Introduction
The composition of parasite communities is affected by, among other factors, the result of the interactions between their evolutionary history and the ecological characteristics of the hosts (Poulin, Reference Poulin1995). During their evolutionary history, hosts lose and/or acquire parasites due to the speciation of native parasites or the acquisition of new parasite species from other hosts (Poulin & Rohde, Reference Poulin and Rohde1997). The ecological characteristics associated with the host, such as diet, habitat and niche position, have a great influence on the composition and structure of the parasite communities (Esch et al., 1990; Poulin, Reference Poulin1995). Moreover, host geographic range is a main factor affecting the interchanges of parasite species that are phylogenetically related (Poulin & Morand, Reference Poulin and Morand1999; González & Oliva, Reference González and Oliva2006). Similarly, it has been demonstrated that depth and temperature both affect the parasite communities of fish (Rohde et al., Reference Rohde, Hayward and Heap1995; Oliva et al., Reference Oliva, Gonzalez and Acuña2004).
Studies of parasite communities that do not account for phylogeny may provide inaccurate results (Brooks, Reference Brooks1980). Studies that analyse the determinants of parasite communities of related hosts show that phylogenetic relationships can confuse the real relationships between host ecology and community parasite richness (Poulin & Rohde, Reference Poulin and Rohde1997). However, few studies have evaluated the importance of ecological and phylogenetic factors of the host simultaneously as determinants of parasite communities (Bush et al., 1990; Poulin, Reference Poulin1996, Reference Poulin2010; Muñoz et al., Reference Muñoz, Grutter and Cribb2006), and those studies have provided contradictory results concerning the relationship between parasitological descriptors and ecological variables and/or host phylogeny. Therefore, additional studies are needed to determine whether these trends in the determination of parasite communities are consistent when the ecology and phylogeny of the host are considered.
The aims of the present study were to compare, using multivariate analyses, the degree of similarity of the endoparasite fauna of five fish species belonging to the order Gadiformes: three representatives of the suborder Gadoidei (Merluccius gayi, Merluccius australis (Merlucciidae), Macruronus magellanicus (Macrouronidae)) and two representatives of the suborder Macrouroidei (Nezumia pulchella (Macrouridae) and Micromesistius australis (Gadidae)), and to evaluate whether endoparasite compositions were mainly influenced by phylogenetic or by ecological relationships (e.g. niche dimensions such as diet, depth, latitude or habitat). Two host species (M. gayi and N. pulchella) share latitudinal and bathymetric distributions along the northern Chilean coast, whereas the other three host species (Merluccius australis, M. magellanicus and Micromesistius australis) are distributed along the central-southern Chilean coast (Froese & Pauly, Reference Froese and Pauly2010). As stated by Lillo et al. (Reference Lillo, Céspedes, Ojeda, Balbontín, Bravo, Saavedra, Barbieri and Vera2005) and Saavedra et al. (Reference Saavedra, Correa, Céspedes, Ojeda, Adarme, Días, Oliva and Rojas2006), these species share similar trophic patterns.
Materials and methods
We used our own database comprising M. magellanicus, Merluccius australis and Micromesistius australis, which were caught in 2006 by an industrial fishery from southern Chile (44°S and 45°S). Additionally, our database was complemented with published information for M. magellanicus, Merluccius australis, Micromesistius australis, M. gayi and N. pulchella (table 1). The latitudinal and bathymetric ranges and sizes for each species are presented in table 2.
Table 1 Prevalence (P) and mean intensity of infection (MI) of endoparasites of five Gadiformes fish species off the Chilean coast.

* Larval stages.
Table 2 Number of analysed fish (n), fish size range (mean±SD), latitudinal range and bathymetric range in gadiform fish species from Chile.

Sources: (1) This study; (2) Fernández (Reference Fernández1985); (3) George-Nascimento & Arancibia (Reference George-Nascimento and Arancibia1994); (4) González (2005) ; (5) George-Nascimento (Reference George-Nascimento1996); (6) Oliva (Reference Oliva2001); (7) Niklitschek et al. (Reference Niklitschek, Canales, Ferrada, Galleguillos, George-Nascimento, Hernandez, Herranz, Lafon, Roa and Toledo2009); (8) Salinas et al. (Reference Salinas, González and Acuña2008).
* From Froese & Pauly (Reference Froese and Pauly2010).
For each parasite species, the prevalence and mean intensity of infection were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Cluster analyses (based on the Bray–Curtis similarity and simple linkage algorithm) were used to determine whether the endoparasite composition (prevalence and intensity of infection) among host species was similar. Correspondence analyses were then employed to evaluate the host–parasite associations. All multivariate analyses were performed using Statistica 6.0 software (StatSoft Inc., Tulsa, Oklahoma, USA).
