Introduction
Increasing numbers of species invasions have led to the examination of factors that influence colonization of introduced species. Hosts introduced to new habitats might experience relatively high fitness, because of a loss of most parasite species they would encounter in endemic habitats (Torchin et al., Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003; Simberloff, Reference Simberloff, Sodhi and Ehrlich2010). This loss could even include parasites that travel with a host to a new habitat, but fail to establish. Hosts also generally experience a low acquisition of new parasite species in newly colonized habitats (Torchin et al., Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003) and, because they are freed from parasitic infection, may demonstrate enhanced competitiveness and invasion success in introduced habitats (Torchin et al., Reference Torchin, Lafferty and Kuris2001; Marr et al., Reference Marr, Mautz and Hara2008). Evidence for parasite release mainly comes from observational studies that have reported reduced parasite species richness in hosts from introduced habitats, relative to native or endemic ranges (Dobson, Reference Dobson1988; Torchin et al., Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003), and hosts within restricted areas are found to harbour fewer parasite species than those in larger communities (Morand & Guegan, Reference Morand and Guegan2000).
The distribution patterns of parasites in introduced host populations can also be influenced by their species-specific patterns of association with hosts in native habitats. Parasite prevalence (the proportion of sampled individuals that are infected by a single parasite species) tends to be higher among host populations of higher densities. It is believed that this variation is driven by transmission potential within host populations of different densities. Thus, one might surmise that introduced host populations initially would have lower parasite prevalence because host species introductions generally involve the translocation of small numbers of individuals into new habitats. However, parasites have been found to achieve levels of prevalence among hosts in introduced habitats comparable to, or higher than, those of endemic populations (Dunn, Reference Dunn2009). Notably, the parasites that are lost during the translocation of hosts into introduced habitats tend to be those of low prevalence in endemic habitats (see Torchin et al., Reference Torchin, Byers and Huspeni2005). Furthermore, hosts acquire few new parasite species in introduced habitats, and these parasites tend not to achieve higher prevalence in introduced hosts than in native hosts (Torchin et al., Reference Torchin, Byers and Huspeni2005). In the case of repeated introductions of few individuals collected from various native host populations, parasite prevalence has been observed to vary among the different introduced host populations depending on the prevalence of source native populations (Kulisic et al., Reference Kulisic, Lepojev, Aleksic-bakrac, Jakic, Pavlovic, Milutinovic and Misic2004).
The impact of parasite release on host fitness is predicted to influence the capacity for invasiveness. Hosts with weak or no defence against parasites are expected to experience a direct ‘regulatory release’ from fewer parasites, whereby fitness traits such as survival and fecundity are enhanced (Colautti et al., Reference Colautti, Ricciardi, Grigorovich and MacIsaac2004). For those hosts that do launch a defence against infection, the removal of fitness costs associated with resistance to infection and convalescence allows more resources to be directed towards somatic and reproductive development, that is, a ‘compensatory release’ is expected. The two forms of release may not be exclusive, yet studies have focused on the compensatory form of release because host developmental traits, such as growth rate and size, can be affected. These developmental traits are known to influence fitness and competitive ability (Goater et al., Reference Goater, Semlitsch and Bernasconi1993; Lampo & Bayliss, Reference Lampo and Bayliss1996) and have been linked to enhanced establishment success and superior competitiveness of introduced species (Wolfe, Reference Wolfe2002; Grosholz & Ruiz, Reference Grosholz and Ruiz2003). There remain gaps in our understanding of parasite release. With rare exception (e.g. Marr et al., Reference Marr, Mautz and Hara2008; Roche et al., Reference Roche, Leung, Franco and Torchin2010), studies testing the parasite release hypothesis have looked at invertebrate models (for example, Ross et al., Reference Ross, Ivanova, Severns and Wilson2010), for which larger sample sizes can be more easily obtained.
