Introduction
Fascioliasis is one of the most important parasitic diseases of bovines, with approximately 700 million animals raised in areas in which there is a high level of risk of infection. Fasciola hepatica is a trematode parasite with a wide geographical distribution (Lotfy et al., Reference Lotfy, Brant, DeJong, Le, Demiaszkiewicz, Rajapakse, Perera, Laursen and Loker2008). Although ruminants are the most important, and most frequently infected, livestock hosts (Dutra et al., Reference Dutra, Molento, Naumann, Biondo, Fortes, Savio and Malone2010), a variety of other mammals (i.e. horses, capybaras, deer and humans) can be infected and/or serve as natural reservoirs for the parasite (Mendes et al., Reference Mendes, Lima and De Melo2008; Ichikawa-Seki et al., Reference Ichikawa-Seki, Shiroma, Kariya, Nakao, Ohari, Hayashi and Fukumoto2017).
Despite high incidence in domestic animals, very few human cases of fascioliasis have been reported in Brazil (Pritsch & Molento, Reference Pritsch and Molento2018). The South of Brazil, which includes the states of Paraná (PR), Santa Catarina (SC) and Rio Grande do Sul (RS), is the region with the highest level of fascioliasis in ruminants in the country (Bennema et al., Reference Bennema, Scholte, Molento, Medeiros and Carvalho2014). Cattle in the state of RS are the most highly affected in the country (14.39%), with the economic impact on the region costing approximately $147 million/year due to losses in carcass weight (Molento et al., Reference Molento, Bennema, Bertot, Pritsch and Arenal2017).
Even though it is largely believed that F. hepatica was introduced in South America by Portuguese and Spanish settlers who zealously transported animals to the region (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009; Ichikawa-Seki et al., Reference Ichikawa-Seki, Shiroma, Kariya, Nakao, Ohari, Hayashi and Fukumoto2017), Carnevale et al. (Reference Carnevale, Malandrini, Pantano, Soria, Rodrigues-Silva, Machado-Silva, Velásquez and Kamenetzky2017) did not find any geographic structuration within Argentinean samples, using the ITS1 and mitochondrial markers. Fasciola hepatica has been reported in Brazil since 1921, but there is little information concerning its genetic variation within local or regional populations. In addition, there is a complete lack of information regarding the geographic organization of F. hepatica genetic variation in Brazil, which could be useful to forecast the eventual dispersal of new, drug-resistant strains, as suggested by Beesley et al. (Reference Beesley, Williams, Paterson and Hodgkinson2017). This study aimed to evaluate the genetic diversity of F. hepatica in cattle from PR and RS and predict the spread of the parasite in the region.
Material and methods
Samples
Adult parasites were collected from cattle after liver inspection in slaughterhouses in 15 localities within RS and two in PR (supplementary table S1 and fig. 1). In total, 91 flukes were analysed in this study. After sampling, the trematodes were immediately stored in absolute ethanol at −80°C for later use, according to Itagaki et al. (Reference Itagaki, Kikawa, Sakaguchi, Shimo, Terasaki, Shibahara and Fukuda2005). For the analysis, individual parasites collected from the same area were considered one population.

Fig. 1. (a) View of Latin America, sample points highlighted in blue; (b) geographic distribution of Fasciola hepatica samples in Brazil included in the study; (c) geographic origins of samples from GenBank included in our analysis (highlighted in red).
Molecular analysis
DNA extraction was performed from single flukes using phenol–chloroform, according to Green & Sambrook (Reference Green and Sambrook2012). We amplified two mitochondrial genes, the cytochrome oxidase subunit 1 (COI) and the nicotinamide dehydrogenase subunit 1 (Nad1), using primer pairs ITA8/ITA9 and ITA2/ITA10, respectively, following the protocol described in Itagaki et al. (Reference Itagaki, Kikawa, Sakaguchi, Shimo, Terasaki, Shibahara and Fukuda2005). After analysis using electrophoresis in an agarose gel, polymerase chain reaction products were purified using 13% Polyethylene Glycol (PEG) precipitation and sequenced in both directions, using an ABI 3500 automated DNA sequencer (with BigDye Terminator Chemistry, Belo Horizonte, (MG), Brazil).