Results
The host species N. pulchella showed the narrowest latitudinal range, whereas Merluccius australis, M. magellanicus and Micromesistius australis demonstrated overlapping latitudinal distributions (table 2). Twenty endoparasite species were detected in the studied hosts. Of these species, seven were in the larval stages, and several parasite species were common among the host species. The larval parasites Anisakis sp. and Hepatoxylon trichiuri demonstrated a higher prevalence of infection in Merluccius australis, Micromesistius australis and M. magellanicus (table 1). There was no relationship between the sample size and parasite richness (r 2 = 0.107, P>0.20).
The cluster analysis based on parasite prevalence (fig. 1) included Micromesistius australis and M. magellanicus in a clade with 50% similarity; Merluccius australis was included in this clade with a similarity of 49% and M. gayi with a similarity of 36%. Finally, N. pulchella demonstrated a parasite similarity of only 23% with the other four host species. The cluster analysis based on the mean intensity of infection (fig. 2) revealed the same pattern.

Fig. 1 Cluster analyses based on prevalence of infection of endoparasite fauna of five Gadiformes species. Code for host species: Nezumia pulchella (NEPU), Merluccius gayi (MEGA), Merluccius australis (MEAU), Micromesistius australis (MIAU) and Macruronus magellanicus (MAMA).

Fig. 2 Cluster analyses based on mean intensities of infection of endoparasite fauna of five Gadiformes species. Code for species as in fig. 1.
The correspondence analysis based on prevalence (fig. 3) demonstrated significant differences in endoparasite composition among the host species (χ2 = 630.07, df = 76, P < 0.001); 66.5% of the variation was explained by the first two dimensions (39% and 27.5% for the first and second dimensions, respectively). Most parasites were associated with Merluccius australis and Micromesistius australis. However, Aporocotyle wilhelmi and Hysterothylacium sp. were strongly associated with M. gayi. Similarly, Elytrophalloides oatesi, Gonocerca phycidis and Cucullanus sp. were only associated with M. magellanicus, whereas Lepidapedon sp. was present only in N. pulchella (fig. 3).
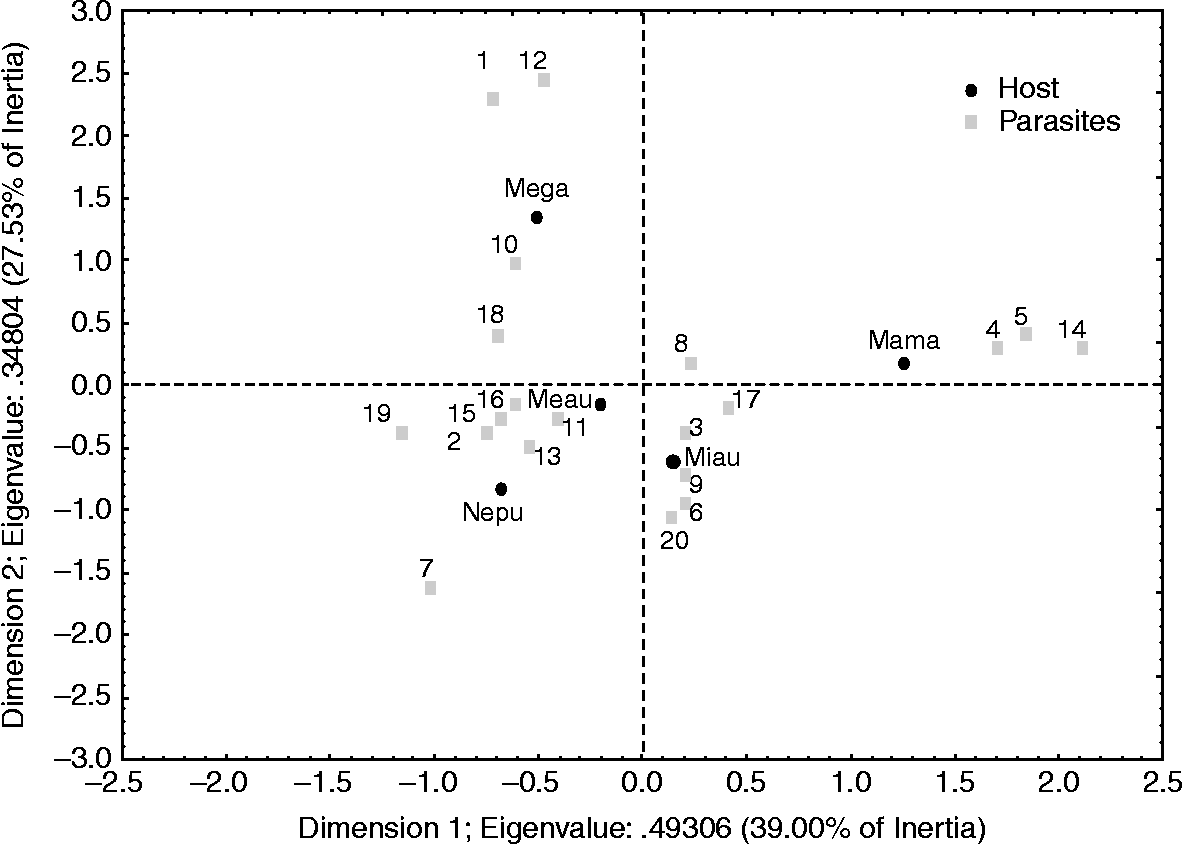
Fig. 3 Correspondence analysis based on prevalence of infection: Nezumia pulchella (Nepu), Merluccius gayi (Mega), Merluccius australis (Meau), Micromesistius australis (Miau) and Macruronus magellanicus (Mama). 1, Aporocotyle wilhelmi; 2, Aporocotyle australis; 3, Derogenes varicus; 4, Elytrophalloides oatesi; 5, Gonocerca phycidis; 6, Hemiuridae gen sp.; 7, Lepidapedon sp.; 8, Anisakis sp.; 9, Ascarophis sp.; 10, Pseudoterranova sp.; 11, Contracaecum sp.; 12, Hysterothylacium sp.; 13, Hysterothylacium aduncum; 14, Cucullanus sp.; 15, Corynosoma sp.; 16, Pseudophyllidea gen sp.; 17: Hepatoxylon trichiuri; 18, Clestobothrium crassiceps; 19, Grillotia heptanchi; 20, Diphyllobotrium sp.
