Introduction
Gastrointestinal nematodes (GINs) have very important economically negative effects on several animal production systems (Nieuwhof & Bishop, Reference Nieuwhof and Bishop2005; Lane et al., Reference Lane, Jubb, Shephard, Webb-Ware and Fordyce2015). The nematode Haemonchus contortus is one of the most relevant GINs that infects small ruminants around the world (Rodríguez et al., Reference Rodríguez, Goldberg, Viotti and Ciappesoni2015). The control of this nematode is performed mainly with synthetic anthelmintics. However, the increased resistance of this parasite to anthelmintics has major economic impacts on livestock worldwide (Kotze et al., Reference Kotze, Hunt and Skuce2014; Albuquerque et al., Reference Albuquerque, Bassetto, Almeida and Amarante2017). The development of natural-based formulations is being considered as an alternative.
Natural products can be used to control strains of H. contortus that are resistant to synthetic compounds (Andre et al., Reference Andre, Ribeiro and Cavalcante2016; Garcia-Bustos et al., Reference Garcia-Bustos, Sleebs and Gasser2019). Among these products, plant essential oils (EOs) and their major compounds, terpenoid and phenylpropanoid, have shown promising anthelmintic effects (Katiki et al., Reference Katiki, Chagas, Takahira, Juliani, Ferreira and Amarante2012; Castilho et al., Reference Castilho, Fantatto, Gaínza, Bizzo, Barbi, Leitão and Chagas2017; Ferreira et al., Reference Ferreira, Benincasa, Fachin, Contini, França, Chagas and Beleboni2018). However, the yield and composition in terms of bioactive volatile compounds depend on genetic, environmental and agronomic factors (Yang et al., Reference Vieira and Simon2018).
The plant Ocimum basilicum L., popularly known as basil, is native to Asia and grows spontaneously in tropical and sub-tropical regions (Khair et al., Reference Khair, Bariyah, Khair-Ul-Bariyah, Ahmed and Ikram2012). The O. basilicum EOs present compounds of interest to the food, cosmetic and also pharmaceutical industries, with a production higher than 40 tons annually (Lawrence, Reference Lawrence, Harley and Reynolds1992; Telci et al., Reference Soares, Penha, Araújo, Cruz, Blank and Costa-Júnior2006). The O. basilicum EOs have been shown to exhibit several biological activities (Govindarajan et al., Reference Govindarajan, Sivakumar, Rajeswary and Yogalakshmi2013; El-Soud et al., Reference El-Soud, Deabes, El-Kassem and Khalil2015; Silva et al., Reference Silva, Lifschitz, Macedo, Campos, Viana-Filho, Alcântara, Araújo, Alencar and Costa-Junior2015; Güez et al., Reference Güez, de Souza and Fischer2017), including action against H. contortus (Castro et al., Reference Castro, Pinto, Mota, Moura, Castro, Madrid, Freitag and Berne2017).
The distinction among numerous basil varieties is largely based on their EO composition, which is of the utmost importance to biological activities and consumers’ preference (Kiferle et al., Reference Kiferle, Ascrizzi, Martinelli, Gonzali, Mariotti, Pistelli, Flamini and Perata2019). Several cultivars of O. basilicum present EOs with linalool, methyl chavicol (estragol), citral and eugenol as its main constituents, in variable concentrations (Vieira & Simon, Reference Van Wyk and Mayhew2000; Pascual-Villalobos & Ballesta-Acosta, Reference Pascual-Villalobos and Ballesta-Acosta2003; Sajjadi, Reference Sajjadi2006; Martins et al., Reference Martins, Nascimento, Filho, Filho, Souza, Aragão and Silva2010; Ottai et al., Reference Ottai, Ahmed and El Din2012). These compounds have been shown to have anthelmintic activity, isolated or in a mixture, and they are also present, in different concentrations, in several other EOs (Katiki et al., Reference Katiki, Barbieri, Araujo, Veríssimo, Louvandini and Ferreira2017; Ferreira et al., Reference Ferreira, Benincasa, Fachin, Contini, França, Chagas and Beleboni2018; Macedo et al., Reference Macedo, Oliveira and André2019). The standardization of efficient cultivars or the combination of natural compounds is extremally important to human and veterinary pharmaceutical industries.
