Introduction
Trichinella spiralis is a serious zoonotic nematode that infects a wide range of mammals such as pigs, mice, birds, horses and humans. Trichinosis causes widespread morbidity and mortality (Al-Attar et al., Reference Al-Attar, El-Kersh, Sadek, Harba, Osheiba and Brakat2020). Currently, T. spiralis infects more than 11 million people in Africa, Central and South America and Asia (Muñoz-Carrillo et al., Reference Muñoz-Carrillo, Maldonado-Tapia, López-Luna, Muñoz-Escobedo, Flores-De La Torre, Moreno-García and Bastidas2018). About 10,000 cases occur each year with a 0.2% mortality rate (Ashour & Elbakary, Reference Ashour and Elbakary2011; García et al., Reference García, Leonardi, Vasconi, Hinrichsen and Lamas2014). Trichinella spiralis was internationally categorized among the top ten foodborne parasites (El Temsahy et al., Reference El Temsahy, Ibrahim, Mossallam, Mahrous, Abdel Bary and Abdel Salam2015). Consequently, trichinosis represents a public health threat that affects human health and causes enormous economic losses in the porcine animal industry and food safety (Gottstein et al., Reference Gottstein, Pozio and Nöckler2009).
After consumption of undercooked meat from infected animals, the larvae take roughly 17 days to enter the striated muscles; moreover, intact encapsulations form on day 30 after infection (Ren et al., Reference Ren, Qin, Zhang, Zheng, Dai, Wu, Dong and Cui2018). The encysted stage is the most virulent stage of T. spiralis, and it is only during this stage that it can be transmitted between hosts (Yadav & Temjenmongla, Reference Yadav and Temjenmongla2006). The capsule wall is resistant to humoral and cellular immune responses as it comprises collagen fibres produced by host fibroblasts. These muscle larvae can survive and persist within the host for its entire life (Ren et al., Reference Ren, Qin, Zhang, Zheng, Dai, Wu, Dong and Cui2018).
The symptoms of trichinosis vary from mild to severe. They include generalized fever, stomach discomfort, diarrhoea, nausea, vomiting and myalgia, as well as myocarditis and encephalitis (Muñoz-Carrillo et al., Reference Muñoz-Carrillo, Maldonado-Tapia, López-Luna, Muñoz-Escobedo, Flores-De La Torre, Moreno-García and Bastidas2018). Furthermore, they include respiratory myositis, obstructive bronchitis, secondary bacterial pneumonia (Compton et al., Reference Compton, Celum, Lee, Thompson, Sumi, Fritsche and Coombs1993), haematuria and renal failure (Neghina et al., Reference Neghina, Neghina, Marincu and Iacobiciu2011). Heart failure, breathing difficulties or kidney failure are the most common causes of death (Bruschi & Murrell, Reference Bruschi and Murrell2002).
The traditional trichinosis therapy with benzimidazole derivatives, such as albendazole and mebendazole, is considered as broad-spectrum and effective (Priotti et al., Reference Priotti, Codina, Leonardi, Vasconi, Hinrichsen and Lamas2017). The administration of 50 mg/kg albendazole starting from the third day post-infection (dpi) for three successive days and from the 31st day dpi for seven successive days in T. spiralis-infected mice resulted in a reduction in adult and larval counts (94.2% and 90.9%, respectively) (Attia et al., Reference Attia, Mahmoud, Farrag, Makboul, Mohamed and Ibraheim2015). However, it has low bioavailability and many adverse effects. In addition, drug resistance may result from its long-term application (Shalaby et al., Reference Shalaby, Moghazy, Shalaby and Nasr2010), and it has limited effectiveness against the parasite's encapsulated larval stages (Caner et al., Reference Caner, Döşkaya, Değirmenci, Can, Baykan, Üner, Başdemir, Zeybek and Gürüz2008). Furthermore, many of these medications are harmful to children below the age of three years, pregnant women and others who are vulnerable to carcinogenicity (Yadav & Temjenmongla, Reference Yadav and Temjenmongla2012). Consequently, alternative anthelmintic drugs derived from traditional medicinal plants (Abu El Ezz, Reference Abu El Ezz2005) and natural products from different origins with minimal side effects that are well tolerated are warranted (Gilleard & Beech, Reference Gilleard and Beech2007).
Propolis is a glue substance gathered by bees from the bark of trees and from certain plant buds (Abdulrhman et al., Reference Abdulrhman, Elbarbary, Amin and Ebrahim2012). Bees use this substance to seal holes in the beehive to defend the colony against pathogens and predators (Hegazi, Reference Hegazi2012). Since 300 BC, man has used propolis as a traditional medicine (Sung et al., Reference Sung, Choi, Lee and Shin2017). Early Egyptians were aware that it can prevent putrefaction (Sforcin, Reference Sforcin2016). Therefore, they used it to preserve their bodies from decomposition (Zabaiou et al., Reference Zabaiou, Fouache, Trousson, Baron, Zellagui, Lahouel and Lobaccaro2017) and heal wounds (Parolia et al., Reference Parolia, Thomas, Kundabala and Mohan2010). Chemical analysis has revealed at least 300 compounds in this material (Drescher et al., Reference Drescher, Klein, Neumann, Yanez and Leonhardt2017). Caffeic acid phenethyl ester (CAPE) is one of the biologically active ingredients of propolis. It has many biological properties, including apoptotic, antimicrobial and antiviral activities (Hassan et al., Reference Hassan, Abou-El-Dobal and Hegazi2016). Egyptian propolis has exhibited anti-helminthic activity against adult Fasciola gigantica. It causes distortion of oral and ventral suckers, tegumental damage and loss of spines (Hegazi et al., Reference Hegazi, Abd El Hady and Shalaby2007). Moreover, disorganization of the cuticle and body musculature was observed in Toxocara vitulorum treated with Egyptian propolis (Hassan et al., Reference Hassan, Abou-El-Dobal and Hegazi2016). Furthermore, it has the potential to improve the amount of protection against Taenia saginata infection in mice when administered concurrently with immunization (Kandil et al., Reference Kandil, Nassar, Nasr, Shalaby, Hendawy and El Moghazy2015).
Selenium (Se) is an essential trace element, a structural component of various antioxidant enzymes, including glutathione peroxidase. It is thought to reduce oxidative stress (Talas et al., Reference Talas, Ozdemir, Yilmaz, Gok and Orun2008). Se has the potential to boost both humoral and cell-mediated immune responses. Most of the available data suggest that Se status affects resistance to bacterial, parasitic and fungal infections (Hoffmann & Berry, Reference Hoffmann and Berry2008).