The correspondence analysis based on the mean intensity of infection (fig. 4) also showed significant differences in endoparasite composition among the host species (χ2 = 1812.1, df = 76, P < 0.001); 68% of the variation was explained by the first two dimensions (38.6% and 29.7% for the first and second dimensions, respectively). Several endoparasites were associated with Merluccius australis, Micromesistius australis, M. magellanicus and M. gayi (fig. 4).
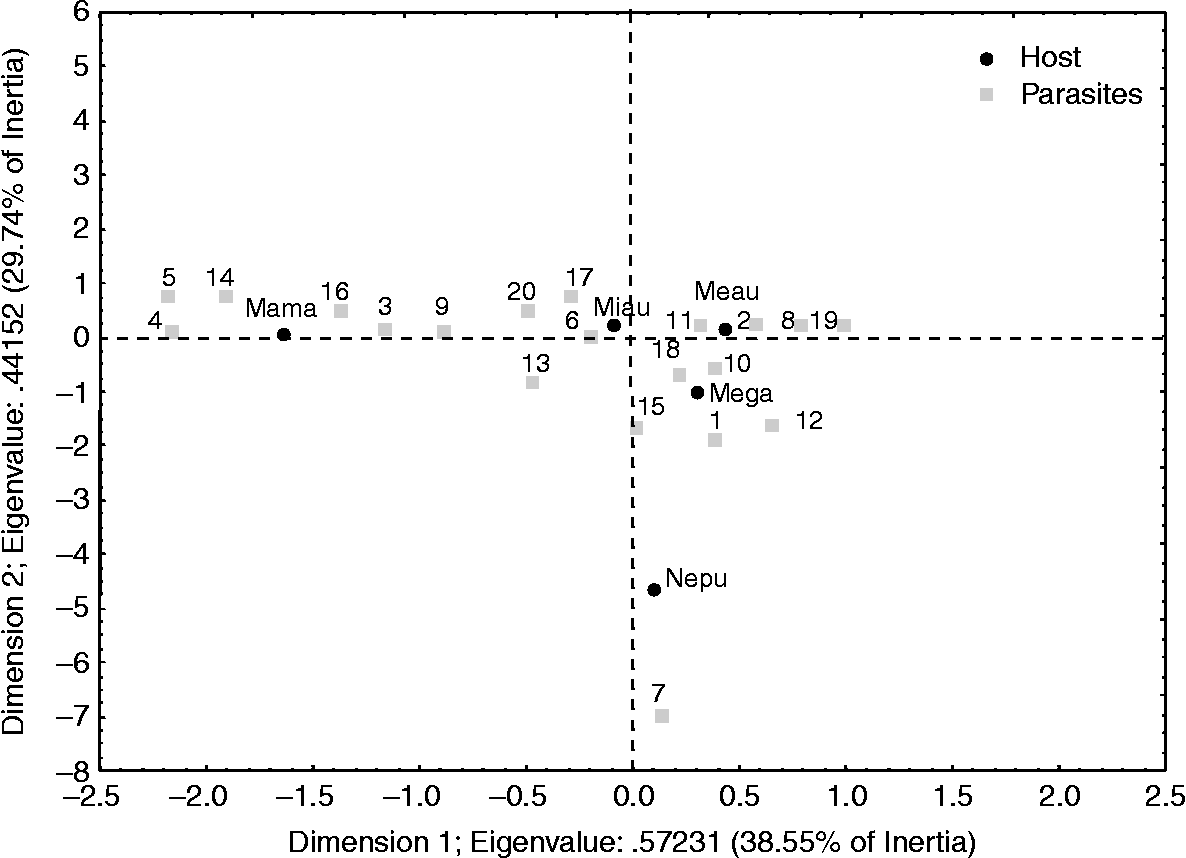
Fig. 4 Correspondence analyses based on mean intensity of infection. Code for host species and parasite species as in fig. 3.
Discussion
Several studies have evaluated the role of ecological factors (e.g. niche dimensions such as diet, depth, latitude and habitat) on the structure and parasite richness of fishes (Aldana et al., Reference Aldana, Pulgar, Ogalde and Ojeda2002; Muñoz et al., Reference Muñoz, Valdebenito and George-Nascimento2002). However, studies investigating the relationship between parasite descriptors and ecological variables of the host, in particular those including phylogeny, are scarce (Poulin & Rohde, Reference Poulin and Rohde1997; Morand et al., Reference Morand, Cribb, Kulbicki, Rigby, Chauvet, Dufour, Faliex, Galzin, Lo, Lo-Yat, Pichelin and Sasal2000; Muñoz et al., Reference Muñoz, Grutter and Cribb2006). Specifically, the endoparasite communities of fishes can be determined by the feeding habits of the host (for instance, specialist versus generalist predators), changes in their ontogenetic feeding, and the availability of different prey (intermediary hosts) in a given environment (Poulin, Reference Poulin1995). The host species Micromesistius australis and M. magellanicus, which belong to different suborders in the Gadiformes, show greater similarity in their endoparasite compositions, which can be best explained by their similar trophic patterns instead of by phylogeny. Micromesistius australis, M. magellanicus and Merluccius australis are related trophically, because they feed mainly on the same species of myctophid fish (e.g. Lampanyctus sp.) and crustacean (Pasiphaea doffleini) (Lillo et al., Reference Lillo, Céspedes, Díaz and Ojeda2004, Reference Lillo, Céspedes, Ojeda, Balbontín, Bravo, Saavedra, Barbieri and Vera2005; Saavedra et al., Reference