Considering that different cultivars of the same plant species may have different EO compositions, with different bioactivity, the objective of this study was to evaluate the action of EOs obtained from different cultivars of O. basilicum, as well as combinations of their major constituents, on H. contortus.
Materials and methods
Plant material and EOs
EOs from 15 commercial cultivars and one experimental hybrid from the Basil Genetic Breeding Program of Universidade Federal de Sergipe were evaluated. The following 15 commercial cultivars were used: Anise, Napoletano, Genovese, Ararat, Edwina, Dark Opal, Italian Large Leaf (Richters), Magical Michael, Mrs Burns, Nufar F1, Purple Ruffles (Richters Herbs, Goodwood, ON, Canada), Italian Large Leaf (Isla), Italian Large Red Leaf, Limoncino (Isla Sementes, Porto Alegre, RS, Brazil) and Maria Bonita (Blank et al., Reference Blank, Souza, Arrigoni-Blank, Paula and Alves2007), and the experimental hybrid Genovese × Maria Bonita. All EOs used were obtained from the study of Pinto et al. (Reference Pinto, Blank, Nogueira, Arrigoni-Blank, Andrade, Sampaio and Pereira2019). The cultivars were planted and collected simultaneously during the rainy season (April–June 2016), and EOs were extracted and analysed according to Pinto et al. (Reference Pinto, Blank, Nogueira, Arrigoni-Blank, Andrade, Sampaio and Pereira2019).
Parasitological procedures
The H. contortus strain used in the present study was isolated from a goat naturally infected, as described in Silva et al. (Reference Silva, Sousa, Guerra, Pessôa, Freitas, Alves and Lima2021). Third larvae stage (L3) of Haemonchus contortus (n = 2000 L3/animal) was used to experimentally infect a donor sheep confirmed to be parasite-free, with five successive negative faecal egg counts (Robert & O'sullivan, Reference Robert and O'Sullivan1950) performed in three-day intervals. After 30 days, the infection was confirmed by faecal egg count, faecal culture and L3 identification (Robert & O'sullivan, Reference Robert and O'Sullivan1950; Van Wyk & Mayhew, Reference Telci, Bayram, Yilmaz and Avci2013). Through previous in vitro tests, the H. contortus strain used was confirmed to be resistant to benzimidazoles and susceptible to levamisole.
The nematode eggs were recovered from faeces, according to Silva et al. (Reference Silva, Sousa, Guerra, Pessôa, Freitas, Alves and Lima2021), and stored in a 15 mL conical tube (eggs primary solution). The total number of eggs collected was estimated in three samples of 20 mL of the primary solution, and then a solution of 1000 eggs/mL was prepared. The experimental procedures were performed according to the guidelines of the Animal Ethics Committee (CEUA) of the Federal University of Maranhão, and were approved under the protocol number 23115018061/2011-01.
Egg-hatching assay
The eggs were added to a saturated sodium chloride solution and centrifuged (1350 g) for three minutes. The floating eggs were collected (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992), washed three times and re-suspended in distilled water. A suspension of 100 eggs/well was placed in a 96-well sterile plate.
The EOs from all cultivars and commercial samples of their major constituents linalool, methyl chavicol, citral and eugenol purchased from Sigma-Aldrich (St Louis, MO, US), were individually diluted in 3% Tween in different concentrations (7.0, 4.9, 3.4, 2.4, 1.7, 1.2, 0.8, 0.6, 0.4 and 0.3 mg/mL). Each samples test was performed in quadruplicate (n = 4), using at least six concentrations. The negative control was performed with 3% Tween. The eggs were incubated for 48 h at 27°C. Eggs and first-stage larvae were counted under an inverted microscope at 40× magnification.
Compound combinations
Linalool, methyl chavicol, citral and eugenol (Sigma-Aldrich) were used to simulate the composition of three cultivars using the two major compounds of each. Cultivars with low and intermediate IC50 (concentration required to inhibit 50% of hatching) and different major compounds, were selected. The efficacy of compounds in combination to simulate Genovese (57% linalool and 27% methyl chavicol), Mrs Burns (38% linalool and 49% citral) and Italian Large Leaf (Richters) cultivars (64% linalool and 11% eugenol) was assessed in an egg-hatching assay. To complete each mixture to 100% of composition, olive oil was used.