Nanotechnologies are now offering new opportunities for innovative medical treatments to fight parasitic infection, as particles with nanometre dimension have novel properties different from those of both isolated atoms and bulk materials (Peng et al., Reference Peng, Zhang, Liu and Taylor2007). The reactive oxygen species (ROS) produced by these particles kill infectious organisms. Furthermore, the small size of nanoparticles allows them to pass through membrane barriers, resulting in greater reactivity (Bhardwaj et al., Reference Bhardwaj, Saudagar and Dubey2012).
Rayman (Reference Rayman2012) reported that Se nanoparticles (SeNPs) have antioxidant and anti-inflammatory properties. SeNPs exhibit anti-leishmanial effects (Dkhil et al., Reference Dkhil, Al-Quraishy and Wahab2015) and play a protective role against jejunal injury in murine schistosomiasis (Dkhil et al., Reference Dkhil, Bauomy, Diab and Al-Quraishy2016). Along the same lines, Nelson et al. (Reference Nelson, Shay, James, Carlson, Urban and Prabhu2016) suggested that increases in dietary Se in mice infected with Nippostrongylus brasiliensis decreased parasite egg production (fecundity) and lowered the number of adult worms in the intestine.
The present study aimed to identify innovative and safe treatments for trichinosis that reduce morbidity and mortality. In mice, Se, SeNPs and Egyptian propolis were compared with albendazole regarding their antiparasitic, anti-inflammatory and anti-angiogenic properties. The efficacy of the drugs was evaluated using parasitological, histological and immunohistochemical assays, as well as scanning electron microscopy (SEM), on adult worms. To the best of our knowledge, this is the first study to evaluate the therapeutic effects of Egyptian propolis and SeNPs on murine trichinosis.
Materials and methods
Animals and parasites
A total of 80 parasite-free, five-week-old Swiss albino male mice weighing 20–25 g were used in this study. The mice were obtained from the animal house of Theodor Bilharz Research Institute in Giza, Egypt, and were cared for according to institutional and national requirements.
Trichinella spiralis was isolated and maintained in rats through repeated passaging. For experiments, 200–250 T. spiralis larvae were orally administrated to each mouse in the Medical Parasitology Department laboratory, Faculty of Medicine, Zagazig University, Egypt (Shoheib et al., Reference Shoheib, Shamloula, Abdin and El-Segai2006).
Experimental design
Eight groups of mice, with ten mice each, were included in this study: group 1, negative control (uninfected mice); group 2, positive control (infected mice that did not receive any treatment); group 3, infected mice treated with albendazole; group 4, infected mice treated with propolis; group 5, infected mice treated with Se; group 6, infected mice treated with a combination of propolis and Se; group 7, infected mice treated with SeNPs; and group 8, infected mice treated with a combination of SeNPs and propolis.
Assessment of samples
Five mice from each group were sacrificed on the seventh dpi. Subsequently, 1 cm of the middle section of the small intestine was removed and immediately preserved in 10% formalin for histological analysis. Trichinella spiralis adult worms were counted using the residual sections of the small intestine. The mature worms were then kept in a fresh fixative for SEM. The remaining mice were euthanized at the 35th dpi, with muscle samples collected from the diaphragm and preserved in 10% formalin for histological and immunohistochemical analyses. The total larval count was estimated from the digestion of the remainder of the muscles.
Drugs and extracts
Albendazole was supplied as Alzental suspension (EIPICO, October city, Egypt), which contained 20 mg/ml. It was given orally in a 50 mg/kg dose for three days post-infection, starting on the third dpi (Attia et al., Reference Attia, Mahmoud, Farrag, Makboul, Mohamed and Ibraheim2015).
Preparation of propolis ethanolic extract
Egyptian propolis resinous material (50 g) was chopped into small pieces and extracted at room temperature with 250 ml of 80% ethanol by shaking (twice after 24 h). The alcoholic extract was evaporated until dryness under a vacuum using a rotatory evaporator at 50°C. The dried propolis ethanolic extract (5 g yield) was suspended in phosphate-buffered saline (PBS) (pH 7.2) (Hegazi et al., Reference Hegazi, Farghali and Abd El Hady2004) and was administered once per day from one dpi at an oral dose of 250 mg/kg and continued until the 35th dpi to cover all stages of T. spiralis infection (Issa, Reference Issa2007).
Se in the organic form of Sel-plex (Alltech, Nicholasville, Kentucky, USA) was suspended in dimethyl sulfoxide and administered at a dose of 0.5 mg/kg orally, once per day from one dpi and continued until the 35th dpi (Gabrashanska et al., Reference Gabrashanska, Teodorova, Petkova, Mihov, Anisimova and Ivanov2010).
SeNPs (50–100 nm particle size) were obtained as a sterilized solution from Nanotech Lab in 6th October City, Egypt. They were dispersed in PBS and ready to use. In summary, SeNPs were synthesized using a simple wet chemical approach that involved reacting a sodium selenosulfate precursor with various organic acids in an aqueous medium under ambient conditions. The SeNPs were stabilized using polyvinyl alcohol. A high-speed centrifuge was used to separate the nanoparticles from their sol, and a sonicator was used to re-disperse them in an aqueous medium. Transmission electron microscopy was used to characterize the shape and size of nanoparticles (Dwivedi et al., Reference Dwivedi, Shah, Singh, Kumar and Bajaj2011). SeNPs were administered at a dose of 0.5 mg/kg orally, once per day from one dpi and continued until the 35th dpi (Gabrashanska et al., Reference Gabrashanska, Teodorova, Petkova, Mihov, Anisimova and Ivanov2010).
Parasitological analysis
Trichinella spiralis adult worm isolation and count
The small intestines of mice were separated after the animals were euthanized. The intestines were split into 1 cm sections and stored at 37°C in 10 ml physiological saline for 2 h after being cleansed with saline. The intestines were rinsed with physiological saline, and the fluid was centrifuged for 3 min at 2000 rpm. The sediment was placed in a Petri dish containing physiological saline following the removal of the supernatant. Under a dissecting microscope, the number of adults was counted by examining the sediment drop by drop (Basyoni & El-Sabaa, Reference Basyoni and El-Sabaa2013).
