 2017 year of sampling, table 2).
The parasitological analysis of fish muscle was performed as described by Schähle et al. (Reference Schähle, Medgyesy and Psenner2016). The prevalence and mean intensity of parasite infestation were calculated according to the definitions by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). The prevalence (P) of parasite infestation (in %) was calculated as:
Where I is number of host infected, and N is total number of host examined. The error of prevalence index (E) was calculated by the following formula:
where P is prevalence, and N is total number of host examined.
Mean intensity of invasion (I) was assessed as the average of number of individuals of a particular parasite species (K) in a single infected host (n):
Mean abundance of invasion (A) was assessed as the average of number of individuals of a particular parasite species (K) in all studied host (n):
Error of intensity and abundance indices (SE) was calculated according to:
Where SD is standard deviation of row of number of individuals of a particular parasite species in a single infected host, and n is total number of infected hosts (intensity) or all hosts (abundance).
One hundred and thirteen individuals of T. crassus were mounted in Berlese's medium and used to measure the morphological parameters of the scolex hooks (fig. 1). All measurements of scolex hooks were done as described in Kuchta et al. (Reference Kuchta, Vlckova, Poddubnaya, Gustinelli, Dzika and Scholz2007) using an Axiolab microscope (Carl Zeiss, Germany) with software Toup View vx86, 3.7.2608 (ToupTekPhotonics, China). All measurements for scolex hooks are given in micrometres (μm).

Fig. 1. Measured morphological parameters of the scolex hooks of T. crassus according to Kuperman (Reference Kuperman1968, Reference Kuperman1973) and Kuchta et al. (Reference Kuchta, Vlckova, Poddubnaya, Gustinelli, Dzika and Scholz2007). 1, width of basal plate; 2, height of basal plate; 3, length of longer lateral prong; 4, length of shorter lateral prong; 5, distance between longer lateral and median prongs; 6, distance between shorter lateral and median prongs; 7, distance between both lateral prongs.
All data are presented as a mean ± standard error (SE). To estimate the differences between parasite intensity (only for the ‘planktivorous’ form) and abundance across different age groups (2017) and sampling years (2017 and 2019–2020), as well as in terms of fish standard length among different sampling years (2017, 2019, and 2020), the Mann–Whitney test was applied using PAST v. 3.16 (Hammer et al., Reference Hammer, Harper and Ryan2001). In the same programme, to explore the correlation between parasite abundance and fish standard length, a Spearman rank correlation test (ρ) was used. The effect of ‘whitefish form’ as a factor on the parasite abundance and intensity was tested using one-way ANOVA at P ≤ 0.05. The effect of ‘age’ as a factor on the parasite abundance and intensity was tested using one-way ANOVA at P ≤ 0.05 only for age groups where there were infected individuals.
DNA extraction, amplification and sequencing
Total DNA was extracted from 15 ethanol preserved individuals of T. crassus using the DNA-sorb B kit (Central Research Institute of Epidemiology, Russia) according to the manufacturer's protocol. To obtain a barcode sequence for T. crassus, we amplified a 593 bp portion of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1). Amplification was performed using the primers and PCR conditions as described in Van Steenkiste et al. (Reference Van Steenkiste, Locke, Castelin, Marcogliese and Abbott2015). The PCR products were purified by adsorption on Agencourt Ampure XP (Beckman Coulter, Indianapolis, IN, USA) columns and subjected to Sanger sequencing using the BigDye Terminator V.3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) with subsequent unincorporated dye removal by the Sephadex G-50 gel filtration (GE Healthcare, Chicago, IL, USA). The Sanger products were analysed on an ABI 3130XL Genetic Analyzer (Applied Biosystems). The purification and sequencing of PCR products were performed in the SB RAS Genomics Core Facility (Novosibirsk, Russia). The manual editing and alignment of sequences were performed with MEGA 7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). The number of haplotypes and levels of DNA polymorphism were calculated using the program DNASP 6 (Rozas et al., Reference Rozas, Ferrer-Mata, Sánchez-DelBarrio, Guirao-Rico, Librado, Ramos-Onsins and Sánchez-Gracia2017). Sequences were deposited into GenBank (NCBI) under the following accession numbers: MT951570–MT951584.