The primary aim of this study was to evaluate parasite release for the invasive American bullfrog (Lithobates catesbeianus/Rana catesbeiana) by comparing populations in introduced and native habitats: we compared helminth species richness, and determined measures of infection (including prevalence, abundance and intensity) for parasite species expected to have fitness costs on their hosts. This study is unique because (1) we examined the American bullfrog, which is one of the most invasive vertebrates in North America (Da Silva et al., Reference Da Silva, Filho and Feio2011) that demonstrates super-competitiveness; (2) bullfrogs are known to carry high numbers of parasite species and thus potentially experience higher fitness costs of parasitism than other species; and (3) samples were collected from native and introduced populations with known ecological histories within North America.
Bullfrogs introduced to British Columbia, Canada, have not been examined to determine whether parasite composition and richness, or levels of prevalence, abundance and intensity, differ from endemic populations, although bullfrogs introduced to other regions are known to carry very different helminth fauna from bullfrogs from endemic populations (Goldberg et al., Reference Goldberg, Bursey and Cheam1998). Reports on parasite burdens of bullfrogs, both in native and other introduced ranges, generally have not made distinctions among ages of frogs in samples (but see Andrews et al., Reference Andrews, Lampley, Gillman, Corey, Ballard, Blasczyk and Dyer1992; Bursey & De Wolf, Reference Bursey and DeWolf1998; Goldberg et al., Reference Goldberg, Bursey and Cheam1998). Yet, research on demographic patterns of bullfrog spread on Vancouver Island, British Columbia, has shown that population growth rate is most sensitive to alterations in survival rates of the post-metamorphic age cohorts, rather than fecundity or larval (tadpole) survival (Govindarajulu et al., Reference Govindarajulu, Altwegg and Anholt2005). The diet, behaviour, immune system function and ecology of herbivore juvenile bullfrogs differ considerably from those of carnivorous adults and will influence exposure and susceptibility to parasites. We therefore made distinctions between age classes among our samples in order to determine any potential impact of parasites on survival rates of this species at its most vulnerable age and further assessed whether there were any age-dependent patterns of parasitism.
Materials and methods
Study system
Bullfrogs were introduced from south-eastern Canada to British Columbia in the early 1900s to support the gourmet culinary and garden ornamentation industries (Culley, Reference Culley1981). Bullfrogs have since been introduced in British Columbia to various backyard ponds, irrigation pools and lakes, where they have rapidly become invasive and are a pest species in the province. Less competitive native frog species that are forced to live sympatrically with bullfrogs may become vulnerable to extinction. Bullfrog invasiveness has been associated with the large size of adults (up to 750 g) with a wide gape size, low predation rates, unregulated increases in density and voracious appetites as tadpoles and adults. Higher fitness associated with parasite release might manifest itself as both higher growth and reproduction, thereby enabling bullfrogs to out-compete other species and alter community structure.
Other aspects of bullfrog natural history are relevant to this study. Bullfrogs are highly aquatic and stay closely affiliated with their natal lakes throughout their lives. Migrations of up to a maximum of about 2 km are reported only along watersheds (Willis et al., Reference Willis, Moyle and Baskett1956). Thus, for this species, migrations between bodies of water tend to occur rarely.
Collection and examination of frogs for parasites
We sampled a total of 76 bullfrogs from across all native (N= 26) and introduced sites (N= 50). Adult (>3 years old) and juvenile (1–2 years post-metamorphosis) bullfrogs were collected with dip and seine nets from five sites in the greater Victoria area, Vancouver Island, British Columbia, in the summer and autumn of 2003. They were euthanized with MS-222 and frozen for future necropsy. We also collected bullfrogs from a site in Ontario in the summer and autumn of 2007.
The five sites around Victoria were 2.5–7 km apart from one another. Trevlac pond was a peat bog/wetland that was mined and converted into a permanent lake. Bullfrogs colonized Trevlac (48 29 44.6N; 123 26 37.6W) in the 1990s and are the most recent introduction of host species among our samples. Better approximations of dates for introductions to the other four lakes are not available. Florence Lake (48 27 31.8N; 123 30 43.9W) and Eagle Lake (48 30 32.2N; 123 27 40.4W) are natural lakes. Wade pond is a man-made agricultural pond (48 33 13N; 123 26 58.0W). The Interurban pond (48 31 11.87N; 123 25 34.45W) is a dugout pond located near a highway.