Total larval burden in muscles
Muscle larval counts in complete carcasses were evaluated after the mice were euthanized on the 35th dpi using the method described by Dunn & Wright (Reference Dunn and Wright1985). Each mouse was dissected, and the muscles digested in 200 ml of distilled water with 1% pepsin hydrochloride. The mixture was incubated for 1 h at 37°C with constant stirring. Encysted larvae were collected using the sedimentation method and washed in distilled water numerous times. The larvae were microscopically counted using a McMaster counting chamber (Lauda-Königshofen, Germany).
SEM of adult worms
Adult worms were fixed in a 2.5% glutaraldehyde solution and incubated overnight at 4°C. Adults were rinsed in 0.1 M sodium cacodylate buffer for 5 min and then fixed in 2% osmium tetroxide for 1 h. The sample was dehydrated in increasing grades of alcohol and dried using a critical point of carbon dioxide drying. The sample was examined using a JEOL SEM instrument (JEOL Co., Tokyo, Japan) after being sputter-coated with gold (Bughdadi, Reference Bughdadi2010).
Histopathological analysis
Tissue samples from the studied groups were fixed in 10% formalin for 24 h, washed with water for 12 h, dehydrated in increasing grades of alcohol, cleared in xylene and embedded in paraffin blocks followed by sectioning at a 5 μm thickness a microtome and staining with haematoxylin and eosin. Subjective semi-quantitative histopathological scoring was used to evaluate the histopathological characteristics of the small intestine and muscle sections (Othman et al., Reference Othman, Abou Rayia, Ashour, Saied, Zineldeen and El-Ebiary2016).
Examination and scoring of the tissue sections (+1 = mild reaction; +2 = moderate reaction; +3 = intense reaction) were carried out. The extent of inflammatory cell infiltrates within the intestinal villi and submucosa core was histopathologically evaluated in small intestinal specimens. The extent of the inflammatory response surrounding the capsule in skeletal muscle specimens was assessed and scored. An average score was calculated after examining five histological sections per mouse in ten low-power fields (100×) from each of the examined histological sections.
Immunohistochemical analysis
Detection of the vascular endothelial growth factor (VEGF) in the skeletal muscles
Muscle slices were deparaffinized and treated with 3% hydrogen peroxide in methanol to suppress endogenous peroxidase activity. An antigen retrieval procedure was performed. The sections were then incubated at room temperature for 30 min with a monoclonal antibody against VEGF (clone: EP1176 y, ready-to-use, GENOVA Diagnostics Company, Asheville, North Carolina, USA). The biotin–streptavidin–peroxidase technique was employed to visualize the antigen–antibody combination. The sections were lightly counterstained with haematoxylin after the colour was developed using a diaminobenzidine solution. Subsequently, the sections were dehydrated and mounted. A brownish cytoplasmic staining was observed in cells positive for VEGF immunostaining.
Immunohistochemical scores (IHSs) were determined by combining the percentage of positively stained cells (quantity score) with the staining intensity score. The scores ranged from 0 to 4, with zero indicating that no immunostaining occurred; 1 = 1–10% of the cells were stained; 2 = 11–50% of the cells were stained; 3 = 51–80% of the cells were stained; and 4 = 81% or more of the cells were stained. The intensity of the staining was graded as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The IHSs ranged from 0 to 12 and were calculated by multiplying the quantity score (0–4) by the staining intensity score (0–3). A score of 9–12 was considered strong immunoreactivity (+3), a score of 5–8 was considered moderate immunoreactivity (+2), a score of 1–4 was considered weak immunoreactivity (+1) and a score of zero was considered negative immunoreactivity (Gou et al., Reference Gou, Chen, Zhu, Jiang, Yang, Cao and Hou2011). The average score was calculated after examining five histological sections per mouse and ten high-power fields (400×) from each examined section.
Statistical analysis
Statistical analyses were conducted using SPSS version 18.0 (IBM, Armonk, New York, USA). The results are expressed as means ± standard deviation. Data were analysed using one-way analysis of variance (ANOVA) test followed by Tukey's post-hoc test for multiple comparisons between groups. The chi-squared test was employed for histopathological and immunohistochemical scoring. Differences were considered statistically significant when P < 0.05, highly significant when P < 0.01 and not significant when P < 0.05.
Results
Parasitological findings
Trichinella spiralis adult worm and larval burden
Compared with the positive control group, all treated groups exhibited a significant decrease in the mean number of adult T. spiralis worms. The strongest reduction was detected in mice that had received the combination of SeNPs and propolis (92.8%), which was very close to that of the mice that received albendazole (92.6%), followed by those that received SeNPs (89.2%), combination of propolis and Se (85.5%), Se (80.3%) and propolis (75.1%) (table 1).
Table 1. Mean T. spiralis adult count in the small intestine and mean larval count in the muscles.

n, number of mice in each group; SD, standard deviation; P, probability.
* Significant difference.
** Highly significant difference. P > 0.05 = not significant.
P1, albendazole treated group vs. positive control group; P2, albendazole treated group vs. propolis group; P3, albendazole treated group vs. Se group; P4, albendazole treated group vs. propolis and Se group; P5, albendazole treated group vs. SeNPs group; P6, albendazole treated group vs. SeNPs and propolis group.
Regarding the mean larval count in muscles, compared with the positive control group, a significant decrease was observed in all treatment groups. Mice that were given a combination of SeNPs and propolis exhibited the strongest reduction (93.1%), followed by mice given SeNPs alone (90.4%), albendazole (89%), combination of propolis and Se (87.9%), Se (83.1%) and propolis (77%) (table 1).
SEM
The results of the examination of the adult worms via SEM are presented in fig. 1. The mature worm cuticle in the control group retained its usual morphology, including the distinctive transverse creases, ridges and annulations, as well as the emergence of hypodermal apertures (fig. 1b, c). A pair of leaflets, two pairs of mastoids and a gonopore made up the bell-shaped attachment apparatus at the end of the male body. The mastoid and reproductive openings were visible, and the leaflets stretched outward and upright (fig. 1a). The cuticle of T. spiralis adult worms was extensively injured in the treated groups, the body had collapsed and there were numerous blebs and vesicles (fig. 1d–h). The cuticle of the adult worms was swollen between circular annulations (fig. 1e). On the surface of the worms, the typical creases, annulations and ridges had disappeared, and sloughing of the cuticle was detected in some locations (fig. 1d, h, i).