Results
Fish
All results of ANOVA (Mann–Whitney test) comparisons among different years for the ‘benthivorous’ and ‘planktivorous’ forms are given in table 1. The SL and BW of the ‘benthivorous’ form were significantly lower in 2020 (SL = 186.4 ± 4.7, BW = 89.0 ± 9.2) than in 2017 (SL = 214.5 ± 4.3, BW = 134.8 ± 7.6) and 2019 (SL = 211.4 ± 3.9, BW = 146.6 ± 8.6). We have found similar trends for the ‘planktivorous’ form: lower SL and BW in 2020 (SL = 133.1 ± 1.5, BW = 26.8 ± 0.9) than in 2017 (SL = 136.4 ± 0.8, BW = 28.2 ± 0.5) and 2019 (SL = 136.1 ± 1.9, BW = 31.3 ± 1.0). Positive significant correlation was found between SL and BW for the ‘benthivorous’ (Spearman ρ = 0.97, P = 6.16 × 10−43, n = 67, 2017 year; ρ = 0.80, P = 0.0000021, n = 25, 2019; ρ = 0.96, P = 2.90 × 10−30, n = 54, 2020) and ‘planktivorous’ (Spearman ρ = 0.77, P = 0.0000013, n = 38, 2017; ρ = 0.76, P = 0.0000013, n = 30, 2019 year; ρ = 0.65, P = 0.000037, n = 33, 2020) forms for all studied years. Thus, further comparisons between parasitological indices were done with SL only.
Table 1. The results of ANOVA and Mann–Whitney test comparisons among different years for the ‘benthivorous’ and ‘planktivorous’ forms.

BW, body weight; SL, standard length; F, F values, Df., degrees of freedom, and p – p values are from ANOVA; (U), U values and (p), p values are for pair wise comparisons calculated by Mann–Whitney test from ANOVA.
Infection rate of T. crassus in muscle of the ‘planktivorous’ and ‘benthivorous’ forms
The relationship between number of cysts and fish SL for the ‘planktivorous’ form is shown in fig. 2a. The average of intensity and abundance of parasite infection in fish muscle ranged from 4.2 ± 0.6 to 4.8 ± 0.5 and from 4.0 ± 0.6 to 4.7 ± 0.5, respectively. In the muscle of the ‘planktivorous’ forms, the prevalence of T. crassus infestation ranged from 94.7 ± 3.6% (2017) and 97.5 ± 2.5% (2019) to 97.0 ± 3.0% (2020) (fig. 2a). A positive correlation was found between SL of the ‘planktivorous’ form and the number of cysts in muscle tissue in 2017 (Spearman ρ = 0.26, P = 0.12, n = 38) and 2019 (Spearman ρ = 0.30, P = 0.11, n = 30), whereas in 2020 the correlation was significantly negative (Spearman ρ = −0.39, P=0.02, n = 33). Fish gender (‘sex’) was not a factor affecting the abundance in fish muscle in 2017 and 2019 (ANOVA, F = 1.36, P = 0.26, df = 37, 2017 year and F = 1.52, P = 0.20, df = 30, year 2019).