We collected road-killed bullfrogs from Bishops Mills, Kemptville, Ontario (44 52 60.0N; 75 40 0.0W). The sample site is an extensive wetland area sectioned by roads, but not fragmented by large bodies of land. High vehicle-induced mortality occurs in this area, indicating significant movement of frogs across roads that divide the body of water. Consequently, we expect that our samples were from a large population of bullfrogs in the area that provided a broader parasite profile than would be observed from a single isolated lake or smaller pond population. The ample information available from other studies on the richness, prevalence and intensity of helminth parasites in bullfrogs in native habitats, made it possible to compare our observations with others, in particular, New Brunswick (McAlpine, Reference McAlpine1997). We collected adult and juvenile frogs over the summer and autumn to coincide with seasonal times of collection of bullfrogs from the Victoria sites, although collections were performed 4 years later. Collections were made within minutes of mortality, before any desiccation of the carcass occurred.
We examined lungs and kidneys of all frogs from Bishops Mills and Victoria sites for trematodes. However, one road-killed frog from Bishops Mills did not have kidneys present upon collection, and thus our sample size when enumerating kidney cysts was 25.
We also examined all organs and tissues under a microscope on a subsample (haphazardly selected) of seven and 13 bullfrogs from Bishops Mills and Victoria, respectively, to get a coarse measure of the general helminth species numbers in the various organs (table 1). We selected only fully intact individuals from Bishops Mills for this full necropsy. No juveniles from Bishops Mills were intact enough to examine all tissues, thus we examined only adults for the full necropsies. We also compared age-specific infection levels reported from bullfrogs in New Brunswick (McAlpine, Reference McAlpine1997). Both juveniles and adults collected from Victoria were examined. Our identifications of the helminths were informed by previous work on amphibians (Dare & Forbes, Reference Dare and Forbes2009) and standard morphological identification keys (e.g. Prudhoe & Bray, Reference Prudhoe and Bray1982).
Table 1 Numbers of juvenile and adult male (M) and female (F) frogs sampled from Bishops Mills, Ontario, and from Victoria sites, British Columbia.

Data analysis
All analyses were conducted with SAS (version 9.1.3, 2008; SAS Institute Inc., Cary, North Carolina, USA). A t-test for samples with unequal variance was performed on helminth species richness among frogs from Victoria and Bishops Mills. For prevalence of lung fluke and echinostome infections, we performed two-sample tests of equality of proportions. This test of equality directly compares the two proportions and generates confidence intervals on the differences in proportions. We have reported results of non-parametric univariate tests (Wilcoxon/Kruskal–Wallis) for data that did not pass Levene's test for homogeneity of variances when testing for differences in abundance and intensity of lung flukes and echinostome infections. Z and P statistics are reported for normal approximations where samples are greater than ten (when comparing among Victoria and Bishops Mills sites) and chi-squared approximations are reported for samples lower than ten (when comparing among the five sites in Victoria).
We tested for age effects on lung fluke and echinostome infections among the Victoria and Bishops Mills sites using one-way analyses of variance (ANOVA). We also tested whether there was any association between sex and parasitism, because sex bias in lung fluke infections in bullfrogs has been reported on rare occasions (Whitehouse, Reference Whitehouse2002). However, sex was not significantly associated with abundance or intensity of either lung or kidney flukes in hosts from either location (P values ranged from 0.12 to 0.82) and was excluded from further consideration in our analyses.
Results
Parasite species richness
We examined 50 individuals from the five sites in Victoria (N juv= 30; N adult= 20). We observed a total of three taxonomic groups (Nematoda, Acanthocephala and Trematoda, to a total of nine separate, unidentified helminth species) from 13 sub-sampled fully necropsied adult and juvenile bullfrogs. We also observed adult trematodes of the genus Haematoloechus spp. (lungs) and metacercariae of Echinostoma spp. (kidney cysts). Species from these last two genus complexes are difficult to distinguish solely from morphological characteristics (Bolek & Janovy, Reference Bolek and Janovy2007). Of note, two of the 13 fully necropsied frogs from the Victoria sites had no parasites whatsoever, and seven frogs only had flukes in their lungs. Furthermore, there were no parasites observed in the body cavity, liver, muscle or urinary bladder of any frogs collected from the Victoria sites.