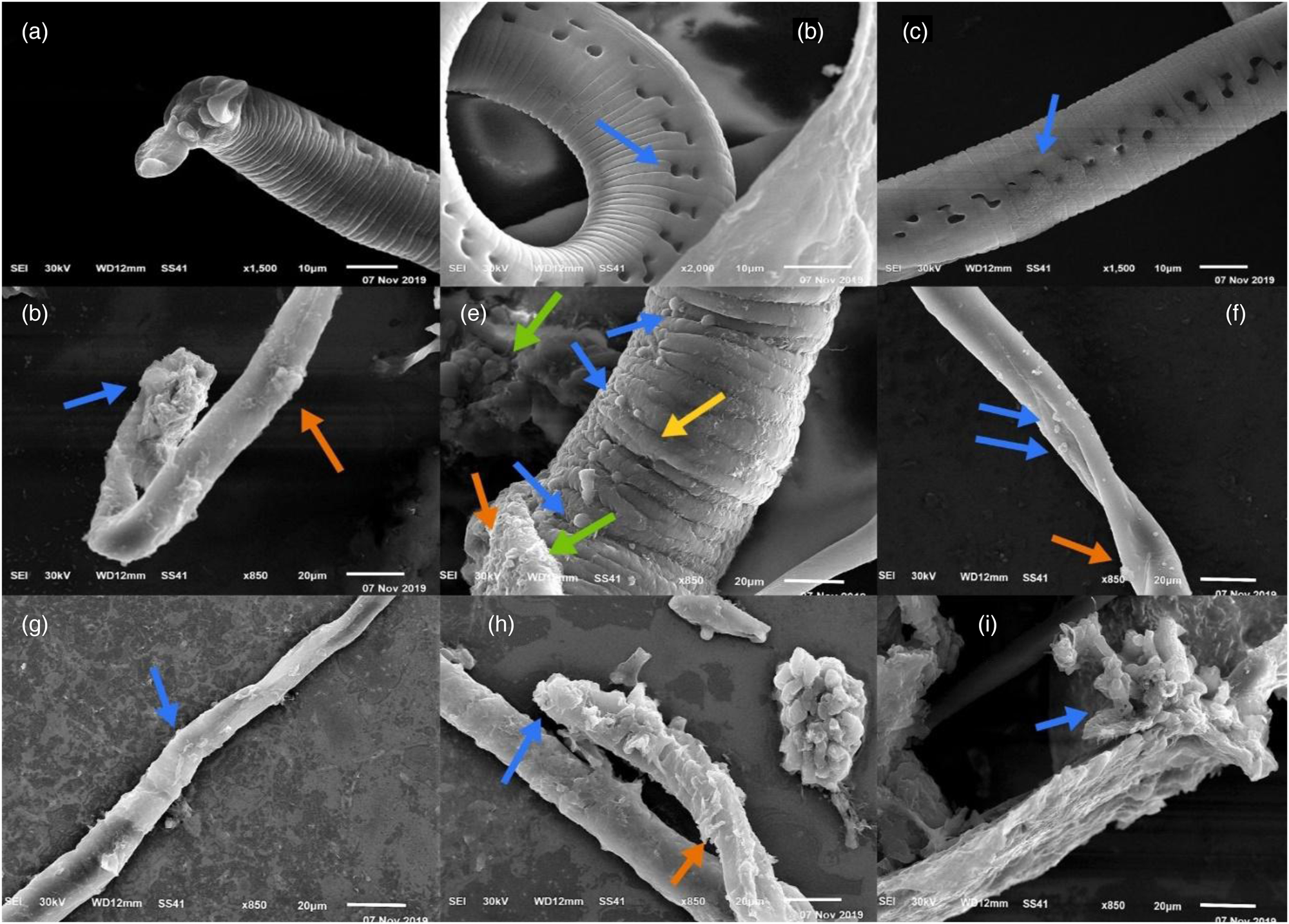
Fig. 1. SEM findings of the adult T. spiralis worm. (a) Infected control group showed an intact mating accessory structure of a control group male worm, and two pairs of mastoids. (b, c) Infected control group showed intact cuticle of an adult worm with circular annulations and opening of hypodermal glands (blue arrow). (d) Albendazole-treated group showing marked destruction of the adult worm. There is sloughing of some cuticle areas (blue arrow) with loss of its circular annulation and large blebs (red arrow). (e) Propolis-treated group showing the body of adult worm was collapsed (green arrows), swollen cuticle (yellow arrow), many vesicles were detected (blue arrows) in the cuticle with sloughing area (red arrow) and no opening of hypodermal glands. (f) Se-treated group showing some vesicles in the cuticle of the adult worm (blue arrows) with loss of annulations and large blebs (red arrow). (g) Combination of propolis and Se-treated group showing the exfoliated cuticle of the adult worm with loss of annulations and small bleb (blue arrow). (h) SeNPs-treated group showing marked destruction of the adult worm. Sloughing of some cuticle areas (blue arrow) and many vesicles (red arrow) and loss of annulations. (i) Combination of SeNPs and propolis-treated group showing adult worm was completely destructed with sloughing areas of the cuticle (blue arrow) and loss of annulations.
Histopathological findings
Small intestine
Compared with the intestine in the negative control group, the histopathological analysis of the positive control group revealed intense inflammatory cell infiltrate mainly in the centre of the villi and extending into the submucosa. Eosinophils and neutrophils made up the majority of the inflammatory infiltrates. Villi flattening, as well as villous tip sloughing, was also observed. Adult T. spiralis cross-sections were visible (fig. 2b). SeNPs (fig. 2g) and the combination of SeNPs and propolis (fig. 2h) yielded a significant reduction in inflammation intensity and elongated villi compared with sections from mice treated with albendazole (fig. 2c). The presence of adult T. spiralis worms on the tip of villi and between villi was observed, respectively, at a higher magnification (fig. 2c1, c2). Mice treated with propolis (fig. 2d), Se (fig. 2e) and combination of propolis and Se (fig. 2f) exhibited moderate inflammation within the core of the intestinal villi. The degree of inflammatory cell infiltration within the intestinal villi and submucosa core is presented in table 2.