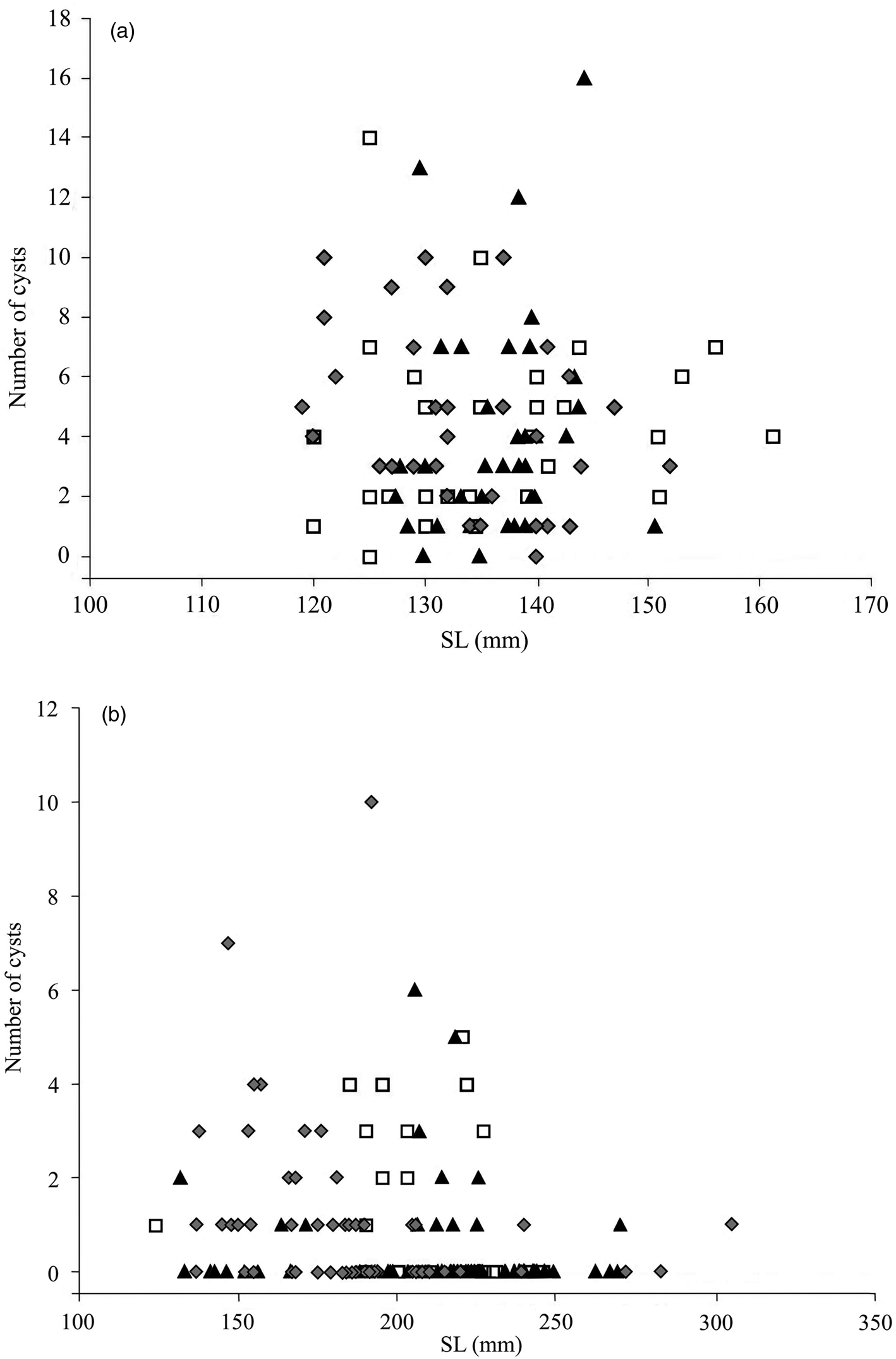
Fig. 2. (a) Abundance of T. crassus in muscle of the ‘planktivorous’ form in Lake Teletskoye in studied years; ▴ 2017, □ 2019, ♦ 2020. (b) Abundance of T. crassus in muscle of the ‘benthivorous’ form in Lake Teletskoye in studied years; ▴ 2017, □ 2019, ♦ 2020.