The isolated compounds and their mixtures were diluted in 3% Tween in decreasing concentrations (3.4, 2.4, 1.7, 1.2, 0.8, 0.6, 0.4 and 0.3 mg/mL). The tests of each compound were performed in quadruplicate using at least six of the above-described concentrations. The negative control was performed with 3% Tween in olive oil, at 25 mg/mL. The egg-hatching assays were performed as described above.
Statistical analysis
The results were used to determine the IC50 with respective 95% confidence intervals using GraphPad Prism 8.0 software (GraphPad Inc, San Diego, CA, US). The data were initially transformed into Log (X), normalized and then non-linear regression was applied to obtain the IC50 values. The differences among the IC50 were assessed using the F test (GraphPad Inc). Linear regression was applied to compare the IC50 values from isolated compounds, their combinations and cultivars, for which the percentages of the four major constituents are listed (GraphPad Inc).
Results
The EOs from different cultivars showed differences in the IC50 (table 1). This difference reached up to 3.96-fold, between the Napoletano cultivar, which presented the highest efficacy (IC50 0.56 mg/mL), and the cultivars with the lowest efficacy such as Purple Ruffles and Italian Large Red Leaf (IC50 2.22 mg/mL) (table 1).
Table 1. Major compounds (%) from essential oils of cultivars and hybrid of Ocimum basilicum and concentrations required for achieving 50% inhibition of egg hatching in Haemonchus contortus (IC50) with respective 95% confidence intervals (95% CI).
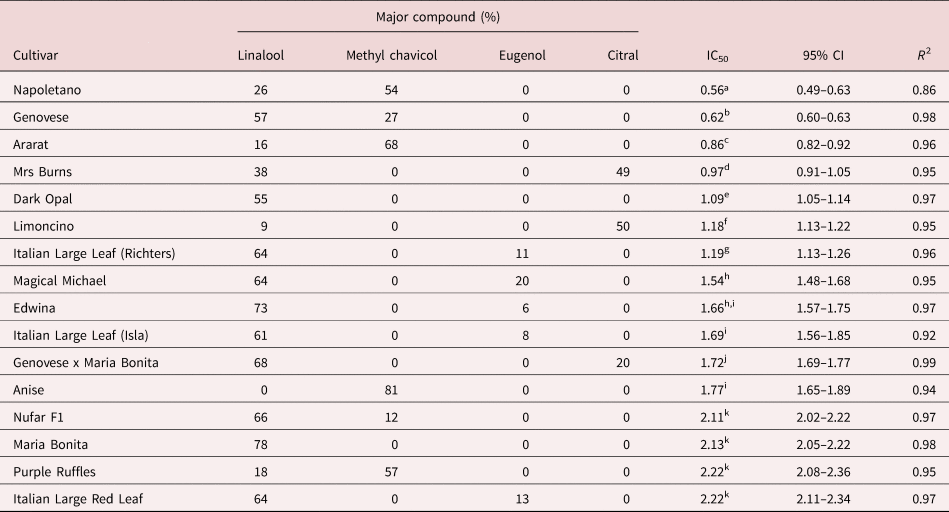
Chemical composition from Pinto et al. (Reference Pinto, Blank, Nogueira, Arrigoni-Blank, Andrade, Sampaio and Pereira2019).
R 2, regression coefficient. The R 2 value quantifies goodness-of-fit at the non-linear regression curve performed to estimate the IC50.
Different superscript letters in the IC50 column indicate significant differences (P < 0.05).
The anthelmintic activity of the major EO constituents – linalool, methyl chavicol, eugenol and citral – was also assessed. Citral was the most effective compound (IC50 0.30 mg/mL) (table 2). Two of the three assessed combinations – eugenol + linalool and methyl chavicol + linalool – showed higher efficacy than their isolated compounds. However, the combination of citral + linalool is less effective than citral alone, and more effective than only linalool.
Table 2. Inhibition concentrations required for achieving 50% of egg hatching in Haemonchus contortus (IC50) with respective 95% confidence intervals (95% CI) from major compounds and their combinations simulating cultivars of Ocimum basilicum.