We collected 26 adult and juvenile frogs from Bishops Mills (N juv= 11; N adult= 15). From the seven frogs we necropsied, we observed a total of 19 helminth species (Nematoda, Cestoda and Trematoda). These helminths were observed in the muscle, body cavity, urinary bladder, liver and gastrointestinal tract. In addition, Haematoloechus spp. was observed in lungs (a single worm) and Echinostoma spp. cysts in kidneys.
Frogs from Victoria carried, on average, 1.46 ± 0.31(standard error) helminth species per individual (range 0–3), whereas frogs from Bishops Mills carried on average 5.6 ± 0.99 helminth species (range 2–9) (t-statistic unequal variances = 4.087, P= 0.004, df = 1,8).
Lung flukes (Haematoloechus spp.)
Ninety per cent of bullfrogs from the Victoria sites had Haematoloechus spp. in their lungs (45/50 infected; 18 adults and 27 juveniles). In contrast, 42.3% of the frogs from Bishops Mills carried Haematoloechus spp. (11/26 infected: 7 adults and 4 juveniles) (test of equality of proportions: Z= 4.48, P< 0.0001, confidence interval (CI) = 0.27–0.68). Frogs from the Victoria sites carried significantly higher abundances and intensities of lung flukes than did frogs from Bishops Mills (abundance: Z= − 5.925, P< 0.0001; intensity: Z= − 4.66, P< 0.0001). The median abundance of worms carried by the 50 frogs from the Victoria sites was 27 (interquartile range (IQR) = 11–40; range of intensity = 29–136 worms/frog) (table 2), while the median abundance of worms carried by the 26 frogs from Bishops Mills was 0 (IQR = 0–1; range of intensity = 1–23 worms/frog).
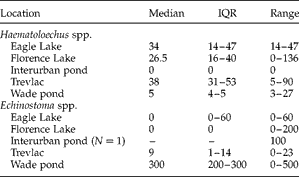
Table 2 Median abundance, interquartile range (IQR) and actual range of Haematoloechus spp. (lung flukes) and Echinostoma spp. (kidney flukes) cysts from bullfrogs collected among five sites in Victoria, British Columbia.
Kidney flukes (Echinostoma spp.)
Location also significantly influenced the prevalence, abundance and intensities of echinostome cysts. However, the pattern of association was opposite to that observed for the lung flukes. That is, the prevalence of echinostome cysts in the kidneys of bullfrogs from the Victoria sites was 34% (17/50 individuals infected: 7 adults and 10 juveniles), whereas all 25 bullfrogs with kidneys present from Bishops Mills harboured echinostome cysts (test of equality of proportions: Z= − 5.43, P< 0.0001, CI = − 0.791 to − 0.53). Frogs from the Victoria sites harboured fewer kidney cysts than did frogs from Bishops Mills (abundance: Z= 6.13, P< 0.0001; intensity: Z= − 2.79, P= 0.005). The median number of cysts carried by frogs from Victoria was 0 (IQR = 0–6; range of intensity: 1–500 cysts/frog), while the median number of cysts carried by frogs from Bishops Mills was 128 (IQR = 100–500; range of intensity: 16–1000 cysts/frog). Bullfrogs from the single agricultural pond (Wade pond) carried an average of 260 ± 81.24 cysts/frog, compared with an average of 35.38 ± 13.75 cysts/frog observed in frogs from the other four locations (table 2).
We also checked for differences in abundance and intensity of lung flukes and echinostomes among the five Victoria sites. Location significantly influenced both the abundance and intensity of lung flukes (abundance: χ2= 10.78, P= 0.02; intensity: χ2= 10.22, P= 0.02). Location also influenced abundance and intensity of echinostomes (abundance: χ2= 22.54, P= 0.002; intensity: χ2= 10.38, P= 0.03).