The relationship between abundance of T. crassus in muscle and fish SL for the ‘benthivorous’ form is shown in fig. 2b. The average of intensity and abundance of parasite infection in fish muscle ranged from 1.9 ± 0.4 to 2.8 ± 0.3 and from 0.4 ± 0.1 to 1.3 ± 0.3, respectively. The prevalence of T. crassus infestation ranged from 22.4 ± 5.1 (2017) and 46.7 ± 9.3 (2019) to 51.9 ± 6.8 (2020) (fig. 2b). Negative correlation between SL of the ‘benthivorous’ form and abundance was found in 2017 (Spearman ρ = −0.13, P = 0.29, n = 67), 2019 (Spearman ρ = −0.19, P = 0.38, n = 25) and 2020 (Spearman ρ = −0.44, P=0.0009, n = 54). The factor ‘sex’ had no effect on the abundance in fish muscle (ANOVA, F = 0.26, P = 0.44, df = 64, 2017 year).
The abundance of T. crassus in muscle of the ‘planktivorous’ form was significantly higher (Mann–Whitney test) than that in the muscle of the ‘benthivorous’ form for all age groups in 2017 year (where these calculations could be performed): age 3+ (U = 3, P = 0.041), age 4+ (U = 5.5, P = 0.0031), and age 5+ (U = 0, P = 0.0015) (table 2). The effect of ‘whitefish form’ as a factor on the level of intensity (ANOVA, F = 5.62, P = 0.022, df = 50, 2017; F = 2.36, P = 0.13, df = 40, 2019; F = 16.40, P = 0.00015, df = 59, 2020) and abundance (ANOVA, F = 56.24, P = 2.33 × 10−11, df = 104, 2017; F = 15.97, P = 0.0002, df = 54, 2019; F = 47.78, P = 8.26 × 10−10, df = 86, 2020) of T. crassus in muscle was significant (intensity for the year 2019 was exception) (fig. 3a, b). The level of prevalence was higher in the years 2019 and 2020 than in 2017 for the ‘benthivorous’ form, whereas for the ‘planktivorous’ form this index did not change during the studied years (fig. 3b).

Fig. 3. (a) The average levels of intensity (□ ‘planktivorous’ form; ■ ‘benthivorous’ form) and prevalence (○-‘planktivorous’ form; ● ‘benthivorous’ form) of T. crassus in muscle of both forms in Lake Teletskoye during studied years. (b) The average levels of abundance (□ ‘planktivorous’ form; ■ ‘benthivorous’ form) of T. crassus in muscle of both forms in Lake Teletskoye during studied years. The asterisk denotes statistically significant differences (ANOVA at P ≤ 0.05).
Table 2. Prevalence, intensity, and abundance of T. crassus in muscle of the ‘benthivorous’ and ‘planktivorous’ forms in Lake Teletskoye in 2017.

ND, no data; N, number of individuals; nd, not determined (see the Materials and methods); the lowercase letters denote significant differences among the age groups for each whitefish forms (ANOVA, Mann–Whitney test).
No significant effects of ‘age’ as a factor on the levels of intensity (only for the ‘planktivorous’ form: ANOVA, F = 1.38, df = 31, P = 0.27) and abundance (ANOVA, F = 1.92, df = 35, P = 0.15 for the ‘planktivorous’ form and ANOVA, F = 1.20, df = 60, P = 0.32 for the ‘benthivorous’ form) for both forms were found. But the significant differences (Mann–Whitney test) for level of abundance between ages 6+ and 8+ (U = 49.5, P = 0.042) for the ‘benthivorous’ form and between ages 4+ and 5+ (U = 10.5, P = 0.031) for the ‘planktivorous’ form were found (table 2). For the ‘planktivorous’ form the significant difference (Mann–Whitney test) for level of intensity were found only between ages 4+ and 5+ (U = 10.5, P = 0.037) (table 2), whereas for the ‘benthivorous’ form it was impossible to estimate these differences due to the low number of infected whitefish in most of the age groups.