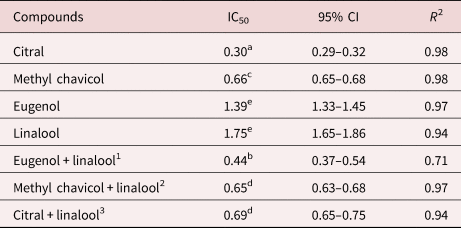
R 2, regression coefficient. The R 2 value quantifies goodness-of-fit at the non-linear regression curve performed to estimate the IC50; all combinations were used with olive oil to complete 100% composition; among the different treatments, IC50 values with the same superscript letter are statistically equivalent (P < 0.05).
1 11% eugenol and 64% linalool, simulating the EO from Italian Large Leaf (Richters) cultivar.
2 27% methyl chavicol and 57% linalool, simulating the EO from Genovese cultivar.
3 49% citral and 38% linalool, simulating the EO from Mrs Burns cultivar.
The best result was obtained with the combination of 11% eugenol plus 64% linalool (IC50 0.30 mg/mL), simulating the Italian Large Leaf (Richters) cultivar (table 2). This compound combination was 2.7 times more effective than EOs from the cultivar. On the other hand, the combination of 38% linalool and 49% citral was 1.4 times more effective than EOs from the cultivar Mrs Burns. The other combination used in the present study – 57% linalool and 27% methyl chavicol – did not differ statistically from the EO of the Genovese cultivar.
A negative correlation was observed at increase the concentration of citral in cultivars, compounds isolated and its combinations decreasing the IC50 value (P = 0.03). No other correlation was found.
Discussion
The EO of O. basilicum has several biological activities, such as antifungal (El-Soud et al., Reference El-Soud, Deabes, El-Kassem and Khalil2015), antimicrobial (Lang & Buchbauer, Reference Lang and Buchbauer2012), antiprotozoal (Almeida et al., Reference Almeida, Alviano, Vieira, Alves, Blank, Lopes, Alviano and Rosa2007; Santoro et al., Reference Santoro, Cardoso, Guimarães, Mendonça and Soares2007), insecticidal (Rodríguez-González et al., Reference Rodríguez-González, Álvarez-García, González-López, Silva and Casquero2019), acaricidal (Martinez-Velazquez et al., Reference Martinez-Velazquez, Castillo-Herrera, Rosario-Cruz, Flores-Fernandez, Lopez-Ramirez, Hernandez-Gutierrez and Del Carmen Lugo-Cervantes2011) and anthelmintic (Castro et al., Reference Castro, Pinto, Mota, Moura, Castro, Madrid, Freitag and Berne2017). However, there are several basil cultivars with considerably different EO composition (Sharopov et al., Reference Sharopov, Satyal, Ali, Pokharel, Zhang, Wink, Kukaniev and Setzer2016). This is the first study to show a statistical difference in the inhibition of H. contortus egg hatch – up to 3.96 times – among EOs from cultivars of the same plant species (table 1).
The egg-hatch test used in the present study has been developed as a phenotypic diagnostic of resistant nematodes for the benzimidazoles, looking at the eggs that fail to hatch (Lacey et al., Reference Lacey, Brady, Prichard and Watson1987; FAO, 2004). The benzimidazoles inhibit embryonation and hatching by interfering with microtubules’ formation (Mandelkow & Mandelkow, Reference Mandelkow and Mandelkow1990; Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992). Additionally, natural compounds altered the egg's surface and increased benzimidazole activity (Silva et al., Reference Silva, Sousa, Guerra, Pessôa, Freitas, Alves and Lima2021). Therefore, the rationale for using egg-hatch assay in the present study was to use it as a model to search for new compounds against nematode infection, and not to target specific use in nematode eggs.