Fig. 2. Histopathological findings of the small intestine sections at seventh dpi Hematoxalyin and Eosin (H&E). (a) Negative control group showing distinct intestinal layers beside villous length, width and submucosal crypts (H&E ×200). (b) Infected control group showing distorted and sloughed villous portion (black arrow), numerous parasites (curved arrow), atrophied crypts (thick arrow) beside intense inflammatory cells in lamina propria and the core of intestinal villi (H&E ×200). (c) Albendazole-treated group showing a few parasitic elements on the tips of villi (black arrow) with proliferative hyperplastic villous enterocytes and crypts (thick arrows), and mild inflammatory cells infiltrate in the core of villi and submucosa (H&E ×200). (c1) Albendazole-treated group showing parasitic element on the tips of villi (black arrow) at a high-power magnification (H&E ×400). (c2) Albendazole-treated group showing parasitic elements in between villi (black arrows) at a high-power magnification (H&E ×400). (d) Propolis-treated group showing a minute parasite present in the mucosa (black arrow) with broad villi, and hyperplastic crypts (thick arrow) with moderate inflammatory cells infiltrate (H&E ×200). (e) Se-treated group showing broad villi and hyperplastic crypts (black arrows) and moderate inflammatory cells (thick arrow) (H&E ×200). (f) Combination of propolis- and Se-treated group showing broad villi with moderate inflammatory cells infiltrate (black arrow) and hyperplastic crypts (thick arrow) (H&E ×200). (g) SeNPs-treated group showing intense hyperplasia of intestinal crypts (thick arrow), a few parasitic elements (black arrows) and mild wide intestinal villi with mild inflammatory cells infiltrate (H&E ×200). (h) Combination of SeNPs- and propolis-treated group showing short villi, little parasitic elements (black arrow) and hyperplastic crypts (thick arrows) mild inflammatory infiltrate (H&E ×200).
Table 2. The extent of inflammatory cell infiltrates in the small intestine (within the core of villi and submucosa) and diaphragm.

N, number of mice in each group; P, probability.
* Significant difference.
** Highly significant difference.
Skeletal muscles
In contrast with the typical muscle fibres of the diaphragm observed in the negative control group (fig. 3a), the histopathological analysis of the skeletal muscles of the positive control group revealed encysted T. spiralis larvae surrounded by nurse cells, a collagen capsule and dense inflammatory cell infiltration; the infiltrates contained primarily histiocytes, plasma cells, lymphocytes and eosinophils, which surrounded the encysted larvae and invaded the damaged muscle fibres in a diffuse manner (fig. 3b).
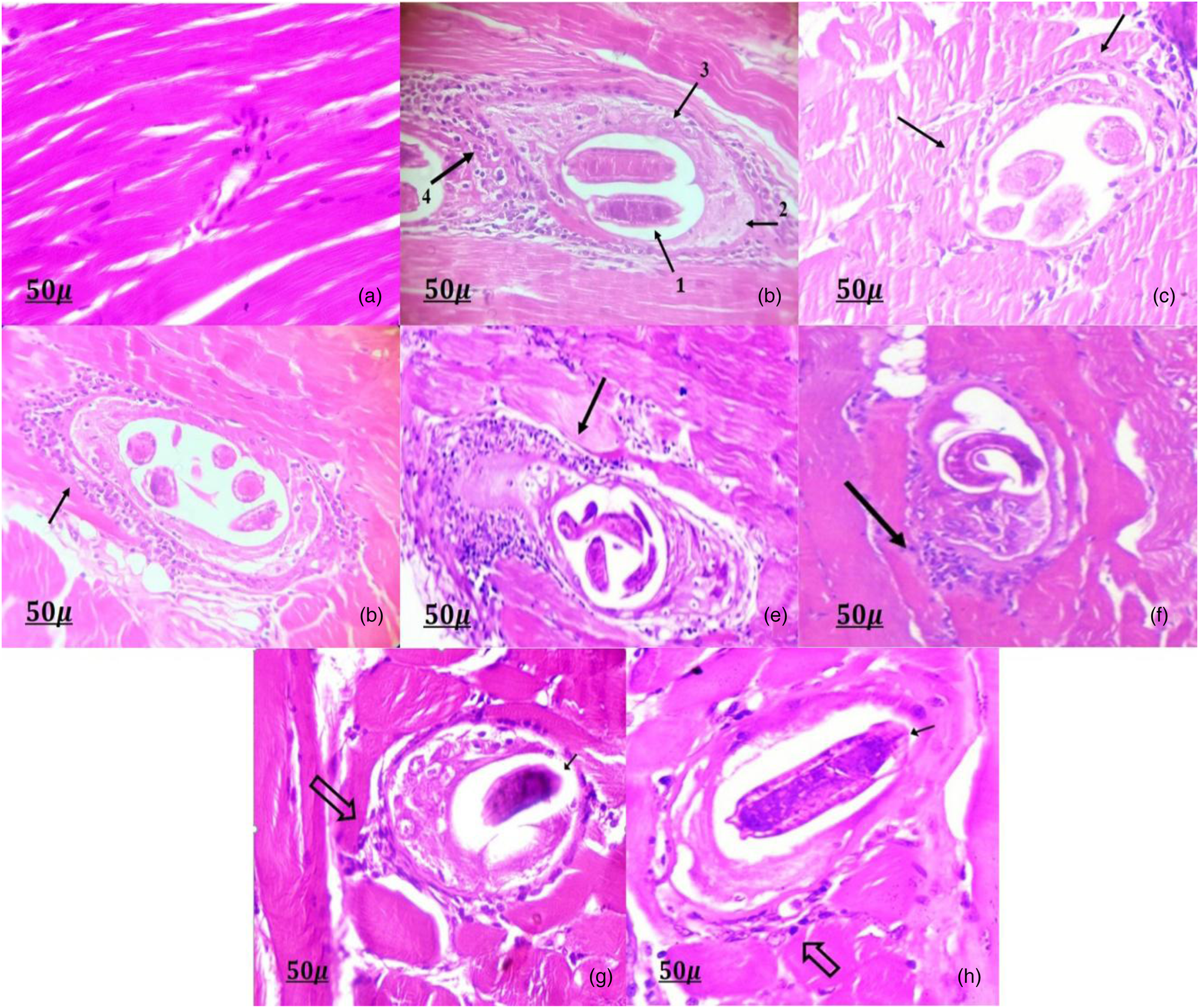
Fig. 3. Histopathological findings of the muscle sections at 35th dpi (H&E ×400). (a) Negative control group showing normal muscle fibres of diaphragm. (b) Infected control group showing (1) encysted larvae of T. spiralis, (2) collagen capsule, (3) nurse cell and (4) marked inflammatory cells infiltrate. (c) Albendazole-treated group showing mild inflammatory cells infiltrate (black arrows) around T. spiralis larva. (d) Propolis, (e) Se and (f) combination of propolis- and Se-treated groups, all of which show moderate inflammatory cells infiltrate (black arrows) around T. spiralis larva. (g) SeNPs and (h) combination of SeNPs- and propolis-treated groups, both showing mild inflammatory infiltrate (thick arrows) around degenerated and calcified larva (black arrows).