Scolex hook measurements and DNA sequencing
Measurements of scolex hooks are summarized in table 3. All scolex hook measurements fit within the ranges described by previous studies. Partial sequences of the mitochondrial cox1 gene (593 bp) were obtained. Among 15 sequences, four haplotypes with five polymorphic sites were found. The haplotype diversity was 0.467 with variance and standard deviation of 0.02184 and 0.148, respectively. Nucleotide diversity was 0.00286.
Table 3. Measurements (in μm) of scolex hooks of Triaenophorus crassus presented by Kuperman (Reference Kuperman1968, Reference Kuperman1973), Kuchta et al. (Reference Kuchta, Vlckova, Poddubnaya, Gustinelli, Dzika and Scholz2007) and present study.

1, width of basal plate; 2, height of basal plate; 3, length of longer lateral prong; 4, length of shorter lateral prong; 5, distance between longer lateral and median prongs; 6, distance between shorter lateral and median prongs; 7, distance between both lateral prongs; ND, no data; n, the number of studied specimens. All measurements are given in μm and presented as a range, followed by a mean ± standard error in parentheses.
Discussion
In the present study, we have analysed the infection level of T. crassus in muscle of two sympatric forms of whitefish that inhabit Lake Teletskoye. We have collected fish groups similar in terms of size, as those described in previous studies (Titova, Reference Titova1954; Bochkarev & Gafina, Reference Bochkarev and Gafina1993; Bochkarev & Zuikova, Reference Bochkarev and Zuikova2006). Apparently, fish with such a range of body weight and standard length represent the dominant cohort of individuals within populations in Lake Teletskoye.
In order to confirm the taxonomic position of T. crassus in whitefish in Lake Teletskoye we have conducted a morphological analysis of 113 specimens according to Kuperman (Reference Kuperman1968, Reference Kuperman1973) and Kuchta et al. (Reference Kuchta, Vlckova, Poddubnaya, Gustinelli, Dzika and Scholz2007). According to our morphological results, the studied plerocercoids belong to the species T. crassus. Moreover, we provide the first data for barcode analysis based on part of the cox1 mitochondrial gene for this species.
The first mention of T. crassus infection of whitefish from Lake Teletskoye was registered in 1954 with the range of prevalence of infection registered as from 20% to 60% (Titova, Reference Titova1954). Unfortunately, due to that fact that the young ‘benthivorous’ form is morphologically very similar to the ‘planktivorous’ form of the same size, in the aforementioned study no information was provided about which form of whitefish exactly was investigated. In a later study it was shown that the levels of T. crassus infection for the ‘benthivorous’ form was relatively low (prevalence 52%, intensity 2.1 and abundance 1.1) compared with the ‘planktivorous’ form (prevalence 100%, intensity and abundance 3.9) (Bochkarev & Gafina, Reference Bochkarev and Gafina1993).