Statistical analysis
Base calling and sequence accuracy procedures were performed using the Staden software package (Staden, Reference Staden1996), and polymorphic sites were confirmed by the visual inspection of sequence chromatograms. Indices of population diversity (number of haplotypes, haplotype diversity (Hd) and nucleotide diversity) and Tajima's D test were calculated using the DNAsp 5.0 (Librado & Rozas, Reference Librado and Rozas2009). Identification of haplotypes and the construction of network trees were performed using the medium joining method with Network 5.0 (Bandelt et al., Reference Bandelt, Forster and Rohl1999). In addition to the samples we collected, we used sequences deposited in GenBank for the geographic comparison of haplotypes. We downloaded sequences from 14 countries (Peru, Argentina, Ecuador, Uruguay, UK, Ireland, Italy, Poland, Egypt, Afghanistan, Iran, China, Australia and Brazil), resulting in a total of 197 sequences of the COI gene (supplementary table S2) and 254 of the Nad1 gene (supplementary table S3).
We used the analysis of molecular variance (AMOVA) to search for the main source of genetic variability of F. hepatica, and F-statistics were used to estimate the proportion of genetic variability among populations (FST), among populations within groups (FSC) and among groups (FCT). The AMOVA was run with populations grouped according to geographical sampling (RS and PR), considering that values close to 1 indicated an extreme differentiation between the populations, and values close to zero indicated a total genetic mix among populations. Both types of analysis were performed using the Arlequin program 3.5.2 (Excoffier & Lischer, Reference Excoffier and Lischer2010).
Results
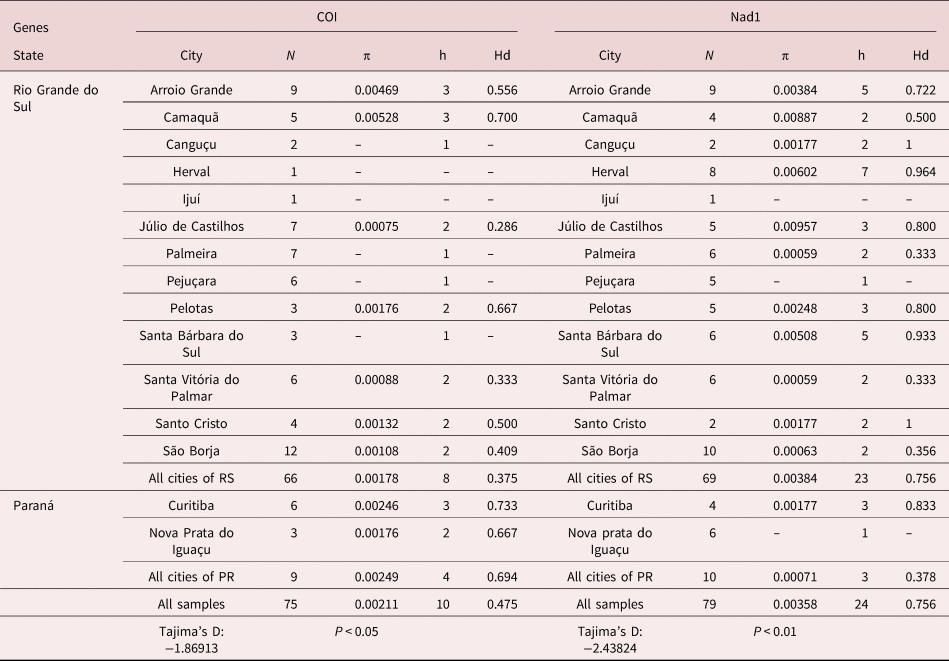
We analysed the COI gene (379 bp) from 75 samples and obtained Hd and nucleotide diversity (π) values of 0.475 and 0.002, respectively. Among these, ten distinct haplotypes were identified. Regarding the Nad1 gene (564 bp), we identified 24 distinct haplotypes from a total of 79 samples, resulting in a Hd value of 0.756 and a nucleotide diversity value of 0.004 (table 1).
Table 1. Indexes of population diversity of Fasciola hepatica for the COI and Nad1 genes.

N, number of samples; π, nucleotide diversity; h, number of haplotypes; Hd, haplotype diversity.
The COI haplotype network built with the samples from our study presented a star-like model, where the C_1 haplotype was the most frequently observed and consisted of 54 samples that were distributed throughout both RS and PR (fig. 2). In addition to C_1, the C_5 haplotype appeared in both areas. Six C_5 haplotypes were shared among cities in RS and two were shared within PR (fig. 2). When the 197 sequences from Genbank were included in our COI network, we observed 46 haplotypes (fig. 3). Two haplogroups were formed; the first haplogroup was the most diverse, containing haplotypes from all analysed countries. In this haplogroup, the most frequently observed haplotype was C_1, in which a total of 125 sequences were included (including 54 from our study). The second haplogroup seemed to be more restricted, including samples mainly from Iran. In this haplogroup, the most frequent haplotype, comprising 42 sequences, was C_2, which included 36 sequences from Iran, two from Poland, one from Peru and three from Brazil (identified in our study).