Sections from mice treated with albendazole (fig. 3c), SeNPs (fig. 3c) and combination of SeNPs and propolis (fig. 3h) exhibited a significant reduction of inflammatory cell infiltration around the larvae. Moreover, the groups treated with propolis (fig. 3d), Se (fig. 3e) and combination of propolis and Se (fig. 3f) showed moderate inflammation around the larvae. The amount of the inflammatory cell infiltrates in the skeletal muscle sections is presented in table 2.
Immunohistochemical findings
The negative control group showed no VEGF reactivity (fig. 4a). Conversely, the positive control group showed a substantial (+3) reaction, primarily in the cytoplasm of the surrounding inflammatory cells of the nursing cells (fig. 4b). A substantial decrease in VEGF expression was observed in the drug-receiving groups: a weak reaction (+1) in the groups treated with albendazole (fig. 4c), SeNPs (fig. 4g) and combination of SeNPs and propolis (fig. 4h), and a moderate reaction (+2) in the groups treated with propolis (fig. 4d), Se (fig. 4e) and combination of propolis and Se (fig. 4f), with a significant difference (P < 0.001) compared with the positive control group. The expression of VEGF in the experimental groups is presented in table 3.

Fig. 4. Immunohistochemical expression of VEGF in muscles of T. spiralis-infected mice (immunoperoxidase ×400). (a) Negative control group showing no staining for VEGF in the muscle. (b) Infected control group showing strong cytoplasmic VEGF expression within the nurse cells and marked inflammatory infiltrate (black arrow). (c) Albendazole-treated group showing weak cytoplasmic VEGF expression within the nurse cells and mild inflammatory cells infiltrate (black arrows). (d) Propolis, (e) Se and (f) combination of propolis- and Se-treated groups, all of which show moderate cytoplasmic VEGF expression within the nurse cells, and moderate inflammatory cells infiltrate (black arrows). (g) SeNPs and (h) combination of SeNPs- and propolis-treated groups, both showing weak cytoplasmic VEGF expression within the nurse cells and mild inflammatory cells infiltrate (black arrows).
Table 3. Immunohistochemical expression of VEGF expression in the muscles of studied groups.

n, number of mice in each group; P, probability.
* Highly significant difference.
Discussion
Although benzimidazole derivatives such as mebendazole and albendazole are the conventional treatment for trichinosis, they have several side effects (Basyoni & El-Sabaa, Reference Basyoni and El-Sabaa2013). The unsatisfactory therapeutic outcomes of these drugs against the encapsulated larvae in the muscle fibres (Caner et al., Reference Caner, Döşkaya, Değirmenci, Can, Baykan, Üner, Başdemir, Zeybek and Gürüz2008) has led to the need to identify novel, effective and safe natural anthelminthic drugs against both adults and the encysted stage. The current study evaluated the efficacy of Se, SeNPs and Egyptian propolis compared with albendazole as antiparasitic, anti-inflammatory and anti-angiogenic agents against murine trichinosis. We used parasitological, histopathological, immunohistochemical assays, as well as SEM, to examine adult worms. The nematocidal efficacy of SeNPs and propolis against T. spiralis infection was highlighted. Because the use of SeNPs and propolis against nematodes is novel, an in vivo investigation was conducted first to determine how these agents affected adult T. spiralis worms.
Experimental trichinosis is an important model of parasitic infection as it affects the host in the enteral and muscular ways. In our study, we used the diaphragm muscle for the detection of larvae because it is a predilection site (highly oxygenated site) for T. spiralis larvae, as reported by Gottstein et al. (Reference Gottstein, Pozio and Nöckler2009). The larvae invade the skeletal muscle fibres during the muscular phase, causing an inflammatory reaction that triggers the myositis associated with this disease stage (Bruschi & Chiumiento, Reference Bruschi and Chiumiento2011). Some infected myocytes grow into nurse cells after entering the muscle cell. Their primary job is to feed the parasite and protect it from the immunological reaction of the host. The angiogenesis initiated by the parasite attracts a network of permeable blood vessels to the surface of its collagenous capsule (Ock et al., Reference Ock, Cha and Choi2013).
The present study demonstrated a highly significant reduction in adult worm counts in all treated groups compared with the positive control group. The strongest reduction was detected in mice that had received the combination of SeNPs and propolis.
Moreover, the percentage of the reduction in the larval count compared with the positive control group was significantly decreased in all treated groups. A highly significant reduction in the larval counts was found for the combination of SeNPs and propolis group. These results may be attributed to the augmented antiparasitic, and immune-stimulant effects of the combination of SeNPs with propolis.
Our results are in agreement with those of Issa (Reference Issa2007), who reported that propolis produced a significant reduction (58.38%) in the number of adult worms of Schistosoma mansoni in immunosuppressed mice and a marked reduction in egg counts in the stool (61.8%). Moreover, Gabrashanska et al. (Reference Gabrashanska, Teodorova, Petkova, Mihov, Anisimova and Ivanov2010) reported that the percentage of larval count reduction was 63% after the administration of Se in the diet of T. spiralis-infected rats.
The effect of albendazole on T. spiralis larvae was lower than that its effect on adults due to the limited bioavailability of oral administration and water solubility of albendazole (Caner et al., Reference Caner, Döşkaya, Değirmenci, Can, Baykan, Üner, Başdemir, Zeybek and Gürüz2008). These results are consistent with those of Salama et al. (Reference Salama, Mostafa, Abd EL-Aal, Mostafa, Hammad, Adel and Moawad2021), who reported that the percentages of reduction in both total adult worm and muscle larval counts in the albendazole treated group were 93.5% and 90.6%, respectively. Moreover, Huang et al. (Reference Huang, Yao and Liu2020) reported the high efficacy of albendazole on adult worms and its reduced efficacy on muscle larval counts at 97.7% and 49.1%, respectively.
Interestingly, our natural alternative medication – that is, Se, SeNPs and Egyptian propolis – had a better effect on larvae vs. adults.