Saavedra, Correa, Céspedes, Ojeda, Adarme, Días, Oliva and Rojas2006). This similarity in diet agrees well with the similarity of the endoparasite fauna of Micromesistius australis and M. magellanicus, which in turn is supported by cluster and correspondence analyses (figs 1–4), emphasizing the higher prevalence of H. trichiuri and Anisakis sp. in these three host fishes. The latter parasite can be transmitted to Merluccius australis via the ingestion of juveniles of M. magellanicus, their main prey (Lillo et al., Reference Lillo, Céspedes, Ojeda, Balbontín, Bravo, Saavedra, Barbieri and Vera2005), which is an intermediate host of Anisakis sp. (Riffo & George-Nascimento, Reference Riffo and George-Nascimento1992). The infection of Micromesistius australis by Anisakis sp. could be explained by the consumption of intermediate hosts (Crustacea) (Sakanari & McKerrow, Reference Sakanari and McKerrow1989). The cestode H. trichiuri is a common parasite of merluccid fish (Mladineo, Reference Mladineo2006). This cestode can infect all three hosts through the ingestion of larval stages harboured by crustaceans (Vásquez-López et al., 2001). The similar trophic patterns of two Gadoidei (Merluccius australis, M. magellanicus) and one Macrouroidei (Micromesistius australis) are caused by their spatial overlap (both latitudinal and bathymetric). In contrast, the distribution of M. gayi overlaps those of the other three species only within a narrow latitudinal range, 28°S–47°S (Aguayo, Reference Aguayo, Alheit and Pitcher1995); this host species demonstrates distinct feeding habits, with its most important prey being the crustaceans Pterygosquilla armata, Pleuroncodes monodon, Cerviminuda johni and Euphausia mucronata, the fishes Engraulis ringens and Strangomera bentincki and other M. gayi (Arancibia & Fuentealba, Reference Arancibia and Fuentealba1993). On the other hand, N. pulchella overlaps with M. gayi both latitudinally and bathymetrically between 20°S and 33°S (Sielfeld & Vargas, Reference Sielfeld and Vargas1996). This spatial overlap is not reflected in the parasite composition of these two fish species, as shown by cluster and correspondence analyses (figs 3 and 4). The lack of similarity between endoparasite fauna of N. pulchella and M. gayi could be explained by the digenean Aporocotyle wilhelmi, which is a parasite specific to M. gayi (Villalba & Fernández, Reference Villalba and Fernández1986). However, the most important endoparasite species of N. pulchella (Macrouridae) is the digenean Lepidapedon sp., which is a common parasite of Gadiformes species (Bray & des Clers, Reference Bray and des Clers1992).