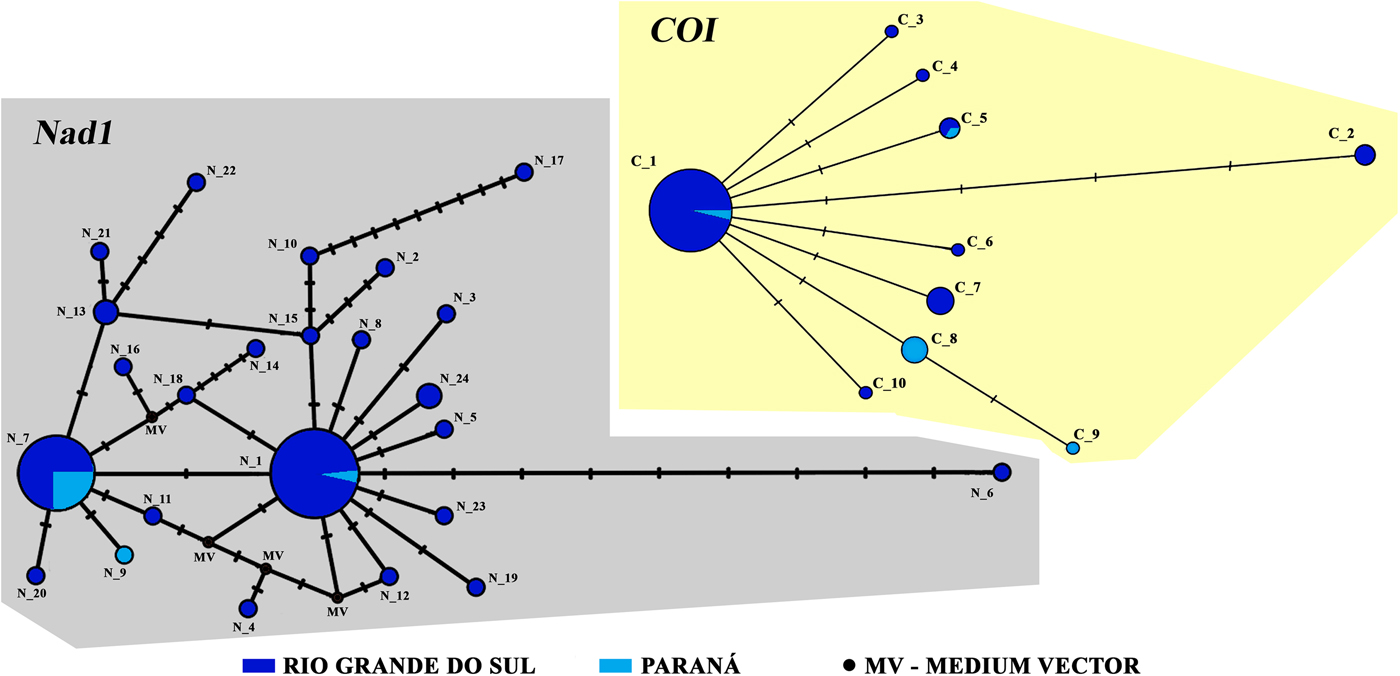
Fig. 2. Network analysis of Nad1 and COI genes of Fasciola hepatica samples from this study. In grey, the distribution of 24 haplotypes of the Nad1 gene are shown; in beige, the distribution ten haplotypes for the COI gene are presented.

Fig. 3. Network analysis for the COI gene. In this analysis, we grouped sequences identified in the study with samples from other regions of the world. The colours correspond to their respective geographical locations.
The haplotype network of the Nad1 gene was performed using only the newly identified, Brazilian samples. The analysis resulted in the identification of 24 haplotypes out of 79 total samples (fig. 2). The most frequent haplotype observed was N_1, comprising 32 individual samples, and the second was the N_7, with 23 samples. Curiously, 22 haplotypes were not shared among any city. When GenBank samples were added to the analysis, we obtained a total of 333 sequences, 88 haplotypes and four haplogroups (fig. 4). The sequences of haplogroups 1 and 2 were composed of the greatest concentration of samples from Europe, Asia and Africa, whereas the haplogroups 3 and 4 were mainly composed of a mixture of samples from South America. There were two highly shared haplotypes, N_1 and N_7, belonging to the South American haplogroups 3 and 4, respectively. Haplotype N_7 was the most frequently occurring; 90 sequences of the haplotype were identified, distributed between Afghanistan, Argentina, Ecuador, Egypt, Peru, Poland, Italy, the UK, Iran and Brazil. Of these 90 sequences, 24 were found in our study. The second most frequent haplotype was N_1, consisting of 78 sequences. This group was formed by individuals from Ecuador, Peru, Egypt, Uruguay, Argentina and our newly identified samples from Brazil (34 sequences). When we compared all the existing haplotypes of this analysis, we had a total of 15 haplotypes found exclusively in the RS and PR states.