Host age effects and parasitism
Differences in ages of bullfrogs may have influenced the above results. However, there were roughly similar proportions of juvenile and adult frogs from across our samples (table 1). Furthermore, age did not have a significant effect on abundance or intensity of lung flukes in frogs from the Victoria sites (ANOVA: abundance: F= 1.53, df = 1,48, P= 0.22; intensity: F= 1.78, df = 1,43, P= 0.19) or Bishops Mills (abundance: F= 1.59, df = 1,24, P= 0.22; intensity: F= 3.19, df = 1,9, P= 0.11). Age also did not significantly affect abundance or intensity of echinostome cysts in the kidneys of frogs from the Victoria sites (abundance: F= 0.00, df = 1,48, P= 0.99; intensity: F= 0.01, df = 1,15, P= 0.93) sites or Bishops Mills (abundance/intensity – all hosts were infected: F= 8.32, df = 1,23, P= 0.08). Thus we were able to examine differences in infections among populations without controlling for age.
Discussion
This study identified differences in parasite species richness and abundance between frogs from native and introduced sites and, more importantly, elucidated striking differences in prevalence of infection for those parasites believed to impose higher costs to host fitness. Despite our likely underestimation of true parasite species richness for both locations, due to the limited sample sizes, bullfrogs from Victoria with an average of 1.46 helminth species had significantly lower parasite species richness than those in Ontario. The observed average of 5.6 helminth species per frog from Bishops Mills falls within the range reported by McAlpine (Reference McAlpine1997) who documented an average of 2.5 helminth species per frog (2.7 for juveniles), with a range that extended up to 21 helminth species per host. The highest numbers of helminth species that individuals in New Brunswick have been known to carry far exceeds any numbers observed in bullfrogs from Victoria. Other studies have also reported lower parasite richness in bullfrogs in other introduced ranges (e.g. south-western USA, Goldberg et al., Reference Goldberg, Bursey and Cheam1998), though no studies have reported such a contrast in levels of lung and kidney fluke infections.
Our observations of the lung flukes that we examined did not support the prediction of the parasite release hypothesis that levels of parasitism by those species thought to cause fitness reductions in their hosts, would be lower for invasive than for native or endemic hosts (Torchin et al., Reference Torchin, Byers and Huspeni2005). While almost all the bullfrogs from Victoria carried Haematoloechus spp. infections in their lungs, fewer than half of the bullfrogs from the native Bishops Mills population were infected. In comparison, other studies have reported prevalence levels of various species of Haematoloechus spp. in endemic populations of bullfrogs that range between 1 and 50% (Andrews et al., Reference Andrews, Lampley, Gillman, Corey, Ballard, Blasczyk and Dyer1992; Bursey & DeWolf, Reference Bursey and DeWolf1998). We also observed a much higher intensity of lung flukes in the bullfrogs from Victoria than has been reported in the literature (Brandt, Reference Brandt1936; Goldberg et al., Reference Goldberg, Bursey and Cheam1998), and contrasts with the range of intensity of infection that we observed in the native Bishops Mills population. Yet, the intensity of lung flukes in bullfrogs from Bishops Mills corresponds to levels reported for populations in New Brunswick (McAlpine, Reference McAlpine1997). Thus it appears that levels of Haematoloechus spp. infection observed around Victoria are uniquely high.
Infection by Haematoloechus and other blood-feeding lung helminths are known to damage the integrity of lung parenchyma and negatively impact lung function, stamina, growth and survival, and cause anorexia of anuran hosts (Goater & Ward, Reference Goater and Ward1992; Goater et al., Reference Goater, Semlitsch and Bernasconi1993). Such damage to lungs may also impede chorusing ability of males during the breeding season, thereby lowering reproductive success of infected individuals. Thus high infections of this parasite likely impose significant fitness costs on hosts and influence which males in a population are able to reproduce successfully.
Haematoloechus flukes are acquired when frog hosts consume infected naiad or teneral anisopteran and zygopteran odonates. These flukes display a wide range of patterns of odonate host specificity, from specialist to broad generalist (Snyder & Tkach, Reference Snyder and Tkach2001). As such, variation in host specificity likely influences the fitness and establishment of lung flukes across the geographic ranges of odonate hosts. Furthermore, patterns of distribution and density of various species of odonates closely define levels of exposure of infective stage cercariae (Kennedy, 1981; Bolek & Janovy, Reference Bolek and Janovy2007). Studies identifying Haematoloechus species in the region, and patterns of bullfrog infections as they relate to the distribution patterns of odonates, are needed to explain the unusual levels of intensity and prevalence of lungworms in bullfrogs in Victoria.