The antiparasitic effect of propolis could be explained by the presence of caffeic acid in this material, which is one of the biologically active ingredients of propolis that induces morphological changes in the parasitic cells, integrity of mitochondria and cellular plasma membrane, consequently promoting apoptosis. Caffeic acids also increase the antiparasitic activity of macrophages by promoting the expression of ROS (Bortoleti et al., Reference Bortoleti, Tomiotto-Pellissier and Gonçalves2019). Furthermore, the antiparasitic effect of propolis could be attributed to the antioxidant effect of the flavonoids that are concentrated in propolis or its immunomodulatory properties via the augmentation of nonspecific host defence mechanisms following macrophage activation (Al-Ghandour et al., Reference Al-Ghandour, Ahmed, Salem, Tealeb, Mohamed and Yousef2020).
The larvicidal effect of propolis could be suggested because of its saponin content. Drescher et al. (Reference Drescher, Klein, Neumann, Yanez and Leonhardt2017) reported that the chemical analysis of propolis led to the detection of at least 300 compounds in its composition, with saponin being among them. The combination of the saponin molecule and the cholesterol molecules existing in the cuticle membranes of the larvae of the Aedes aegypti mosquito causes the disarrangement of this membrane and a rupture of the larval body surface (Wiesman & Chapagain, Reference Wiesman and Chapagain2006).
The antiparasitic effect of Se in the form of a considerable reduction in the number of muscle larvae is explained by the antioxidant defence system and active immunological response in T. spiralis-infected rats (Bass & Szejda, Reference Bass and Szejda1979; Gabrashanska et al., Reference Gabrashanska, Teodorova, Petkova, Mihov, Anisimova and Ivanov2010). The intensification of the antioxidant processes is related to the presence of the larvae, which causes an increase in phagocyte production, thus leading to ROS generation (Hughes, Reference Hughes1988; Gabrashanska et al., Reference Gabrashanska, Teodorova, Petkova, Mihov, Anisimova and Ivanov2010). The use of Se has a favourable effect on the immune system (McKenzie et al., Reference McKenzie, Rafferty and Beckett1998; Hoffmann & Berry, Reference Hoffmann and Berry2008). Kumar et al. (Reference Kumar, Garg, Mudgal, Dass, Chaturvedi and Varshney2008) found that the administration of Se as sodium selenite to lambs positively affected their immunological response and improved their antioxidant status. Se provided by Se supplementation can increase the function of cytotoxic effector cells and may also be important for maintaining T-cell maturation and functions, as well as T-cell-dependent antibody production (Bae & Kim, Reference Bae and Kim2020).
Furthermore, Nelson et al. (Reference Nelson, Shay, James, Carlson, Urban and Prabhu2016) reported that mice infected with N. brasiliensis that were fed on dietary Se exhibited a rapid and biased Th2-type response, producing elevated levels of interleukin (IL)-4 and IL-13. These cytokines are thought to play a major role in intestinal physiology by causing the rapid expulsion of parasites from the intestine and decreasing in parasite egg production (fecundity). This is likely achieved through increased selenoprotein activity in alternatively activated macrophages in the small intestine and IL-13 upregulation in the jejunum of infected mice, which was responsible for helminth clearance. This is likely because IL-13 also increases the endogenous production of 15d-PGJ2 in macrophages (Berry et al., Reference Berry, Balard and Coste2007).
As a possible larvicidal mechanism, SeNPs may denature the special sulphur-containing proteins and phosphorus-containing compounds, such as DNA, leading to the denaturation of vital organelles, decreasing membrane permeability and reducing or disrupting adenosine 5′-triphosphate synthesis, which ultimately leads to cell death in mosquito larvae (Sowndarya et al., Reference Sowndarya, Ramkumar and Shivakumar2017; Krishnan et al., Reference Krishnan, Ranganathan, Maadhu, Thangavelu, Kundan and Arjunan2020).
Regarding our electron microscopic results, the adult worm cuticle was damaged, with loss of annulations and absence of hypodermal gland opening in all treated groups. There were many vesicles and blebs in the adult worm cuticle in all treated groups, with the exception of the combination group of SeNPs and propolis, in which the cuticle was completely destroyed. Sloughing of some cuticle areas was detected in the albendazole, SeNPs and combination of SeNPs and propolis groups. Contrarily, the cuticle retained its normal morphology with the opening of hypodermal glands and circular annulations in the positive control group.
Other researchers studied the destructive effects of Egyptian propolis against several adult parasites. Hassan et al. (Reference Hassan, Abou-El-Dobal and Hegazi2016) reported that the in vitro incubation of mature T. vitulorum with Egyptian propolis induced injury to the tegument and deformity of sensory papillae and excretory pore. In addition, Hegazi et al. (Reference Hegazi, Abd El Hady and Shalaby2007) studied the effect of Egyptian propolis on adult F. gigantica. They found that it distorted the oral and ventral suckers and caused tegumental lesions and loss of spines.
Furthermore, the change in the structural architecture of treated T. spiralis was consistent with that reported in nematode species treated with chemical medicines in vitro, as demonstrated by Shalaby et al. (Reference Shalaby, El Namaky, Khalil and Kandil2012), based on the hypothesis that anthelmintics are delivered to parasites via oral consumption or diffusion through the external surface (Martin et al., Reference Martin, Robertson and Bjorn1997). Passive diffusion of anthelmintics through the cuticle or tegument might be responsible for the destructive changes and deformation of the body surface of trematodes, nematodes and cestodes (Schmahl et al., Reference Schmahl, Mehlhorn, Harder, Klimpel and Krieger2007).
In this study, the treatment of Trichinella worms with Se, SeNPs, propolis and albendazole disrupted the aforementioned physiological processes as a result of irreversible cuticle damage, which allowed the drugs to penetrate deeply into the tissues and cause greater and widespread damage, resulting in adult worm death. Blebbing is an attempt by the parasite to replace the damaged surface membrane in response to drug action (Stitt & Fairweather, Reference Stitt and Fairweather1993). Therefore, tegumental changes can be considered as a good indicator of the possible anti-nematocidal activity of a drug.
Regarding the histopathological changes in the small intestine, the groups treated with albendazole, SeNPs and combination of SeNPs and propolis showed elongation of villi with proliferative hyperplastic villous enterocytes and crypts, as well as a significant decrease in inflammation intensity compared with the positive control group, which exhibited dense inflammatory cell infiltration mainly in the core of the villi and extending into the submucosa with flat sloughed villous tips and atrophied crypts. Conversely, the groups treated with propolis, Se and combination of propolis and Se exhibited moderate inflammatory cell infiltration in the core of villi and hyperplastic crypts.