Fig. 4. Network analysis for the Nad1 gene. In this analysis, we grouped sequences identified in the study with samples from other regions of the world. The colours correspond to their respective geographical locations.
The results of the analysis of population structure showed that most of the genetic diversity observed was within populations (COI: 57.4%; Nad1: 77.5%). The FST index value for the COI and Nad1 genes were 0.425 and 0.225, respectively. FCT index values for COI and Nad1 were 0.368 and 0.089, respectively. When the COI gene was considered, there was 36.8% similarity among groups (table 2). The Tajima's D test of neutrality produced negative values, which were significant for both COI (−1869, P < 0.05) and Nad1 (−2438, P < 0.01) genes (table 1).
Table 2. AMOVA results based on the COI and Nad1 genes of Fasciola hepatica from Southern Brazil.

Discussion
This is the first report of the genetic characterization of F. hepatica from infected cattle isolated from different regions of Brazil. Diversity indices, evaluated using two mitochondrial genes for analysis, produced findings similar to others that were carried out in different countries. For example, a study in Peru analysed the Nad1 fragment from 78 individual parasites and found eight haplotypes (Hd = 0.685 and π = 0.00175) (Ichikawa-Seki et al., Reference Ichikawa-Seki, Ortiz, Cabrera, Hobán and Itagaki2016). A study conducted in Argentina examining 22 individuals, identified seven haplotypes for the COI gene. When two other mitochondrial genes were analysed – Nad4 and Nad5 – four and three haplotypes were identified, respectively (Carnevale et al., Reference Carnevale, Malandrini, Pantano, Soria, Rodrigues-Silva, Machado-Silva, Velásquez and Kamenetzky2017). Elliott et al. (Reference Elliott, Muller, Brockwell, Murphy, Grillo, Toet, Anderson, Sangster and Spithill2014) analysed 208 specimens in a study that yielded only six COI haplotypes (Hd = 0.482 and π = 0.003), and 18 Nad1 haplotypes (Hd = 0.832 and π = 0.005) in Australia.
A possible explanation for both high Hd and low nucleotide diversity could be related to the arrival of F. hepatica in Peru, Argentina, Australia and Southern Brazil, with a very small number of individuals, each from a much larger parental population, creating a Founder's effect. To better explain the large number of haplotypes observed, we suggest that the introduction of F. hepatica in Brazil occurred in several separate human/cattle immigration waves. A similar scenario was pointed out to explain the findings of Semyenova et al. (Reference Semyenova, Morozova, Chrisanfova, Gorokhov, Arkhipov, Moskvin, Movsessyan and Ryskov2006), in which researchers analysed populations from eastern Europe and western Asia with two different lineages. Lineage 1 was shared with Europe, Caucasus, Asia and Oceania, and lineage 2 was shared with European, Armenian and American populations (the USA and Uruguay).
We hypothesize that the introduction of F. hepatica to Brazil could have happened in accordance with two different scenarios. First, it could be due to land migration of wild animals by the Great American Interchange (i.e. wild ruminants from Peru). Second, effects could be due to Portuguese and Spanish colonization (i.e. movement of Catholic settlements and commerce). As nucleotide substitutions are rare, we suppose that there has not been enough time to generate many nucleotide substitutions with regard to ancestral haplotypes (C_1 and N_7). The same pattern has been observed in other helminth parasites after the introduction to new areas, including with Echinococcus granulosus in South America (Sharma et al., Reference Sharma, Fomda, Mazta, Sehgal, Singh and Malla2013). Also, the exclusive haplotypes found in our samples generally contained only one substitution compared to the more frequent haplotypes. The neutrality test (table 1) and all the networks calculated for both genes obtaining a star-like model, indicating population expansion or lineage sorting (Avise, Reference Avise2000).