The much lower prevalence of echinostome infection among the bullfrogs from Victoria relative to bullfrogs from Bishops Mills suggests release from these flukes. The high prevalence of echinostome infections among the Bishops Mills population is consistent with reports of prevalences of 80% or higher in New Brunswick populations of bullfrogs (McAlpine, Reference McAlpine1997). The ability to achieve such a high prevalence is likely connected to density and mode of transmission of the infective cercarial stage of echinostomes. Hosts that acquire parasites through active feeding habits of invertebrates generally carry lower burdens than parasites acquired through passive means (Shaw & Dobson, Reference Shaw and Dobson1995). However, echinostome cercariae swim up the cloaca of hosts and travel to the renal system of amphibians during larval and post-metamorphic stages (Schotthoefer et al., Reference Schotthoefer, Cole and Beasley2003). Thus for echinostomes, infection of a host is passive. Population densities of bullfrogs in Victoria (4.1–530 frogs/ha) (Govindarajulu et al., Reference Govindarajulu, Price and Anholt2006) fall within the range of densities observed in Ontario populations (4.2–1377 frogs/ha) (Shirose et al., Reference Shirose, Brooks, Barta and Desser1993). Host density is therefore not likely to be a limiting factor in transmission. Assuming the parasites possess the same ability to infect hosts in either introduced or endemic habitats, differences in infection levels are then presumably due to density- and/or distribution-dependent attributes of suitable intermediate gastropod hosts.
Echinostome infections have been associated with low juvenile amphibian recruitment (Beasley et al., Reference Beasley, Faeh, Wikoff, Eisold, Nichols, Cole, Schotthoefer, Staehle, Greenwell, Brown and Lannoo2003). Mortality rates between 40 and 100% have been reported with echinostome infections of larval frogs (Schotthoeffer et al., Reference Schotthoefer, Cole and Beasley2003; Holland et al., Reference Holland, Skelly, Kashgarian, Bolden, Harrison and Capello2007). Tadpoles of other species are known to exhibit oedema and inhibited growth (Fried et al., Reference Fried, Pane and Reddy1997), although older frogs do not display the same lack of resistance to infection (Schotthoeffer et al., Reference Schotthoefer, Cole and Beasley2003). The lower intensity of cysts among the introduced populations suggests that any regulatory effect of echinostome infection on early tadpole stage individuals is likely lower than that experienced by hosts in Bishops Mills. In such a manner, introduced bullfrogs from Victoria may experience parasite release from this one trematode species.
As expected, echinostome intensity varied among bullfrogs from Victoria, but it is interesting to note that the highest infections were found in bullfrogs collected from the only agricultural pond (Wade pond). This exceptional observation supports other studies that have shown linkages between agricultural activity and higher levels of fluke infection in amphibians (Johnson et al., Reference Johnson, Chase, Dosch, Hartson, Gross, Larson, Sutherland and Carpenter2007). However, the mechanistic bases for these linkages remain equivocal.
Our main questions were confined to a comparison of parasite profiles between a single region from the bullfrog's introduced range in Victoria, and native ranges that we sampled and evaluated from the literature. However, the five sample sites in Victoria appeared to be distinct, with considerable variability in parasitism, parasite intensity and species richness among the sites.
Bullfrogs in our study were classified by age cohort, as behavioural patterns of individuals that may influence exposure to these parasites, such as feeding habits, may be linked to stage of development and not size. Juvenile bullfrogs in New Brunswick are more heavily parasitized than adults, with higher abundances and higher numbers of helminth species (McAlpine, Reference McAlpine1997). However, we observed no differences in any parasite measure between juvenile and adult bullfrogs from either Victoria or Bishops Mills. Our small sample sizes precluded robust statistical analyses that would control for an interaction between size and age. We also did not observe any linkages to host sex in our samples for either Haematoloechus spp. or Echinostoma spp. Other studies have also shown no differences between sexes in helminth species richness or abundance in adult or juvenile bullfrogs (McAlpine, Reference McAlpine1997).