In addition to the positive control group, albendazole, SeNPs and combination of SeNPs and propolis groups exhibited a considerable reduction of larval deposition in muscles and a highly significant reduction in inflammatory cell infiltration around the larvae. Contrarily, the propolis, Se and combination of propolis and Se groups exhibited a moderate reduction in muscle larval count and moderate inflammation around the larvae.
This was in agreement with the findings of Dkhil et al. (Reference Dkhil, Khalil, Diab, Bauomy, Santourlidis, Al-Shaebi and Al-Quraishy2019), who reported that the jejunum showed evident amelioration with a few lesions detected in the jejunal tissues in the SeNPs-treated group against murine intestinal schistosomiasis. In addition, Al-Ghandour et al. (Reference Al-Ghandour, Ahmed, Salem, Tealeb, Mohamed and Yousef2020) reported that the intestine of mice with giardiasis and treated with propolis exhibited a moderate inflammatory cell infiltrate in the lamina propria, broad villi and hyperplastic crypts on the 12th dpi, with Giardia trophozoites detected between villi.
In accordance with the results of the present study, Salama et al. (Reference Salama, Mostafa, Abd EL-Aal, Mostafa, Hammad, Adel and Moawad2021) found that natural remedies such as Capsicum frutescens and Citrus limon extracts had a strong nematicidal effect on murine trichinosis, with percentage reductions in adult worm count of 68.54% and 58%, respectively, and percentage reductions in larval count of 71.6% and 61%, respectively. Compared with the positive control group, the C. frutescens group showed elongation of villi and a considerable reduction in inflammation intensity in the small intestine. The C. frutescens extract was more effective than was the C. limon extract. Compared with the positive control group, the C. frutescens group had a significantly lower number of larvae deposited in the muscles and a considerably lower amount of inflammatory cell infiltration around the larvae. Conversely, the C. limon group had a slight decrease in muscle larval count and moderate inflammation around the larvae.
The inflammatory changes observed in the intestine and muscles of infected mice treated with propolis ware attributed to its components. CAPE is a major constituent of propolis. It is a potent modulator of arachidonic acid (AA), prevents the release of AA from the cell membrane and inhibits the expression of the LOX and COX genes, which encode enzymes involved in the AA metabolic pathways (Mirzoeva & Calder, Reference Mirzoeva and Calder1996). COX-2 is an enzyme that promotes the production of prostaglandins and thromboxane, which cause marked inflammation and pain (Cannon & Cannon, Reference Cannon and Cannon2012). In in vitro and in vivo conditions, the ethanol extract of propolis inhibited leukotriene and prostaglandin production. The effect of propolis on COX may be the result of the action of its flavonoids, which have been demonstrated to suppress prostaglandin endoperoxide synthase (Pahlavani et al., Reference Pahlavani, Malekahmadi, Firouzi, Rostami, Sedaghat, Moghaddam, Ferns, Navashenaq, Reazvani, Safarian and Ghayour-Mobarhan2020).
Nelson et al. (Reference Nelson, Shay, James, Carlson, Urban and Prabhu2016) reported that mice infected with N. brasiliensis fed on dietary Se, were characterized by a rapid and biased Th2-type response, producing elevated levels of IL-4 and IL-13 and causing rapid expulsion of parasites from the intestine. Interestingly, a robust Th2 response inhibits the generation of a Th1 response, thus protecting the host from excess inflammation as well as priming the intestine for increased infiltration of macrophages, basophils and eosinophils.
Moreover, Ren et al. (Reference Ren, Zhang, Lin, Ma and Yan2019) reported that supplementation with SeNPs reduced the inflammatory process in rats with rheumatoid arthritis (RA) by decreasing proinflammatory cytokines (TNF-α, IL-1 and IL-6) and increasing the levels of antioxidants in the ankle joints of rats with RA. Furthermore, COX-2 expression was reduced by modulation of COX-2 gene expression in the groups supplemented with SeNPs.
Angiogenesis within the nurse cells is necessary to maintain the viability and healthy development of muscle larvae. The formation of circulatory rete around nurse cells is required for larval nutrition and waste removal (Ock et al., Reference Ock, Cha and Choi2013). During this process, VEGF acts as the chief vascular endothelial stimulating factor (Capó et al., Reference Capó, Despommier and Polvere1998).
Regarding the angiogenic results of this study, the VEGF expression was significantly reduced in all treated groups compared with the infected control group: a weak reaction occurred in the group treated with albendazole, SeNPs and combination of SeNPs and propolis; in turn, a moderate reaction was observed in the groups treated with propolis, Se and combination of propolis and Se. Its anti-angiogenic effect is the most beneficial anti-cancer function of propolis. It inhibits tumour angiogenesis by triggering death in tube-forming endothelial cells (Ohta et al., Reference Ohta, Kunimasa, Kobayashi, Sakamoto and Kaji2008). Kaempferol and galangin are propolis components that exhibit anti-angiogenic activities (Kim et al., Reference Kim, Liu, Guo and Meydani2006). Increased production of the anti-angiogenic factor tissue inhibitor of metalloproteinases-1 in splenocytes in mice with Ehrlich ascites cell carcinoma could also explain the anti-angiogenic effect of Se (Azab et al., Reference Azab, Hanafy, El Fateh and Badr2015). Furthermore, Rajkumar et al. (Reference Rajkumar, Sandhya, Koganti and Burgula2020) suggested the potential use of SeNPs in preventing neovascularization in tumours.
In conclusion, this study demonstrated for the first time that the combination of SeNPs and propolis has antiparasitic, anti-inflammatory and anti-angiogenic effects on trichinosis. Consequently, this combination could be used as a natural alternative therapy to albendazole for treating trichinosis. This combination damaged the adult worm cuticle. Furthermore, it caused a significant reduction in adult worm and total larval counts. Moreover, it reduced the expression of the angiogenic marker VEGF in muscles, which could be beneficial in reducing Trichinella-associated myositis and depriving the parasite from its nutrition, thus causing its death. The results of this study represent a novel therapeutic approach of using SeNPs and propolis against murine trichinosis. Further studies are necessary to evaluate the therapeutic effect of propolis-loaded nanoparticles in experimental T. spiralis-infected mice as an alternative natural product for the treatment of T. spiralis.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The ethics committee of the Faculty of Medicine of Zagazig University approved the study's procedure (approval number: ZU-IACUC/3/F/30/2019).