The large number of haplotypes identified in our study may be associated with the optimal conditions for the intermediate host, since the landscape is formed by lowland areas with a large number of water sources (Dutra et al., Reference Dutra, Molento, Naumann, Biondo, Fortes, Savio and Malone2010; Bennema et al., Reference Bennema, Molento, Scholte, Carvalho and Pritsch2017). Epidemiological studies show that the dynamics of ruminant diseases should be combined with the understanding of climatological and environmental data, since these factors directly influence the continuity of the parasite cycle (Charlier et al., Reference Charlier, Ghebretinsae, Levecke, Ducheyne, Claerebout and Vercruysse2016). Thus, we believe that, once brought into Americas, parasites faced numerous challenges (different climate and host adaptation). Accordingly, some local hosts may have offered ideal environments for parasite establishment. The lowlands of the Pampa region in the South of RS represents a complete habitat to the intermediate host, as well as being used to sustain large cattle herds.
The highest portion of the genetic diversity was found within populations (table 2), in accordance with population dynamics of the usual, definitive cattle host in this region, and could be due to cattle movement that contributes to the mixture of populations of F. hepatica within areas observed. However, an important proportion of genetic diversity of the species was found among groups (flukes sampled in each Brazilian state comprised a different group). This observation can be explained by the limited cattle movement occurring between these two states, while the cattle movement within each state was considerably high. These findings are supported with calculated FCT index (COI: 0.368; Nad1: 0.089) and FST (COI: 0.425; Nad1: 0.225) values, showing a geographic structuring among and within RS and PR samples.
In a study analysing flukes from the UK, Beesley et al. (Reference Beesley, Williams, Paterson and Hodgkinson2017) found that the widespread movement of definitive hosts could significantly contribute to the dispersal of F. hepatica variants, leading to the low FST values. Walker et al. (Reference Walker, Johnston, Hoey, Fairweather, Borgsteede, Gaasenbeek, Prodöhl and Trudgett2011) reported low levels of genetic structure in fluke populations from the Netherlands. The aforementioned study contrasts with our data; differences that are probably due to our wide geographical sampling area, differences in cattle migration/commerce and the timing of the establishment of fluke populations from South America, which were established more recently than those in Europe (supplementary tables S4 and S5).
Taken together, this comparison of nucleotide and Hd indicates that the colonization of Southern Brazil was made by several F. hepatica haplotypes. This agrees with a statement made by Ichikawa-Seki et al. (Reference Ichikawa-Seki, Ortiz, Cabrera, Hobán and Itagaki2016), arguing that the F. hepatica population in Peru was originated by numerous haplotypes, from multiple regions, but mainly originating from Europe.
Analysing the network tree constructed using the whole set of sequences, we observed a tree topology consisting of two main groups, with neither seeming to be characteristic of any specific region of the world. The great mixture among the samples suggests a high level of parasite circulation among populations from Europe, Asia and Africa. Moreover, this tree topology shows that the south Brazilian populations of F. hepatica were originated by at least three haplotypes shared by different areas of the world. The high dispersion capacity of the definitive hosts, i.e. dispersion caused by the transport of animals for breeding, demonstrates an increase in the dispersion of some parasite genotypes, which occasionally became more frequent, increasing opportunities for parasite adaptation and causing problems in the management of disease (Auld & Tinsley, Reference Auld and Tinsley2015).
Our data regarding the genetic diversity of F. hepatica demonstrated that the parasite possesses a relatively high level of Hd, and presents a tree network topology with a great mixture in both lines of ancestral sequences mainly from Europe, Asia and Africa, and derivate population sequences from South America. This may be explained by the carriage of different variants of F. hepatica to the Americas through the introduction of infected animals. As F. hepatica faced intense circulation in Europe, Asia and Africa before the American colonization, our data cannot determine the exact centre of origin of our samples. The partition of the AMOVA and the value of FST support the lack of geographical structure in Brazil, which are in agreement with the observed cattle production systems in this region.
The molecular characterization of F. hepatica from Brazil can be used as a key factor to understand epidemiological aspects of the disease. In addition, understanding the geographical structuration observed in different regions, related to the fact that flukes can infect many mammals (including humans), may provide insights to aid local management and regional health programs designed to combat the parasites. Furthermore, nuclear markers, such as microsatellites or genes associated with parasite adaptation, should be used for future studies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X19000774
Acknowledgements
The authors are grateful to DVM Daniela Gallas and DVM José Luis Teixeira for helping with the abattoir liver samplings. Jéssyca B. Schwantes received a Master of Science fellowship by CAPES.
Financial support
This study was funded by the Fundação de Amparo a Pesquisa do Rio Grande do Sul, FAPERGS (project number 16/2551-0000231-2).
Conflicts of interest
None.