The phylogenetic information provided by comparative analysis avoids confusing effects among analysed ecological variables (Harvey & Pagel, Reference Harvey and Pagel1991). Phylogenetic effects could be hidden by ecological effects, except in the presence of strong host ecological effects and high probabilities of acquiring or losing parasites, or in the case of marked changes in ecological characteristics during speciation events (Vickery & Poulin, Reference Vickery and Poulin1998). According to Morand et al. (Reference Morand, Cribb, Kulbicki, Rigby, Chauvet, Dufour, Faliex, Galzin, Lo, Lo-Yat, Pichelin and Sasal2000), host phylogenetic relationships have a strong influence on patterns of parasite richness. However, a consistent pattern explaining the variations in endoparasite composition and richness among fish species of Labridae (Cheiliniae) has not been observed, unless the species are phylogenetically related and present very similar diets and body sizes (Muñoz et al., Reference Muñoz, Grutter and Cribb2006). These observations suggest that the phylogenetic relationships of hosts do not have a significant effect on the structure of their parasite communities, and thus, the mixed effects of host descriptors (diet, weight) and phylogeny are the main contributors to endoparasite composition. Recently, Poulin (Reference Poulin2010) suggested that similarity of parasite fauna decreases with the phylogenetic distance of the host species. In the present study, the results of multivariate analyses (figs 1–4) supported the observation that the fish species most closely related phylogenetically (Merluccius gayi and Merluccius australis) did not show greater parasite fauna similarities. In a similar way, the two species belonging to the suborder Macrouroidei (M. magellanicus and N. pulchella), as defined by Roa-Varón & Ortí (Reference Roa-Varón and Ortí2009), show the higher divergence in the composition of their parasite fauna. Moreover, our results suggest that the most closely related parasite fauna is shared by Micromesistius australis and M. magellanicus – species that belong to different suborders in the Gadiformes. Those findings might be explained by the latitudinal/bathymetric segregation of these species and, consequently, the differential prey availabilities, indicating that the composition of the endoparasite fauna is mainly influenced by feeding habits (predator–prey relationships).
In summary, among the Gadiformes species studied herein, the high degree of endoparasite similarity was determined principally by their ecological characteristics (trophic overlap); consequently, phylogenetic relationships could play a secondary role in the determination of their endoparasite fauna. Nevertheless, and similarly to the results reported by Muñoz et al. (Reference Muñoz, Grutter and Cribb2006), the Gadiformes fish species described herein harbour mainly generalist endoparasites (Anisakis sp., H. trichiuri, Derogenes varicus), which infect several demersal fishes (Genypterus spp., Dissostichus eleginoides, Hippoglossina macrops, among others) (George-Nascimento & Huet, Reference George-Nascimento and Huet1984; Oliva et al., Reference Oliva, Gonzalez and Acuña2004, Reference Oliva, Fernández, Oyarzun and Murillo2008). In addition, there is a possibility that our results could be biased, because generalist larval species such as H. trichiuri, Anisakis sp. and Pseudoterranova sp. could be different species that are morphologically similar, a finding that has been demonstrated genetically for some anisakid species (Mattiucci & Nascetti, Reference Mattiucci and Nascetti2007). Therefore, molecular studies may be necessary to identify such species and to evaluate conclusively the effects of ecological and phylogenetic factors on the composition of endoparasite communities in Gadiformes fish species.