Parasitological analysis of the ‘planktivorous’ form performed during three years in Lake Teletskoye has revealed that the prevalence of T. crassus infestation ranged from 94.7% to 97.5%. The average of intensity and abundance of parasite infection was 4.2–4.8 and 4.0–4.7, respectively. We have found a positive significant correlation between standard length of the ‘planktivorous’ form of whitefish and number of cysts in its muscle in the years 2017 and 2019, whereas in 2020 the correlation was negative. Similar results were obtained by Schähle et al. (Reference Schähle, Medgyesy and Psenner2016) when the infection level of T. crassus was studied in muscle of whitefish from Lake Achensee (Austria). It could be explained by an accumulation of cysts in fish muscle with age, because it is known that the plerocercoids of T. crassus may exist in fish muscle for more than one year. The negative correlation we explain by the generally smaller size of the fish collected in 2020 and the small number of fish with standard length more than 150 mm (only one individual). Parasitological analysis of the ‘benthivorous’ forms observed in the same years has revealed a significantly lower range of prevalence (22.4–51.9%), intensity (1.9–2.8) and abundance (0.4–1.3) of T. crassus infestation compared with the ‘planktivorous’ form. We have found the negative correlations between standard length of the ‘benthivorous’ form and number of cysts in its muscle during sampling years. Such differences in infection level of this fish are explained by the different feeding habits of the studied whitefish forms. Indeed, it has been shown that the ‘planktivorous’ form has a narrow trophic specialization and feeds on zooplankton (different copepods and cladoceras) in both summer and winter periods, whereas the diet of the ‘benthivorous’ form is more taxonomically diverse (larvae of insects, mollusks, gammarids, etc.) (for more details see Bochkarev & Zuikova (Reference Bochkarev and Zuikova2006)). Significantly higher levels of plerocercoid infection in the muscle of the ‘planktivorous’ form is explained by a diet heavily biased on zooplankton, at least during periods of high levels of infection within this first intermediate host. Moreover, the ‘planktivorous’ form continues to feed on zooplanktonic items during seasons where vegetation persists (from May to October) (Bochkarev & Zuikova, Reference Bochkarev and Zuikova2006). According to the present study, the plerocercoid of T. crassus was found in almost all age groups of the ‘benthivorous’ and ‘planktovorous’ forms. But the relatively low level of plerocercoid infection in the muscle of the ‘benthivorous’ form is explained by a lower level of zooplanktonic organisms in its diet (Bochkarev & Zuikova, Reference Bochkarev and Zuikova2006). A higher level of diversity of the cladoceran Eurycercus lamellatus (14.2%) in the diet of the ‘benthivorous’ form has been documented previously (Bochkarev & Zuikova, Reference Bochkarev and Zuikova2006). Moreover, the infected cladoceran E. lamellatus can also be a potential intermediate host for the Triaenophorus parasite. Another possible way for the ‘benthivorous’ fish to be infected by this cestode (for which the first intermediate host is zooplankton) is to consume a reservoir host (e.g. invertebrate benthic organisms). Indeed, many invertebrates may feed on zooplankton organisms thereby becoming reservoir hosts for cestodes. For example, the occurrence of Proteocephalus larvae was shown in alder fly larvae (Megaloptera), which are predators and feed on copepods infected with Proteocephalus procercoids (Scholz, Reference Scholz1999).
Our findings regarding the relation of trophic specialization and different levels of T. crassus infection are also confirmed by a recent study (Pulkkinen et al., Reference Pulkkinen, Valtonen, Niemi and Poikola1999; Schähle et al., Reference Schähle, Medgyesy and Psenner2016). It was shown that prevalence of T. crassus in whitefish (Coregonus lavaretus) from three different sampled areas in Lake Saimaa (Finland) ranged from 20% to 100%, whereas T. crassus was rarely recorded in Coregonus albula; only two fish were infected of the 901 studied (Pulkkinen et al., Reference Pulkkinen, Valtonen, Niemi and Poikola1999). In another study the infection level of T. crassus in C. lavaretus from Lake Achensee was 100% and for C. albula – less than 16% (Schähle et al., Reference Schähle, Medgyesy and Psenner2016). These differences in prevalence values are also explained by the different habitat and trophic position of the studied fish.
In conclusion, in this study we investigated the relationships between feeding habits and different levels of T. crassus infection in sympatric pairs of whitefish from Lake Teletskoye. Our study showed that the trophic specialization of whitefish into ‘planktivorous’ and ‘benthivorous’ forms affected the transmission of Triaenophorus parasites through food webs with different levels of infection. Based on the results regarding different infection levels of T. crassus in these two forms of whitefish, we can conclude that parasites can be an indicator of trophic niche specialization and reflect changes in habitat selection by fish. Further, as both forms are capable of being infected though at distinctly different levels, this difference may ultimately be a driver of the evolution of T. crassus, however confirmation of such nascent speciation will require additional more detailed genetic analyses.
Funding
This research was supported by the Russian Foundation for Basic Research (grant number 19-34-60028).