Anurans typically carry high parasite burdens; bullfrogs in particular are known to carry exceptionally high numbers and intensities of metazoan parasites (Brandt, Reference Brandt1936; Muzzall, Reference Muzzall1991). In some amphibian populations, the prevalence of trematode infections can be as high as 85% and at intensities high enough to impose regulatory effects on the populations (Zelmer & Esch, Reference Zelmer and Esch2000). Thus the release from parasites that impose high fitness costs could have a considerable positive effect on survival, to the extent that in the absence of this regulatory factor, competitive species can rapidly increase and displace other species.
The parasite release hypothesis as a mechanism for determining host establishment and regulating invasiveness is widely debated, and the actual extent to which parasites have an impact (direct or indirect) on host invasiveness is still unknown (Roche et al., Reference Roche, Leung, Franco and Torchin2010). It is important to note that parasite species may not impose significant fitness costs on their hosts. Future research needs to address the effects of release from parasite species for which there are known fitness costs. Hosts that harbour reduced parasite species and lower numbers of parasites may still incur fitness costs, yet the magnitude of the cost will depend both on parasite virulence (Drake, Reference Drake2003) and nature of trade-offs associated with defence. For highly virulent parasites, fitness costs of infection are likely to be high, even with non-lethal infections (Read, Reference Read1994). Thus measures of parasite abundance, prevalence and/ or intensity provide only a partial picture. Research to date has only provided profiles of abundance, intensity and prevalence of parasitic infection in endemic and introduced habitats. Still needed are measures of the fitness costs associated with those translocated parasite species that are able to establish in new habitats, and importantly, the ultimate effect that these parasites have on the establishment of their hosts.
Ecologically, bullfrogs present in Vancouver do not differ from bullfrogs in native ranges. Govindarajulu et al. (Reference Govindarajulu, Price and Anholt2006) revealed that life history traits, including breeding times, length of winter torpor and timing of spring emergence, are similar to those of Ontario populations. It is presumed that accelerated growth rates and enhanced body sizes that are characteristic of invasive species, are likely only apparent in bullfrogs within the first 3 years following colonization. This is because of the high reproductive capacity of the species that enables rapid population explosions, which, in turn, produce intraspecific density-dependent pressures that regulate developmental rates and sizes of post-metamorphic individuals (Govindarajulu et al., Reference Govindarajulu, Altwegg and Anholt2005). Thus, any gross responses of bullfrog populations to parasitism are likely only apparent within the first 5 years of introduction into a new habitat, when rapid growth and increase in population density is more sensitive to the impact of infection. Other confounding intraspecific competitive or predation pressures associated with higher population densities may mask the influence of parasitism at a later time. The high Haematoloechus spp. infections appear to be compensated by the host's other adaptive traits. However, the influence of parasite release may act synergistically with other factors and contribute to the post-invasion establishment of introduced hosts, although parasitism may not, by itself, be a regulating factor.
Our study has shown that helminth species composition of bullfrogs in Victoria, British Columbia, differs dramatically from that of endemic populations in Ontario and New Brunswick; these changes have occurred and persisted independently of increases in host densities and low vagility. We have also identified unusual levels of lung and kidney fluke infections in bullfrogs from the Victoria sites that likely impact host fitness in different ways, and at different stages of development. Differences in infection levels between the introduced and endemic ranges likely relate to variation in other ecological factors, such as intermediate host species composition and density. Despite the expected inhibitory effects of lung and kidney fluke infections, bullfrogs appear able to compensate for infection and still invade new habitats successfully. This study highlights the importance of species-specific approaches to determining whether parasite release occurs.
Acknowledgements
We thank Frederick Schueler for his invaluable assistance in the collection of bullfrogs from Bishops Mills. We also thank Purnima Govindarajulu for the bullfrog samples from British Columbia and for sharing her wealth of information on the ecology of bullfrogs. Funding for this research was provided by a Natural Sciences and Engineering Research Council (NSERC) Post Graduate Scholarship-Doctorate (PGS-D) for O.D. and an NSERC Discovery grant to M.R.F.