Inhibition of H. contortus egg hatch was previously demonstrated by the EO of one O. basilicum cultivar and associated with methyl chavicol and linalool as major compounds of the EO tested (Castro et al., Reference Castro, Pinto, Mota, Moura, Castro, Madrid, Freitag and Berne2017). In the present study, the EOs from Napoletano, Genovese and Ararat cultivars showed the highest anthelmintic activity, and they also contain methyl chavicol and linalool, as major compounds (table 1). However, the EOs from Nufar F1 and Purple Ruffles cultivars exhibited low efficacy against H. contortus while having a similar chemical composition with methyl chavicol and linalool as major compounds. Despite methyl chavicol showing a relatively good efficacy in inhibiting H. contortus egg hatch, Anise cultivar, which possesses 81% methyl chavicol, does not present good efficacy when compared to other cultivars with low amounts of this compound. Interestingly, the hybrid cultivar Genovese + Maria Bonita presented an intermediate anthelmintic effect when compared with separate Genovese and Maria Bonita cultivars.
Citral, a natural combination of the isomers neral and geranial, has been shown to be effective against several nematodes, including H. contortus, both isolated and as the major compound of EO (Hierro et al., Reference Hierro, Valero and Navarro2006; Macedo et al., Reference Macedo, Oliveira and André2019). A negative correlation between the citral concentration and efficacy was found when the results of all EO cultivars were analysed (P = 0.03), whereas isolated citral showed the best activity when tested alone (table 2).
The composition of EOs extracted from basil varies considerably. It can be classified into four, five or seven chemical groups or chemotypes according to the main components and the statistical analysis performed (Martins et al., Reference Martins, Nascimento, Filho, Filho, Souza, Aragão and Silva2010; Liber et al., Reference Liber, Stanko, Politeoc, Strikic, Kolakb, Milosc and Satovicb2011; Giachino et al., Reference Giachino, Tonk, Bayram, Yuce, Telci and Furan2014; Pinto et al., Reference Pinto, Blank, Nogueira, Arrigoni-Blank, Andrade, Sampaio and Pereira2019). The variability of chemical composition from different chemotypes has been found in diverse regions of the world (Hassanpouraghdam et al., Reference Hassanpouraghdam, Gohari, Tabatabaei and Dadpour2010). Differences in EO efficacy from the same vegetal species with different chemical compositions against parasites have been reported (Peixoto et al., Reference Peixoto, Costa-Júnior and Blank2015; Costa-Júnior et al., Reference Costa-Júnior, Miller, Alves, Blank, Li and Pérez de León2016; Lima et al., Reference Lima, Carvalho, Peixoto, Blank, Borges and Costa Junior2016). However, the efficacy could not be correlated with the chemotype or the EO's main compound, and seems to be associated with a blend of compounds (Cruz et al., Reference Cruz, Costa-Junior and Pinto2013; Soares et al., Reference Silva, Sousa, Guerra, Pessôa, Freitas, Alves and Lima2016).
The combinations of the components eugenol + linalool and methyl chavicol + linalool showed more efficacy than the isolated compounds (table 2), demonstrating that the combined compounds potentialized egg-hatch inhibition. Linalool represents the main component of many species of Ocimum, and is considered responsible for biological activities, representing reasons for its relevance (Ravid et al., Reference Ravid, Putievsky, Katzir and Lewinsohn1997).
Despite the benefits of using O. basilicum EOs in human and animal health, the present study has considerable importance for the bioprospection of pure or combinations of natural compounds to control ruminant nematodes. Our results clearly show differences in the bioactivity of EOs from different O. basilicum cultivars, related to the citral concentration. Additionally, the combinations using linalool and other compounds showed higher inhibition of H. contortus eggs than linalool alone, demonstrating the potential use of these compounds for the development of products for nematode control.
Acknowledgement
We thank Dr Paul Michels (The University of Edinburgh, UK) for his valuable suggestions, assistance and critical review of this manuscript.
Financial support
We thank the FAPEMA (Maranhão State Research Foundation) for financial support and for awarding a fellowship to H.N. Costa-Junior. We thank the FINEP (Funding Authority for Studies and Projects) and FAPEMA for supporting the IECT (Science and Technology Institute of Maranhão) Biotechnology. We thank CAPES (Higher Education Personnel Improvement Coordination) for awarding a fellowship to N.C.S. Silva and CNPq (Brazilian National Council for Scientific and Technological Development) for awarding a fellowship to L.M. Costa-Junior. This study was financed, in part, by CAPES (finance code 001).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.




